Abstract
The generation of Ab-secreting plasma cells depends critically on CD4 T-follicular helper (TFH) cells during the germinal center reaction. Germinal center TFH cells share functional properties with circulating CXCR5+ CD4 T cells, referred to herein as peripheral TFH (pTFH) cells. Because deficient Ab production and CD4 T-cell loss are recognized features of HIV infection, in the present study, we investigated pTFH cells in 25 HIV-infected patients on antiretroviral therapy. pTFH frequency was equivalent in patients and healthy controls (HCs), and these cells displayed a central memory phenotype. Sixteen patients and 8 HCs in this group were given a single dose of H1N1/09 influenza vaccine during the 2009 H1N1 influenza outbreak. In the vaccine responders (n = 8) and HCs, pTFH cells underwent expansion with increased IL-21 and CXCL13 secretion in H1N1-stimulated PBMC culture supernatants at week 4 (T2). These changes were not seen in vaccine nonresponders (n = 8). In coculture experiments, sorted pTFH cells supported HIN1-stimulated IgG production by autologous B cells only in vaccine responders. At T2, frequencies of pTFH were correlated with memory B cells, serum H1N1 Ab titers, and Ag-induced IL-21 secretion. Characterization of pTFH cells may provide additional insight into cellular determinants of vaccine-induced Ab response, which may have relevance for vaccine design.
Introduction
T-follicular helper (TFH) cells are a recently identified subset of CD4 T cells that provide critical help to Ag-primed B cells in germinal centers (GCs) to undergo proliferation, isotype switching, and somatic hypermutation,1,2 resulting in long-lasting Ab responses.3 The GC reaction requires contact between surface molecules of Ag-primed B cells and TFH cells, as well as the cytokine IL-21, which is produced abundantly by TFH cells.4-6 A key surface molecule on TFH is the CXC chemokine receptor type 5 (CXCR5), which binds to its ligand CXCL137 for homing to the lymphoid follicles.8 Approximately 10%-15% of circulating CD4 T cells in humans express CXCR5 and have a predominantly memory phenotype.8,9 These circulating CXCR5+ CD4 T cells are referred to herein as peripheral TFH (pTFH) cells because they manifest functional properties of the GC TFH cells, including a capacity for abundant IL-21 secretion and the ability to promote B-cell differentiation in vitro.9-11 Therefore, investigation of CXCR5+ CD4 T cells in the peripheral blood could provide a window into GC TFH in the clinical setting.
Progressive CD4 T-cell loss is a characteristic feature of chronic HIV infection12 and is accompanied by dysfunction of other cell types, including B cells.13,14 Major B-cell defects identifiable in the peripheral blood of HIV-infected persons include expansion of transitional B cells with shrinkage of the memory B cells and poor Ab responses to vaccines, including influenza vaccines.15,16 After potent combination antiretroviral therapy (cART), there is usually a dramatic recovery of CD4 T cells in association with control of HIV replication.17 However, despite cART, the phenotype of B cells does not reach complete normality and the ability to respond to vaccines often remains compromised in HIV-infected patients.13,16,18 In the 2009-2010 influenza season, the novel H1N1 influenza epidemic prompted vaccination of vulnerable populations, which included persons with HIV infection. We reported recently that in a small cohort of HIV-infected patients, almost half did not mount a serologic response to the H1N1/09 vaccine.19,20 Chief among the immunologic defects was a failure of expansion of memory B cells and a lack of increase in serum IL-21 after vaccination in vaccine nonresponders compared with vaccine responders. In the present study, we investigated characteristics of pTFH in the same cohort of H1N1/09 influenza vaccine recipients and in additional patients with chronic HIV infection outside of the vaccine cohort. We demonstrate for the first time that a successful induction of the vaccine Ab response is correlated with an expansion of pTFH cells and that these cells are critical for supporting autologous B-cell differentiation. Our studies provide novel insights into immune defects in otherwise stable HIV-infected patients on cART and into immunologic components of a successful response to the H1N1/09 influenza vaccine.
