Abstract
Shiga toxin (Stx) causes diarrhea-associated hemolytic uremic syndrome by damaging renal microvascular endothelium. The pentameric B subunits of Stx types 1 and 2 (Stx1B and Stx2B) are sufficient to stimulate acute VWF secretion from endothelial cells, but Stx1B and Stx2B exert distinct effects on Ca2+ and cAMP pathways. Therefore, we investigated other signaling components in StxB-induced VWF exocytosis. Incubation of HUVECs with StxB transiently increased phospholipase D (PLD) activity. Inhibition of PLD activity or shRNA-mediated PLD1 knockdown abolished StxB-induced VWF secretion. In addition, treatment with StxB triggered actin polymerization, enhanced endothelial monolayer permeability, and activated RhoA. PLD activation and VWF secretion induced by Stx1B were abolished on protein kinase Cα (PKCα) inhibition or gene silencing but were only moderately reduced by Rho or Rho kinase inhibitors. Conversely, PLD activation and VWF exocytosis induced by Stx2B were reduced by Rho/Rho kinase inhibitors and dominant-negative RhoA, whereas attenuation of PKCα did not affect either process. Another PLD1 activator, ADP-ribosylation factor 6, was involved in VWF secretion induced by Stx1B or Stx2B, but not histamine. These data indicate that Stx1B and Stx2B induce acute VWF secretion in a PLD1-dependent manner but do so by differentially modulating PKCα, RhoA, and ADP-ribosylation factor 6.
Introduction
Ingestion of Shiga toxin (Stx-producing Escherichia coli is a primary cause of hemorrhagic colitis, which in some cases progresses to diarrhea-associated hemolytic uremic syndrome (D+HUS). The classic clinical features of D+HUS include microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. D+HUS is a common cause of renal failure in children and is fatal in ∼ 3%-5% of cases.1
Stx-producing E coli make 2 main subtypes of Stx, Stx1 and Stx2, both of which are complex holotoxins with an AB5 structure. The B subunits form a noncovalent pentamer that mediates toxin adhesion to globotriaosylceramide (Gb3) on the cell membrane. Binding is followed by internalization, retrograde transport to the endoplasmic reticulum, and translocation of the A subunit into the cytoplasm. The A subunit has N-glycosidase activity and cleaves adenine at position 4324 from the 28S ribosomal RNA of the 60S ribosome. This cleavage inhibits protein synthesis and initiates a cascade of reactions called the ribotoxic stress response, which ultimately leads to cell death.2
Stxs activate a panel of signaling molecules in tissue culture cells. In renal ACHN (human kidney adenocarcinoma) cells, binding of Stx to Gb3 triggers a cascade of reactions that lead to cytoskeleton remodeling.3 In HeLa cells, Stx induces phosphorylation of clathrin heavy chain and promotes Stx endocytosis.4 Stx also induces the activation of the mitogen-activated protein kinase p38 and protein kinase C-δ (PKCδ), both of which appear to modulate Stx intracellular transport.5,6
An earlier study reported that incubation of HUVECs or human glomerular microvascular endothelial cells with nanomolar concentrations of Stx1 or Stx2 induces rapid VWF secretion, as well as the formation of platelet-bound VWF strings.7 However, the signaling pathways involved in Stx-mediated endothelial activation and VWF secretion are incompletely understood. We recently reported that the B subunits of Stx1 (Stx1B) or Stx2 (Stx2B) are sufficient to stimulate the acute secretion of ultra large VWF and to support platelet adhesion on human endothelial cells. Furthermore, Stx2B can induce a thrombotic thrombocytopenic purpura-like syndrome in Adamts13 deficient mice but not in heterozygous littermates.8 In addition, Stx1B increases intracellular Ca2+ and activates PKCα, whereas Stx2B instead activates protein kinase A.9 These findings show the existence of StxB-induced signaling pathways that may contribute to endothelial damage and thrombotic microangiopathy.
Phospholipase D1 (PLD1) has been implicated in histamine and forskolin-induced VWF release from HUVECs10 ; therefore, it might participate in responses to other secretagogues. PLD hydrolyzes phosphatidylcholine, resulting in the production of phosphatidic acid (PA). PA promotes the formation of negative membrane curvature and is an essential mediator of many of the downstream effects of PLD, including the regulation of cell migration, proliferation, and membrane traffic. PA can be converted to other lipid second messengers, such as diacylglycerol. Interestingly, PLD1 has been shown to play a role in several Ca2+-mediated exocytosis events, including mast cell degranulation, the release of insulin from pancreatic β cells, and regulated secretion from chromaffin and PC12 cells. PKCα as well as the small GTPases RhoA and ADP-ribosylation factor 6 (Arf6) have been shown to regulate PLD activity by different mechanisms,11 but the role of these GTPases in agonist-induced VWF secretion was unknown.
In this report, we show that Stx1B and Stx2B activate different signaling pathways that preferentially use distinct kinases or GTPases. Nevertheless, these pathways converge on PLD1 to induce VWF secretion.