Methods
Human subjects
Twenty-five HIV-infected persons and 17 HIV-negative healthy controls (HCs) were enrolled in a study between November 2009 and June 2010 to characterize IL-21–producing CD4 T cells. All HIV-infected patients were being followed in the special immunology clinic at the University of Miami and were on potent cART according to the standard of care. The cART included 2 nucleoside reverse transcriptase inhibitors with a Ritonavir-boosted protease inhibitor, the nonnucleoside reverse transcriptase inhibitor Efavirenz, or the integrase inhibitor Raltegravir. Characteristics of the study population are summarized in Table 1. At study entry, the mean plasma HIV RNA was 57.7 ± 79.4 copies/mL, with < 40 copies/mL in 22 of 25 (88%) patients and detectable virus loads of 70, 76, and 369 copies/mL in 3 of 25 (12%) patients. Mean CD4 counts were 574 ± 345 cells/mm3, with the majority of patients having > 350 cells/mm3. From this cohort, additional investigations were conducted in a subgroup of 16 patients and 8 HCs who were enrolled in an H1N1/09 vaccine evaluation study.19 Patients in the vaccine study group were virologically controlled and had a CD4 count of 637 ± 402 cells/mm3. Study participants were given a single intramuscular dose (15 μg; 0.5 mL) of inactivated monovalent A/California/07/2009 H1N1 vaccine (Novartis Vaccines and Diagnostics). H1N1 hemagglutination inhibition Ab titers > 1:40 developed in all HCs and in 8 of 16 (50%) HIV-infected patients. Based on the serologic response, patients were classified as vaccine responders and nonresponders. The mean age and frequencies of CD20+ B cells were statistically equivalent among the patient groups and HCs; CD4 and CD8 T-cell counts and plasma HIV RNA were similar among the responders and nonresponders. The studies were approved by the institutional review board of the University of Miami.
Processing of blood samples
All study participants had peripheral blood samples drawn after informed consent. In the H1N1-vaccinated subgroup, blood samples were obtained immediately before vaccination (T0), on week 4 (range 4-5 weeks, T2) and week 24 (range 24-26 weeks, T3) after vaccination. PBMCs were isolated by density gradient isolation21 and processed fresh for immunophenotyping and cytokine expression after polyclonal stimulation; remaining PBMCs were cryopreserved for study at a later date. Serum samples were stored at −80°C. Hemagglutination inhibition Ab titers against H1N1 were performed at BIOQUAL in baseline and T2 serum samples using virus and turkey RBCs.15
Multicolor flow cytometry for phenotyping and intracellular staining
Phenotypic characterization of pTFH and B cells.
For pTFH staining, mAb panels incorporated CD3-AmCyan, CD4-PerCPCy5.5, CD8-APCCy7, CXCR5–Alexa Fluor 647, CD27–Alexa Fluor 700, CD57-FITC (BD Biosciences); CD45RO-ECD (Beckman Coulter); and inducible costimulator (ICOS)–PECy7 (eBiosciences). For B-cell characterization, the staining panel consisted of CD3-AmCyan (for exclusion of T cells), CD20-APC, CD27–Alexa Fluor 700, CD10-PECy7, and CD21-PECy5 (BD Biosciences). LIVE/DEAD Fixable Violet Dead Cell Stain (ViViD) Kit (Molecular Probes) was included in all staining panels for exclusion of dead cells, as described previously.19 Appropriate isotype control Abs were used for different cell markers to minimize effects due to nonspecific staining. Freshly isolated PBMCs or sorted cells (1 × 106 cells) stained with mAbs were incubated in the dark for15 minutes, washed, and resuspended in PBS with 1% paraformaldehyde. Samples were acquired on an LSRII flow cytometer (BD Biosciences) after proper instrument setting, calibration, and compensation.22,23 At least 500 000 events in the lymphocyte gate were acquired per sample, and frequencies of desired subsets were determined in gated live (ViViD−) cell populations. Gating of live T lymphocytes (CD3+CD14−CD56−CD19−) was followed by sequential gating to identify a CD4+CXCR5+ subset designated as pTFH cells. Further characterization of pTFH cells was performed based on the expression of ICOS and differentiation markers for naive (TN; CD27+CD45RO−), central memory (TCM; CD27+CD45RO+), effector memory (TEM; CD27−CD45RO+), effector (TE; CD45RO+CD57+), and terminal effector (TTE; CD45RO−CD57+) T-cell subsets.24 Frequencies of pTFH and CD4+ICOS+ cells were determined relative to CD4 T cells in the peripheral blood. In separate experiments, pTFH cells were also stained with CD4-Qdot655 (Invitrogen), PD-1-PE, Bcl6-FITC, and CCR7-APC (BD Biosciences). Memory B cells and plasmablasts were identified as described previously.19 Briefly, live cells were first gated for CD3−CD20+ B cells and gated sequentially to identify CD21hiCD27+CD10− memory B cells. Plasmablasts were identified as ViViD−CD3−CD20lo/−CD21lo/−CD10−CD27+CD38+Ki67+ cells.
Intracytoplasmic cytokine production.
For evaluation of cytokine production, PBMCs were cultured in complete medium (RPMI 1640 supplemented with 10% heat-inactivated FBS). Polyclonal stimulation was performed with 50 ng/mL of phorbol 12-myristate 13-acetate (PMA) and 1 μg/mL of ionomycin (both from Sigma-Aldrich) in freshly isolated PBMCs. Brefeldin A (10 μg/mL; Sigma-Aldrich) was added to all cultures together with the aforementioned stimuli and cells were incubated at 37°C for 5 hours. After incubation, cells were washed and stained with Abs for surface markers for CD3, CD4, CD8, CXCR5, ICOS, and with ViViD for 15 minutes in the dark. Cells were then washed, fixed, permeabilized, and stained intracellularly with IL-21–PE (eBiosciences) mAb. After 30 minutes of incubation in the dark, cells were washed, resuspended in PBS with 1% paraformaldehyde, and acquired on an LSRII flow cytometer after proper instrument setting, calibration, and compensation.22,23 Data analysis was performed using FlowJo Version 8.8.6 software (TreeStar).
Cell sorting
Thawed cryopreserved PBMCs were rested overnight. B cells were enriched by positive selection using enrichment beads (EasySep Human CD19 Positive Selection Kit; STEMCELL Technologies) to obtain populations at > 97% purity. Purified pTFH and non-pTFH cell subsets were obtained at purities of ≥ 99% by sterile sorting on a BD FACSAria cell sorter. Briefly, PBMCs depleted of B cells were stained with ViViD, CD3-PerCP, CD4-Qdot655, CXCR5–Alexa Fluor 647, and CD45RO-ECD to sort ViViD−CD3+CD4+CD45RO+ memory CD4 T cells that were pTFH (CXCR5+) or non-pTFH (CXCR5−) cells.
pTFH and B-cell cocultures
Purified B cells (2 × 104) were cocultured at a 1:1 ratio with sorted pTFH or non-pTFH cells in duplicate wells in the presence of 5 μg/mL of H1N1 Ag with 1 μg/mL of anti-CD28 mAb for 7 days. Medium alone and B cells alone were used as negative controls, and 1 μg/mL of staphylococcal enterotoxin B (Sigma-Aldrich) was used as a positive control. Culture supernatants were analyzed for IgG by ELISA and cells were characterized by Ab staining by flow cytometry.
PBMC cultures for cytokine and chemokine secretion
Cryopreserved PBMCs obtained at week 4 after vaccination were thawed, rested overnight, and cultured in the presence of 5 μg/mL of H1N1 Ag, medium alone, or anti-CD3 plus anti-CD28 (1 μg/mL each) for 5 days. Culture supernatants were harvested and stored at −80°C until ready to be assayed by ELISA as described in the next section. To establish the optimum assay time, kinetics of IL-21 and CXCL13 production were determined in 1-, 2-, 3-, 5-, and 7-day culture supernatants of Ag-stimulated PBMC cultures of 3 vaccinated healthy controls at T2.