Methods
Stx B subunit preparations
Stx1B and Stx2B (BEI Resources) were treated with Detoxi-Gel endotoxin removal columns (Pierce). Residual endotoxin was assayed with Limulus amoebocyte lysate (PYROGENT Plus test kit; Cambrex) or QCL-1000 Chromogenic LAL End point Assay kit (Lonza) and was below 1 ng/mg of protein.
Endothelial cell culture and transfection
Pooled HUVECs (Lonza) were cultured in EGM-2 medium supplemented with endothelial growth factors (Lonza). HUVECs at passage 2-4 were transfected with HUVEC nucleofection kits (Lonza) or lipofectamine LTX with Plus reagent (Invitrogen).
PKCα silencing was performed with a mix of 2 small interfering RNA (siRNA) duplexes as described previously.9 Plasmids encoding shRNA that target PLD1 (mCherry-H1-PLD1) and firefly luciferase (mCherry-H1-Luc) were provided by Dr Guangwei Du (The University of Texas Health Science Center at Houston).12,13 Plasmids encoding wild-type Arf6–cyan fluorescent protein (CFP),14 dominant-negative Arf6(T27N)–CFP (dnArf6-CFP),14 and dominant-negative RhoA(T19N)–enhanced green fluorescent protein (EGFP; dnRhoA-EGFP)15 were from Addgene.
PLD assays
When indicated, before stimulation with StxB preparations of HUVECs in 24-well dishes were incubated in Medium 199 for 10 minutes with 25mM n-butanol or t-butanol (Sigma-Aldrich) or for 1 hour with 2μM Gö6976 or 100μM Y27632 (Calbiochem), or 1 μg/mL C3 exoenzyme (List Biologicals).
After treatment with agonists and/or inhibitors, HUVECs were scraped into ice-cold lysis buffer (25mM Tris-HCl, pH 8.0, 150mM NaCl, 0.1% SDS, 1% NP40, 1% deoxycholate) and incubated for 20 minutes. Cell debris was removed by centrifugation, and PLD activity in supernatant fluids was measured with an Amplex Red phospholipase D Assay kit (Invitrogen). Values for PLD activity were normalized to total protein concentration measured with a Coomassie Plus Protein assay (Pierce) and standardized with bovine serum albumin.
VWF secretion
Acutely secreted VWF on the surface of HUVECs was assayed by ELISA as described.8 Cell surface–associated VWF strings were assayed by immunofluorescence in perfusion chambers as described.8,9 VWF propeptide secreted into medium by HUVECs was assayed by ELISA as described16 with the use of monoclonal antibodies 239.2 and 239.3 for capture and biotinylated monoclonal antibodies 242.4 and 242.6 (Blood Research Institute) for detection. The calibrator was purified recombinant VWF propeptide. Bound antibodies were detected with alkaline phosphatase–conjugated streptavidin and p-nitrophenyl phosphate substrate (Thermo Scientific). Color development was stopped with 2N NaOH, and absorbance was measured at 405 nm.
Immunofluorescence
Confluent HUVECs on glass-bottomed dishes (MatTek) were incubated with Stx1B or Stx2B for 5 or 15 minutes, washed 3 times with PBS, fixed with 1% paraformaldehyde for 15 minutes, and permeabilized with 0.1% Triton X-100 for 10 minutes. HUVECs were incubated with mouse monoclonal antivinculin antibody (Sigma-Aldrich) diluted 1:1000, followed by Alexa Fluor 594–labeled goat anti–mouse IgG (Invitrogen) diluted 1:400 for 30 minutes to visualize focal adhesions, and FITC-phalloidin to label F-actin. Images were acquired at room temperature with an Axiovert 200M inverted microscope with a 40×/0.55 NA objective, standard filter sets, AxioCam MRm camera, and Axiovision Version 4 software (Carl Zeiss).
Photoshop CS4 (Adobe Systems) or Canvas X (ACD Systems) was used to automatically adjust levels (using the “autolevel” function) to match the dynamic range of fluorescence images to that of the computer display. Images for a given experiment were processed identically.
Trans-endothelial permeability
HUVECs were seeded on collagen-coated transwell permeable supports (Corning; 3.0-μm polyester membrane, 6.5-mm insert) in 24 wells at 2 × 105 cells per well and cultured for 3-4 days to produce a confluent monolayer. Cells were serum-starved for 1 hour and treated with Medium 199 containing Stx1B or Stx2B for 15 minutes or 1 hour. After treatment, the transwell inserts were transferred to clean wells in 24-well dishes, with each well containing 0.5 mL of PBS. We added 70 000 kDa FITC-Dextrin (Sigma-Aldrich) in 150 μL of phenol red-free Medium 199 to the top of the transwell inserts, and the mixture was incubated in the dark for 10 minutes. Inserts were then removed, and 100 μL of buffer from each well was transferred to a 96-well plate. The plate was then read with a fluorescence plate reader with excitation at 485 nm and emission at 535 nm. Fluorescence intensities of StxB-treated samples were compared with control samples, and the percentages of increases were averaged from multiple experiments.