ELISA
IgG, IL-21, and CXCL13 levels were analyzed in cell culture supernatants using commercially available ELISA kits for IgG (limit of detection, 7.8 ng/mL; Bethyl Laboratories), for IL-21 (limit of detection, 32 pg/mL; eBiosciences), and for CXCL13 (limit of detection, 7.8 pg/mL; R&D Systems) as per the manufacturers' instructions. For IgG ELISA, culture supernatants were assayed undiluted and at a 1:5 dilution and incubated for 1 hour at room temperature. For IL-21, undiluted culture supernatants were incubated overnight at 4°C, as described previously.19 For CXCL13, culture supernatants diluted 1:3 were incubated for 2 hours at room temperature. Plates were washed and developed as per manufacturer's instructions. Enzyme activity was determined at a wavelength of 450 nm.
Statistical analysis
Differences between 2 groups were analyzed by the Student t test or the 2-sample Wilcoxon rank-sum (Mann-Whitney) test according to data distribution. Statistical analysis was performed with Stata Statistical Software Release Version 8.0 (Stata). Correlation between variables was determined by linear regression analysis using Prism Version 5 software (GraphPad). P < .05 was considered significant.
Results
HIV-infected patients and healthy donors have similar frequencies of circulating TFH cells
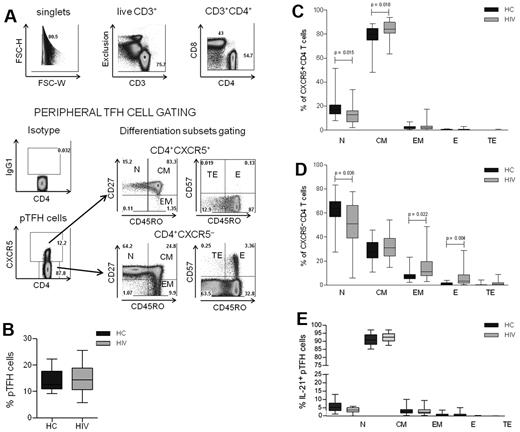
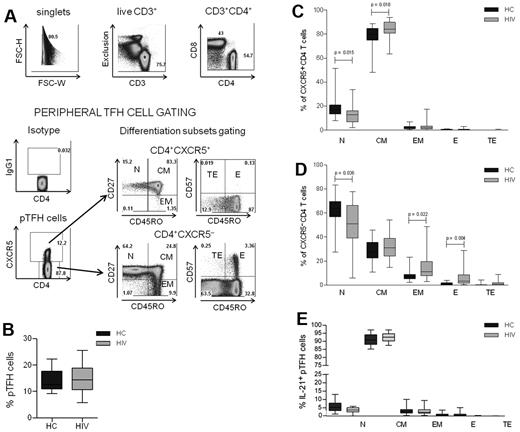
CD4 T-cell numbers and function are altered in HIV infection,25 but the effects of the virus infection on TFH cells are less well known.26 To characterize the pTFH compartment during HIV infection, PBMCs from 25 HIV-infected subjects and 17 healthy controls described in Table 1 were investigated. The gating strategy that was used to identify pTFH cells (defined as CD4+CXCR5+ cells) and maturation subsets is shown for an HIV-infected patient in Figure 1A. No differences were observed in the frequency of pTFH cells in HIV-infected patients and HCs (Figure 1B). The pTFH cells were predominantly TCM cells, (Figure 1C), whereas CXCR5− CD4 T cells were predominantly of the TN phenotype with few TCM and TEM cells (Figure 1D). In the CXCR5− CD4 T-cell compartment, frequencies of more differentiated TEM and TE CD4 T cells were greater, and those of CD4 TN cells were less in patients than in HCs, reflecting CD4 T-cell profile alterations that are observed commonly in HIV-infected patients.27,28 In the CXCR5+ CD4 cell population, the frequency of TN cells was also decreased in patients compared with controls, but the frequency of TCM cells was higher in patients than in HCs. Consistent with their CD45RO+CD27+ CM phenotype, the mean frequency and median fluorescence intensity of CCR7 expression was 90.4% ± 3.7% and 406.1 ± 157, respectively, in pTFH cells and was significantly higher (P < .0001) than in non-pTFH cells (mean frequency, 36.4% ± 10.6%; median fluorescence intensity, 91.1 ± 45.6). Among pTFH cells, the mean frequency of PMA + ionomycin–stimulated IL-21–expressing cells was 18.2% ± 7.1%, and there was no difference between HCs and patients (not shown). As shown in Figure 1E, the majority of IL-21+ pTFH cells were of the TCM phenotype.