Analysis of RhoA and Arf6
Confluent HUVECs were treated with Stx1B or Stx2B for 5 minutes, and cells were washed gently with cold PBS and lysed in ice-cold lysis buffer. GTP-bound RhoA or Arf6 was isolated with Rhotekin-RBD protein agarose beads (Cytoskeleton) or an Active Arf6 Pull-Down and Detection Kit (Thermo Fisher Scientific), respectively, according to the manufacturer's instructions. Proteins in cell lysate or eluted from agarose beads were separated on NuPAGE 4%-12% Bis-Tris gels (Invitrogen), and Western blot analyses were performed with mouse monoclonal anti-RhoA (no. SC-418; Santa Cruz Biotechnology) or mouse monoclonal anti-Arf6 antibody (no. 26186; Thermo Fisher Scientific), and goat anti–mouse IgG conjugated to HRP (no. P0161; Dako).
WPB exocytosis
Weibel Palade body (WPB) exocytosis was quantified essentially as described by Rondaij et al.17 Briefly, HUVECs were nucleofected in duplicate with constructs encoding GFP, dnRhoA-EGFP, dnArf6-CFP, or Arf6-CFP. Seventy-two hours later, cells were treated with 200 ng/mL Stx1B, 200 ng/mL Stx2B, or 100μM histamine for 1 hour; washed 3 times with PBS; fixed with 1% paraformaldehyde for 15 minutes; and permeabilized with 0.1% Triton X-100 for 10 minutes. Dishes were then incubated with rabbit polyclonal anti-VWF antibody (082; Dako North America) diluted 1:1000 for 45 minutes, followed by Alexa Fluor 594–conjugated goat anti–rabbit IgG (Invitrogen) diluted 1:400 for 30 minutes. Images were acquired with an Axiovert 200M inverted microscope with a 40×/0.5 NA objective, standard filter sets, a charge-coupled device camera, and Axiovision Version 4 software (Carl Zeiss). Transfected cells were identified by GFP or CFP fluorescence, and Alexa Fluor 594–labeled WPBs in transfected cells were counted for comparison with the number of WPBs per cell between conditions.
Statistics
Differences between mean values were assessed with the unpaired Student t test.
Results
Activation of PLD1 is necessary for StxB-induced VWF secretion
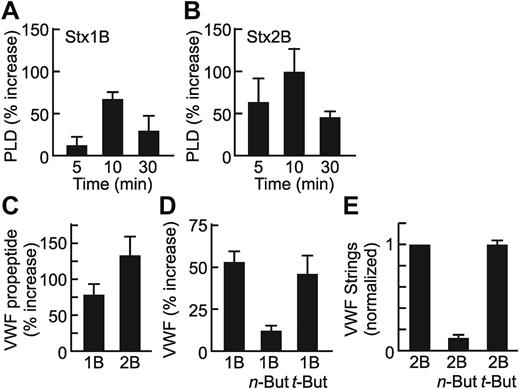
PLD1 is required for histamine-evoked WPB exocytosis from HUVECs,10 which suggests that PLD1 might participate in the acute secretion of VWF induced by the B subunits of Stx1 or Stx2.8 In fact, treatment of HUVECs with recombinant Stx1B or Stx2B caused a rapid and transient increase in PLD activity that was maximal at ∼ 10 minutes (Figure 1A-B). Histamine-induced PLD activation followed a similar time course (data not shown).
PLD activity is required for Stx B-induced VWF secretion. Confluent HUVECs were incubated with 0.2 μg/mL Stx1B (A) or Stx2B (B) for the indicated time, and PLD activity in cell lysates was assayed. Values are normalized to untreated cells (0 minutes) and shown as the mean ± SE for 3 experiments. (C) Confluent HUVECs in 24-well dishes were stimulated for 30 minutes with 0.2 μg/mL Stx1B or Stx2B. Secreted VWF propeptide was quantified by ELISA for each condition. Values shown are the mean ± SE from 3 replicates. Mean VWF propeptide concentrations were 6 ng/mL, 11 ng/mL, and 15 ng/mL for control, Stx1B-, and Stx2B-treated cells, respectively. (D) Confluent HUVECs in 24-well dishes were pretreated for 30 minutes with or without 25mM n-butanol or t-butanol, then stimulated for 30 minutes with 0.2 μg/mL Stx1B. Cell surface-associated VWF was quantified by ELISA in triplicate for each condition. Values shown are the mean ± SE from 4 independent experiments. (E) Confluent HUVECs were perfused at 2.5 dyn/cm2 with 200 ng/mL Stx2B, with or without preincubation with n-butanol or t-butanol. VWF strings were counted in 10 fields, and values are shown as the mean ± SE, normalized to the Stx2B condition for each experiment. Experiments were conducted ≥ 3 times with similar results. For each panel, values for Stx1B or Stx2B are significantly different from control cells (P < .05). (D-E) Inhibition by n-butanol is significant (P < .01).