Characterization of peripheral TFH cells in HCs and HIV-infected patients. (A) Representative dot plots show gating strategy for pTFH cells, defined as CD4+CXCR5+ cells in an HIV-infected patient. Isotype control Ab was used to set the gate for CXCR5. T-cell differentiation subsets in the CD4+CXCR5+ and CD4+CXCR5− subsets were based on the surface expression of CD45RO, CD27, and CD57 as TN (CD45RO−CD27+), TCM: (CD45RO+CD27+), TEM (CD45RO+CD27−), TE (CD45RO+CD57+), and TTE (CD45RO−CD57+). (B) Frequency of pTFH (CD4+CXCR5+) cells are indicated as a percentage of total CD4 T cells in HCs and HIV-infected patients. (C-D) Distribution of CD4+CXCR5+ (C) and CD4+CXCR5− (D) cells within the differentiation subsets. Box plots represent median with 25th and 75th percentile borders. Error bars represent 10th and 90th percentile means from 17 HCs and 25 HIV-infected aviremic patients. (E) Phenotyping for differentiation subsets in IL-21+ pTFH cells obtained after 5 hours of stimulation with 50 ng/mL of PMA, 1 μg/mL of ionomycin, and 10 μg/mL of brefeldin A.
Characterization of peripheral TFH cells in HCs and HIV-infected patients. (A) Representative dot plots show gating strategy for pTFH cells, defined as CD4+CXCR5+ cells in an HIV-infected patient. Isotype control Ab was used to set the gate for CXCR5. T-cell differentiation subsets in the CD4+CXCR5+ and CD4+CXCR5− subsets were based on the surface expression of CD45RO, CD27, and CD57 as TN (CD45RO−CD27+), TCM: (CD45RO+CD27+), TEM (CD45RO+CD27−), TE (CD45RO+CD57+), and TTE (CD45RO−CD57+). (B) Frequency of pTFH (CD4+CXCR5+) cells are indicated as a percentage of total CD4 T cells in HCs and HIV-infected patients. (C-D) Distribution of CD4+CXCR5+ (C) and CD4+CXCR5− (D) cells within the differentiation subsets. Box plots represent median with 25th and 75th percentile borders. Error bars represent 10th and 90th percentile means from 17 HCs and 25 HIV-infected aviremic patients. (E) Phenotyping for differentiation subsets in IL-21+ pTFH cells obtained after 5 hours of stimulation with 50 ng/mL of PMA, 1 μg/mL of ionomycin, and 10 μg/mL of brefeldin A.