PLD activity is required for Stx B-induced VWF secretion. Confluent HUVECs were incubated with 0.2 μg/mL Stx1B (A) or Stx2B (B) for the indicated time, and PLD activity in cell lysates was assayed. Values are normalized to untreated cells (0 minutes) and shown as the mean ± SE for 3 experiments. (C) Confluent HUVECs in 24-well dishes were stimulated for 30 minutes with 0.2 μg/mL Stx1B or Stx2B. Secreted VWF propeptide was quantified by ELISA for each condition. Values shown are the mean ± SE from 3 replicates. Mean VWF propeptide concentrations were 6 ng/mL, 11 ng/mL, and 15 ng/mL for control, Stx1B-, and Stx2B-treated cells, respectively. (D) Confluent HUVECs in 24-well dishes were pretreated for 30 minutes with or without 25mM n-butanol or t-butanol, then stimulated for 30 minutes with 0.2 μg/mL Stx1B. Cell surface-associated VWF was quantified by ELISA in triplicate for each condition. Values shown are the mean ± SE from 4 independent experiments. (E) Confluent HUVECs were perfused at 2.5 dyn/cm2 with 200 ng/mL Stx2B, with or without preincubation with n-butanol or t-butanol. VWF strings were counted in 10 fields, and values are shown as the mean ± SE, normalized to the Stx2B condition for each experiment. Experiments were conducted ≥ 3 times with similar results. For each panel, values for Stx1B or Stx2B are significantly different from control cells (P < .05). (D-E) Inhibition by n-butanol is significant (P < .01).
Treatment of HUVECs with Stx B subunits causes the rapid secretion of VWF propeptide into the medium16,18 (Figure 1C) and of multimeric VWF8 that can be detected on cell surfaces (Figure 1D) or by immunofluorescence of living cells as long VWF strings (Figure 1E). The alcohol n-butanol is a shunt substrate of PLD that prevents the production of PA. Pretreatment of HUVECs with n-butanol blocked the secretion of VWF induced by Stx1B (Figure 1D) or Stx2B (Figure 1E; P < .01), whereas t-butanol, a tertiary alcohol that is not a PLD substrate, had no effect.
PLD occurs in 2 isoforms, PLD1 and PLD2, and both are found in endothelial cells.10 We focused on PLD1 because it has a much lower basal activity19 and is specifically involved in WPB exocytosis induced by histamine.10 Compared with mock-transfected and control shRNA-transfected HUVECs, transfection to express PLD1 shRNA caused an ∼ 60% reduction in PLD1 antigen (Figure 2A) and markedly inhibited the secretion of VWF induced by either Stx1B (Figure 2B) or Stx2B (Figure 2C; P < .01). These data indicate that the activation of PLD1 and production of PA are required for acute VWF secretion mediated by Stx B subunits.
PLD1 knockdown inhibits VWF secretion. (A) HUVECs were transfected with a plasmid that encodes shRNA that targets human PLD1 or firefly luciferase (shLUC). Cell lysate were prepared 72 hours after transfection and probed for PLD1. Membrane was stripped and reprobed for β-actin. (B-C) Seventy-two hours after transfection, HUVECs were incubated with medium that contained Stx1B (B) or Stx2B (C). VWF secretion and deposition on HUVEC surface was measured as described in “Methods.” Results from 3 independent experiments were averaged and plotted. Values were significantly different from the control (Mock) condition (P < .01).
PLD1 knockdown inhibits VWF secretion. (A) HUVECs were transfected with a plasmid that encodes shRNA that targets human PLD1 or firefly luciferase (shLUC). Cell lysate were prepared 72 hours after transfection and probed for PLD1. Membrane was stripped and reprobed for β-actin. (B-C) Seventy-two hours after transfection, HUVECs were incubated with medium that contained Stx1B (B) or Stx2B (C). VWF secretion and deposition on HUVEC surface was measured as described in “Methods.” Results from 3 independent experiments were averaged and plotted. Values were significantly different from the control (Mock) condition (P < .01).
Stx1B and Stx2B induce actin stress fiber formation, increase RhoA activity, and enhance endothelial monolayer permeability
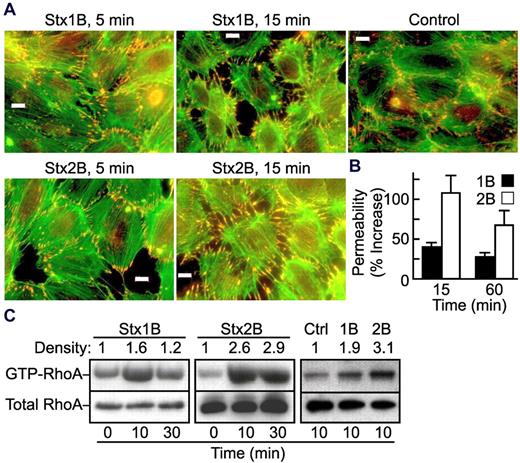
PKCα, Arf, and RhoA directly activate PLD1 by binding to different regions of the enzyme.11 Among them, Rho family GTPases are known to regulate cell structure by modifying actin polymerization and cell-cell junctions.20 We first determined whether Stx B subunits cause cytoskeletal remodeling in HUVECs. Confluent HUVECS have some peripheral actin filaments visualized by phalloidin staining and tight intercellular junctions marked by vinculin (Figure 3A). Treatment with either Stx1B or Stx2B induced the formation of actin stress fibers, the disassembly of tight junctions, and the appearance of intercellular gaps. Such changes occurred within 5 minutes and were more prominent at 15 minutes (Figure 3A). Phalloidin staining continued to increase for ≥ 1 hour (data not shown).