Circulating TFH cell expansion occurs in vaccine responders and is correlated with H1N1-specific Ab production and B-cell differentiation
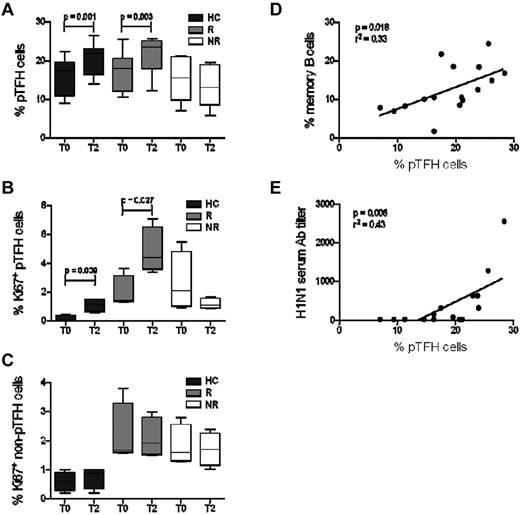
Because TFH cells provide help for B-cell differentiation and Ab production,1,2 investigations were performed to determine whether there were differences in pTFH cells in H1N1/09 vaccine responder and nonresponder HIV-infected patients. Although prevaccination frequencies of circulating pTFH cells were comparable among the patient groups and HCs, a significant expansion of pTFH cells was detected at T2 in HCs and responders (Figure 2A) concurrently with an increase in frequency of Ki67 expression, marking the fraction of proliferating cells (Figure 2B). Although the absolute numbers of pTFH cells also showed an increase in the vaccine responders, the values did not reach significance (not shown). The non-pTFH cells did not show an increase in frequency, absolute numbers, or Ki67 expression (Figure 2C). Frequencies of Ki67+ and CD38+HLA-DR+ CD4 and CD8 T cells at baseline did not differ between HIV responders and nonresponders (not shown), suggesting that underlying global immune activation is not the cause of selective pTFH expansion in HIV-infected responders.
pTFH cell expansion in vaccine responders is correlated with Ab production and B-cell differentiation. (A) Frequency of pTFH (CD4+CXCR5+) cells indicated as a percentage of total CD4 T cells in HCs and HIV-infected patients who were responders (R) or nonresponders (NR) to H1N1/09 influenza vaccine. Peripheral TFH cell frequencies were measured prevaccination (T0) and 4 weeks after vaccination (T2). (B-C) Frequency of Ki67+ pTFH (B) and non-pTFH (CD4+CXCR5−; C) cells at T0 and at T2. Box plots represent median with 25th and 75th percentile borders. Error bars represent the 10th and 90th percentile means from 8 HCs, 8 HIV+ responders, and 8 HIV+ nonresponders. (D-E) Linear correlation between frequency of pTFH cells with frequency of memory B cells (CD20+ CD21high CD10− CD27+) at T2 (D) and with H1N1 serum Ab titer at T2 (E).
pTFH cell expansion in vaccine responders is correlated with Ab production and B-cell differentiation. (A) Frequency of pTFH (CD4+CXCR5+) cells indicated as a percentage of total CD4 T cells in HCs and HIV-infected patients who were responders (R) or nonresponders (NR) to H1N1/09 influenza vaccine. Peripheral TFH cell frequencies were measured prevaccination (T0) and 4 weeks after vaccination (T2). (B-C) Frequency of Ki67+ pTFH (B) and non-pTFH (CD4+CXCR5−; C) cells at T0 and at T2. Box plots represent median with 25th and 75th percentile borders. Error bars represent the 10th and 90th percentile means from 8 HCs, 8 HIV+ responders, and 8 HIV+ nonresponders. (D-E) Linear correlation between frequency of pTFH cells with frequency of memory B cells (CD20+ CD21high CD10− CD27+) at T2 (D) and with H1N1 serum Ab titer at T2 (E).
At T2, the percentage of pTFH cells correlated with the frequency of memory B cells (Figure 2D) and with the serum H1N1 Ab titer (Figure 2E). These data indicate that pTFH cell proliferation and expansion are linked to H1N1 influenza vaccine responsiveness and to the generation of memory B cells in HCs and in HIV-infected patients.
Circulating TFH cells from vaccine responders are functional and able to help B cells secrete IgG on H1N1 Ag stimulation
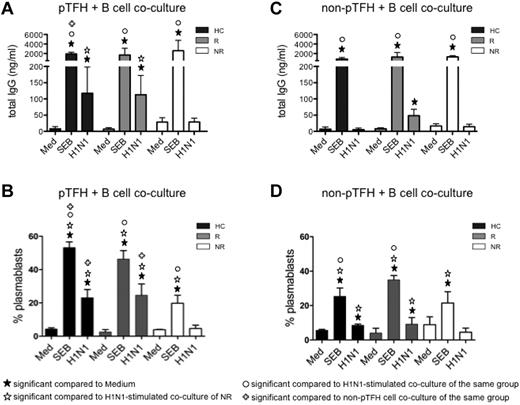
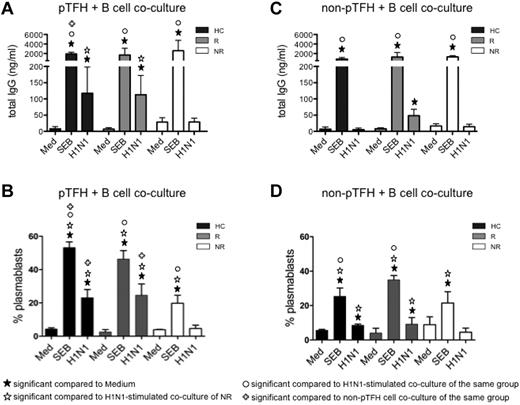
To establish the functional characteristics of pTFH in vaccine recipients, we determined their capacity to help B cells. Sorted pTFH and non-pTFH cells were cocultured with autologous B cells for 7 days. As shown in Figure 3A, pTFH cells from HCs and responders were able to support H1N1 Ag–stimulated IgG production in autologous B cells, whereas pTFH cells of vaccine nonresponders failed to do so, indicating that pTFH cells resemble GC TFH cells in their functional properties. Consistent with these observations, frequencies of proliferating plasmablasts also were higher in H1N1-stimulated pTFH cocultures from HCs and responders (Figure 3B). Non-pTFH CD4 T cells were less efficient in inducing H1N1 Ag-stimulated IgG in culture supernatants (Figure 3C) or plasmablast differentiation in cocultures with B cells (Figure 3D). Ag-induced IgG production was dependent on cognate interactions between T and B cells, because B cells cultured alone (data not shown) or cocultured with pTFH or non-pTFH cells in the absence of stimulus did not produce IgG. The inability of B cells to be triggered by H1N1 Ag in the presence of non-pTFH cells was not because of the poor survival of B cells in the cultures. On day 7, frequencies of live (ViViD−) CD20+ B cells cocultured with pTFH and non-pTFH cells were similar among the study groups (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). No differences were noted among staphylococcal enterotoxin B–induced IgG levels in HCs, responders, or nonresponders in cocultures of autologous B cells with pTFH or non-pTFH cells, although the magnitude of response was higher in the presence of pTFH than non-pTFH cells. These data suggest that defects in Ag-specific functionality of pTFH cells were associated with Ab nonresponsiveness in nonresponders.
pTFH cells promote B-cell Ab production and differentiation on H1N1-specific stimulation. Cryopreserved PBMCs obtained 4 weeks after H1N1/09 vaccination were thawed and rested overnight. B cells (CD20+) were isolated with magnetic beads and pTFH (CD3+CD4+CD45RA−CXCR5+) and non-pTFH (CD3+CD4+CD45RA−CXCR5−) cells were purified by cell sorting. B cells were cocultured with either pTFH or non-pTFH cells at a 1:1 ratio in medium alone or in the presence of 5 μg/mL of H1N1 vaccine Ag or 1 μg/mL of staphylococcal enterotoxin B for 7 days. (A,C) Culture supernatants from the pTFH + B-cell cocultures (A) or non-pTFH + B-cell cocultures (C) were harvested and IgG production was measured by ELISA. (B,D) Cells were harvested and frequency of plasmablasts (defined as CD20lowCD21low/−CD27+CD10−Ki67+ events) within the B cells was evaluated by flow cytometry in the pTFH + B-cell cocultures (B) or the non-pTFH + B-cell cocultures (D). Bars represent the means and error bars indicate the SD of 3 HCs, 3 HIV+ H1N1/09 vaccine responders (R), and 3 HIV+ vaccine nonresponders (NR).