Stx1B and Stx2B activate RhoA and increase endothelial monolayer permeability. (A) Confluent HUVECs were treated with Stx1B or Stx2B for 5 or 15 minutes. Cells were then fixed, permeabilized, and stained for actin (green) and vinculin (red) as a marker for focal adhesions. Bars, 10 μm. (B) Confluent HUVECs on transwell filters were treated with 200 ng/mL Stx1B or Stx2B for 15 minutes or 1 hour. Monolayer permeability was measured by the penetration of FITC-dextrin to the bottom part of the transwell chambers. Results are shown as the mean ± SE for 5 independent experiments. Stx2B increased trans-endothelial permeability more than did Stx1B at both time points (P < .05). (C) HUVECs were treated with Stx1B or Stx2B for 10 or 30 minutes before lysis buffer was added. Cell lysates were probed for total RhoA or GTP-bound RhoA. The experiment was performed 3 times with similar results.
Stx1B and Stx2B activate RhoA and increase endothelial monolayer permeability. (A) Confluent HUVECs were treated with Stx1B or Stx2B for 5 or 15 minutes. Cells were then fixed, permeabilized, and stained for actin (green) and vinculin (red) as a marker for focal adhesions. Bars, 10 μm. (B) Confluent HUVECs on transwell filters were treated with 200 ng/mL Stx1B or Stx2B for 15 minutes or 1 hour. Monolayer permeability was measured by the penetration of FITC-dextrin to the bottom part of the transwell chambers. Results are shown as the mean ± SE for 5 independent experiments. Stx2B increased trans-endothelial permeability more than did Stx1B at both time points (P < .05). (C) HUVECs were treated with Stx1B or Stx2B for 10 or 30 minutes before lysis buffer was added. Cell lysates were probed for total RhoA or GTP-bound RhoA. The experiment was performed 3 times with similar results.
The integrity of the endothelial monolayer was also assessed by measuring trans-endothelial penetration of FITC-dextran before and after treatment with Stx B subunits. Both Stx1B and Stx2B increased the permeability of a HUVEC monolayer at 15 minutes and 1 hour, and Stx2B was significantly more potent than Stx1B (P < .05; Figure 3B). Changes in cell structure and monolayer permeability were accompanied by increases in the level of active GTP-bound RhoA over a similar time course (Figure 3C), suggesting that activation of RhoA could mediate Stx B subunit-induced PLD activation.
Interference with Rho signaling inhibits StxB-induced VWF secretion
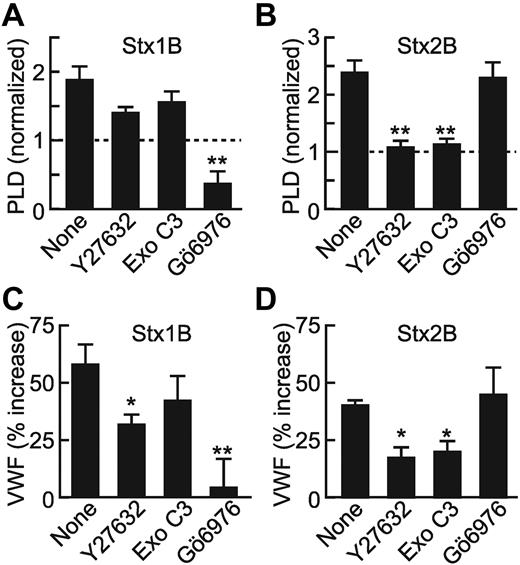
The contribution of RhoA to StxB-induced signaling was investigated further by preincubation of HUVECs with different inhibitors. Y27632 is a selective inhibitor of Rho-associated kinase (ROCK). Exoenzyme C3 ribosylates and inactivates RhoA, RhoB, and RhoC. Y27632 and exoenzyme C3 had modest effects if any on Stx1B-induced PLD activation (Figure 4A; P > .05) and VWF secretion (Figure 4C) but abolished Stx2B-mediated PLD activation (Figure 4B) and reduced Stx2B-induced VWF secretion ∼ 50% (Figure 4D; P < .01). These data support the involvement of RhoA in Stx2B-induced and PLD-dependent VWF secretion.
Differential effects of PKCα and Rho inhibitors on Stx1B- and Stx2B-induced VWF secretion and PLD activation. HUVECs were preincubated with buffer or ROCK inhibitor Y27632 (100μM), Rho family GTPase inhibitor exoenzyme C3 (1 μg/mL), or PKCα inhibitor Gö6976 (2μM) before stimulation with Stx1B (A) or Stx2B (B) for 10 minutes. PLD activity was quantified as described in “Methods.” Graphs represent the average results of 3-5 independent experi-ments. HUVECs were treated with inhibitors before stimulation with Stx1B (C) or Stx2B (D). Cell-surface VWF under each condition was quantified as described in “Methods.” Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. Data reflect the average results of 4 independent experiments. Comparisons with the reference condition are significantly different as indicated: *P < .05; **P < .01.
Differential effects of PKCα and Rho inhibitors on Stx1B- and Stx2B-induced VWF secretion and PLD activation. HUVECs were preincubated with buffer or ROCK inhibitor Y27632 (100μM), Rho family GTPase inhibitor exoenzyme C3 (1 μg/mL), or PKCα inhibitor Gö6976 (2μM) before stimulation with Stx1B (A) or Stx2B (B) for 10 minutes. PLD activity was quantified as described in “Methods.” Graphs represent the average results of 3-5 independent experi-ments. HUVECs were treated with inhibitors before stimulation with Stx1B (C) or Stx2B (D). Cell-surface VWF under each condition was quantified as described in “Methods.” Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. Data reflect the average results of 4 independent experiments. Comparisons with the reference condition are significantly different as indicated: *P < .05; **P < .01.
Inhibition or knockdown of PKCα abolished Stx1B- but not Stx2B-induced PLD activation and VWF secretion
Gö6976, an inhibitor of PKCα and PKCβ, reduced basal and Stx1B-stimulated PLD activity in HUVECs (Figure 4A) and prevented Stx1B-stimulated VWF release (Figure 4C; P < .01), but it had no effect on Stx2B-stimulated PLD activity (Figure 4B) or VWF release (Figure 4D). Reducing intracellular PKCα by 80% (Figure 5A) inhibited VWF secretion induced by Stx1B (P < .01), but it did not inhibit VWF secretion induced by Stx2B (Figure 5B). Treatment of HUVECs with a control siRNA that targeted GFP did not alter either process. These results are consistent with our earlier study,9 which reported that PKCα is selectively involved in Stx1B signaling.
Effect of PKCα knockdown and dominant-negative RhoA on VWF secretion induced by Stx1B and Stx2B. (A) HUVECs were transfected with siRNA against PKCα or GFP (Ctrl). Whole-cell lysates were prepared 72 hours later, and PKCα level was assessed by Western blot analysis. The membrane was stripped and reprobed for β-actin. (B) HUVECs were transfected with siRNA against PKCα or GFP, and cell surface VWF was assayed by ELISA 72 hours later. Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. The graph represents the results of 3 independent experiments. **P < .01. (C-D) HUVECs were transfected with cDNA encoding EGFP or EGFP-DN RhoA. Seventy-two hours later, confluent cells were stimulated for 1 hour with either Stx1B (C) or Stx2B (D), washed, and stained with fluorescent VWF antibody. Numbers of WPBs per cell (stimulated or unstimulated) were counted for 10-30 cells from multiple optical fields. Experiments were conducted 4 times with similar results. Pairwise comparisons with or without Stx1B or Stx2B are significantly different (P < .01) except for the dnRho conditions.
Effect of PKCα knockdown and dominant-negative RhoA on VWF secretion induced by Stx1B and Stx2B. (A) HUVECs were transfected with siRNA against PKCα or GFP (Ctrl). Whole-cell lysates were prepared 72 hours later, and PKCα level was assessed by Western blot analysis. The membrane was stripped and reprobed for β-actin. (B) HUVECs were transfected with siRNA against PKCα or GFP, and cell surface VWF was assayed by ELISA 72 hours later. Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. The graph represents the results of 3 independent experiments. **P < .01. (C-D) HUVECs were transfected with cDNA encoding EGFP or EGFP-DN RhoA. Seventy-two hours later, confluent cells were stimulated for 1 hour with either Stx1B (C) or Stx2B (D), washed, and stained with fluorescent VWF antibody. Numbers of WPBs per cell (stimulated or unstimulated) were counted for 10-30 cells from multiple optical fields. Experiments were conducted 4 times with similar results. Pairwise comparisons with or without Stx1B or Stx2B are significantly different (P < .01) except for the dnRho conditions.
Effect of dominant-negative RhoA on StxB-induced VWF secretion
To analyze stimulated WPB exocytosis on a single cell level, we transfected HUVECs with cDNA that encoded dominant-negative RhoA-GFP or GFP alone. Numbers of WPBs were quantified before and 1 hour after StxB stimulation, as previously described.17 We found that both Stx1B and Stx2B caused a > 60% depletion of WPBs in mock-transfected and GFP-transfected cells. However, StxB-induced WPB depletion was significantly inhibited (P < .01) ∼ 70% in cells expressing dominant-negative RhoA (Figure 5C-D). These results indicate that RhoA plays a role in VWF secretion induced by both Stx1B and Stx2B.
Role of Arf6 in StxB-induced VWF secretion
Arf6 is a small GTPase that is frequently involved in vesicle trafficking at the plasma membrane. In addition, Arf6 regulates agonist-induced, PLD-dependent exocytosis by controlling the synthesis of fusogenic lipids.21 We affinity purified GTP-bound Arf6 from lysate of HUVECs treated briefly with Stx B subunits and found that both Stx1B and Stx2B increased the level of active Arf6 ∼ 2-fold (Figure 6A). Overexpression of dominant-negative (T27N) Arf6 impaired StxB-induced VWF secretion (Figure 6B), as well as WPB exocytosis mediated by Stx1B (Figure 6C) and Stx2B (Figure 6D; P < .01), but not by histamine (Figure 6E). Cells overexpressing wild-type Arf6 have a lower number of WPBs, which is associated with a reduced secretory response (Figure 6D-E).
Arf6 is involved in StxB but not histamine-evoked WPB exocytosis. (A) HUVECs were treated with Stx1B or Stx2B for 5 minutes before lysis buffer was added. Cell lysates were directly probed for Arf6, or pull-down assay was conducted to concentrate GTP-bound Arf6. The experiment was performed 5 times with similar results. (B) HUVECs in 24-well dishes were transfected with wild-type or dominant-negative (T17N) Arf6, and cell-surface ELISA assays were conducted 72 hours later after StxB treatment. Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. The experiment was repeated twice with similar results. (C-E) HUVECs were transfected with cDNA encoding EGFP, CFP-Arf6, or CFP-DN Arf6. Seventy-two hours later, confluent cells were stimulated for 1 hour with Stx1B (C), Stx2B (D), or histamine (E); washed; and stained with fluorescent VWF antibody. Numbers of WPBs per cell were counted for 10-30 cells from multiple optical fields. Experiments were performed 3 times with similar results. Pairwise comparisons with or without Stx1B or Stx2B are significantly different (P < .01), except for the dnArf6 and Arf6 conditions.
Arf6 is involved in StxB but not histamine-evoked WPB exocytosis. (A) HUVECs were treated with Stx1B or Stx2B for 5 minutes before lysis buffer was added. Cell lysates were directly probed for Arf6, or pull-down assay was conducted to concentrate GTP-bound Arf6. The experiment was performed 5 times with similar results. (B) HUVECs in 24-well dishes were transfected with wild-type or dominant-negative (T17N) Arf6, and cell-surface ELISA assays were conducted 72 hours later after StxB treatment. Values for each condition were normalized to the amount of VWF secreted by unstimulated cells. The experiment was repeated twice with similar results. (C-E) HUVECs were transfected with cDNA encoding EGFP, CFP-Arf6, or CFP-DN Arf6. Seventy-two hours later, confluent cells were stimulated for 1 hour with Stx1B (C), Stx2B (D), or histamine (E); washed; and stained with fluorescent VWF antibody. Numbers of WPBs per cell were counted for 10-30 cells from multiple optical fields. Experiments were performed 3 times with similar results. Pairwise comparisons with or without Stx1B or Stx2B are significantly different (P < .01), except for the dnArf6 and Arf6 conditions.
Discussion
Stx-producing E coli cause D+HUS more frequently when they express Stx2 rather than Stx1,22,23 and this difference has not been fully explained. Both Stx1 and Stx2 damage cells by the same N-glycosidase mechanism,24 but their relative potency varies depending on the assays used. Stx1 binds Gb3 on cell surfaces ∼ 10-fold more tightly than does Stx2. Nevertheless, Stx2 is ∼ 400-fold more toxic to mice25 and is more potent for inducing VWF secretion from endothelial cells.8 Differences in the propensity to cause ribotoxic stress may account for some of the observed variation in pathogenicity. However, differences in signaling events at the cell surface also may contribute.
The induction of ribotoxic stress requires ≥ 30 minutes to complete the processes of Stx internalization, retrograde transport to the endoplasmic reticulum, translocation and activation of the cytotoxic A subunit in the cytoplasm, and cleavage of adenine from ribosomes.2 In contrast, endothelial cells exposed to Stx secrete VWF within 5 minutes.7,8 Stx B subunits are sufficient to induce this acute secretory response and can cause fatal microvascular thrombosis in susceptible mice.8 Whether Stx-induced VWF secretion contributes to the pathophysiology of HUS in humans has not been established. However, linkage analysis has indicated that the risk of developing D+HUS is associated with the 145M allele of platelet glycoprotein Ibα with an odds ratio of 3.08 (P < .001).26 This variant of glycoprotein Ibα binds VWF with higher affinity than the more common 145T allele, which suggests that increased VWF-dependent platelet adhesion promotes tissue injury in children infected with Stx-producing E coli.
Stx B subunits induce the secretion of VWF by activating signaling pathways that require binding to cell surface Gb3,8 but these pathways diverge initially for Stx1B and Stx2B. Stx1B-induced VWF secretion depends on a transient rise in intracellular Ca2+ and activation of PKCα (Figures 4 and 5); in contrast, Stx2B-induced VWF secretion is independent of intracellular Ca2+ and PKCα but depends on protein kinase A.9 In this respect Stx1B signaling resembles that induced by histamine or thrombin, and Stx2B signaling resembles that induced by epinephrine.17
The signaling differences between Stx1B and Stx2B reflect differences in their primary structure. The 2 B subunits are ∼ 50%-60% identical in amino acid sequence, and crystal structures show that most of the nonconserved residues are exposed on lateral surfaces that do not interact with Gb3 but could mediate preferential interactions with different cell surface components that cluster in caveolae or associate with glycosphingolipids.27,28
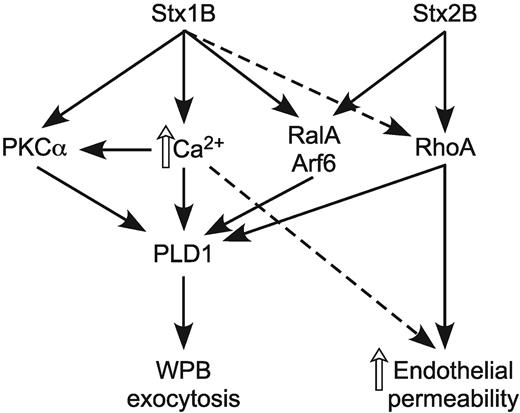
Our results show these distinct Stx1B and Stx2B signaling pathways eventually converge to cause the exocytosis of WPBs by activating PLD1 (Figures 1 and 2), as also reported with histamine and forskolin.10 In this respect, PLD1 serves as a common effector of WPB exocytosis shared by Ca2+-dependent and Ca2+-independent endothelial agonists. PKCα and G proteins of the Rho and Arf families are known regulators of PLD1,11,29 and PLD1 activation by both Stx1B and Stx2B depends on Arf6 (Figure 6). PLD1 interacts directly with Arf6 and the small G protein RalA,30 and RalA increases the activity of Arf6-regulated PLD1.30 In HUVECs, RalA and the GTP exchange factor RalGDS are required for WPB exocytosis in response to elevations of Ca2+ or cAMP.17 Therefore, StxB-induced VWF secretion probably involves both RalA and Arf6 (Figure 7).
StxB induces WPB exocytosis in a PLD1-dependent manner. Stx1B increases intracellular Ca2+ and activates PKCα, RalA, Arf6, and to a lesser extent, RhoA. In contrast, Stx2B does not affect PKCα and Ca2+ signaling but activates RalA, Arf6, and RhoA. All of these pathways can contribute to the activation of PLD1, which leads to WPB exocytosis and VWF secretion. Stx2B is a relatively potent activator of RhoA and therefore has a more pronounced effect on actin cytoskeleton remodeling and endothelial monolayer permeability.
StxB induces WPB exocytosis in a PLD1-dependent manner. Stx1B increases intracellular Ca2+ and activates PKCα, RalA, Arf6, and to a lesser extent, RhoA. In contrast, Stx2B does not affect PKCα and Ca2+ signaling but activates RalA, Arf6, and RhoA. All of these pathways can contribute to the activation of PLD1, which leads to WPB exocytosis and VWF secretion. Stx2B is a relatively potent activator of RhoA and therefore has a more pronounced effect on actin cytoskeleton remodeling and endothelial monolayer permeability.
Although both Stx1B and Stx2B depend on Arf6 to activate PLD1, they differ in the involvement of PKCα and Rho. In most cell types agonist-induced PLD1 activation requires PKCα,11,29 and in HUVECs the activation of PLD1 by Stx1B does appear to depend on PKCα. However, inhibition or knockdown of PKCα has no effect on Stx2B-induced PLD1 activity or VWF secretion (Figures 4 and 5). These results and our previous studies of StxB signaling9 indicate that Stx2B can activate PLD1 in endothelium, independent of PKCα, Ca2+, or phosphatidylinositol-4,5 bisphosphate hydrolysis (Figure 7).
Rho and Arf proteins can act synergistically to activate PLD1 in several systems,29 and, like Arf6, RhoA can bind directly to PLD1.13 Our results show that Stx2B is more potent than Stx1B as an activator of RhoA in HUVECs (Figure 3C) and that Stx2B-induced PLD1 activation and VWF secretion depend more strongly on RhoA (Figure 4). The greater activation of RhoA is consistent with the greater effect of Stx2B on endothelial intercellular junctions (Figure 3A) and monolayer permeability (Figure 3B).
The distinction between Stx1B and Stx2B with respect to RhoA activation can be compared with the effects on HUVECs of selective agonists for protease-activated receptors.31 Stimulation of PAR1 or PAR2 caused rapid VWF secretion that was not impaired by inhibition of ROCK (with Y27632) or Rho (with C3 exoenzyme), indicating that RhoA activation is not required for this response. Stimulation of PAR2 had no effect on monolayer permeability, but stimulation of PAR1 markedly increased endothelial monolayer permeability, and this response was prevented by inhibition of ROCK.31 Thus, strong activation of RhoA by either PAR1 or Stx2B is associated with large effects on the integrity of endothelial monolayers and may increase the propensity of Stx2 to cause D+HUS. If so, then modifiers of RhoA signaling may influence the course of infection with Stx-producing E coli.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Guangwei Du (The University of Texas Health Science Center at Houston) for shRNA-mCherry constructs targeting PLD1 and firefly luciferase.
This work was supported by the National Institutes of Health (grants HL72917, HL89746, and HL112303).
National Institutes of Health
Authorship
Contribution: J.H. designed and performed research, analyzed and interpreted data, and wrote the manuscript; S.L.H designed and performed research and reviewed the manuscript; and J.E.S. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Evan Sadler, Department of Medicine, Washington University School of Medicine, 660 S Euclid Ave, Box 8125, St Louis, MO 63110; e-mail: esadler@wustl.edu.