pTFH cells promote B-cell Ab production and differentiation on H1N1-specific stimulation. Cryopreserved PBMCs obtained 4 weeks after H1N1/09 vaccination were thawed and rested overnight. B cells (CD20+) were isolated with magnetic beads and pTFH (CD3+CD4+CD45RA−CXCR5+) and non-pTFH (CD3+CD4+CD45RA−CXCR5−) cells were purified by cell sorting. B cells were cocultured with either pTFH or non-pTFH cells at a 1:1 ratio in medium alone or in the presence of 5 μg/mL of H1N1 vaccine Ag or 1 μg/mL of staphylococcal enterotoxin B for 7 days. (A,C) Culture supernatants from the pTFH + B-cell cocultures (A) or non-pTFH + B-cell cocultures (C) were harvested and IgG production was measured by ELISA. (B,D) Cells were harvested and frequency of plasmablasts (defined as CD20lowCD21low/−CD27+CD10−Ki67+ events) within the B cells was evaluated by flow cytometry in the pTFH + B-cell cocultures (B) or the non-pTFH + B-cell cocultures (D). Bars represent the means and error bars indicate the SD of 3 HCs, 3 HIV+ H1N1/09 vaccine responders (R), and 3 HIV+ vaccine nonresponders (NR).
PBMC from vaccine responders produce IL-21 upon in vitro H1N1 Ag stimulation
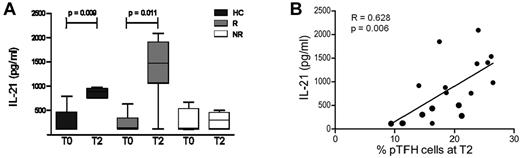
IL-21 secretion by TFH cells is known to promote the differentiation of B cells into Ab-secreting cells.29 Because the frequency of pTFH cells is correlated with vaccination-induced H1N1 Ab titer and with frequency of memory B cells, we investigated the H1N1 Ag–specific IL-21 response in vaccinated subjects. H1N1 Ag–stimulated IL-21 secretion in PBMC cultures of vaccinated HCs was evident as early as day 1 and was detected through day 5 (supplemental Figure 2A). Culture supernatants of PBMCs stimulated with H1N1 Ag for 5 days showed a significant increase in IL-21 at T2 as compared with prevaccination levels in HCs and responders, but not in nonresponders (Figure 4A) and were correlated with frequency of pTFH at T2 (Figure 4B). These findings are in agreement with our previous observation in this study population that serum IL-21 levels were correlated with H1N1 influenza Ab responses.19
H1N1 Ag promotes IL-21 production by PBMCs from vaccine responders on in vitro stimulation. (A) Cryopreserved PBMCs obtained at T0 and 4 weeks after H1N1/09 vaccination (T2) were thawed, rested overnight, and cultured at 1 × 106 cells/mL for 5 days at 37°C in the presence of 5 μg/mL of H1N1 vaccine Ag. On day 5, supernatants were harvested and IL-21 levels were determined by ELISA. (B) Graph shows the linear correlation between the frequency of peripheral TFH cells at T2 with IL-21 levels in H1N1-stimulated PBMC culture supernatants. Bars represent the means and error bars indicate the SD of 5 HCs, 5 HIV+ responders (R), and 4 HIV+ nonresponders (NR). *P < .05.
H1N1 Ag promotes IL-21 production by PBMCs from vaccine responders on in vitro stimulation. (A) Cryopreserved PBMCs obtained at T0 and 4 weeks after H1N1/09 vaccination (T2) were thawed, rested overnight, and cultured at 1 × 106 cells/mL for 5 days at 37°C in the presence of 5 μg/mL of H1N1 vaccine Ag. On day 5, supernatants were harvested and IL-21 levels were determined by ELISA. (B) Graph shows the linear correlation between the frequency of peripheral TFH cells at T2 with IL-21 levels in H1N1-stimulated PBMC culture supernatants. Bars represent the means and error bars indicate the SD of 5 HCs, 5 HIV+ responders (R), and 4 HIV+ nonresponders (NR). *P < .05.
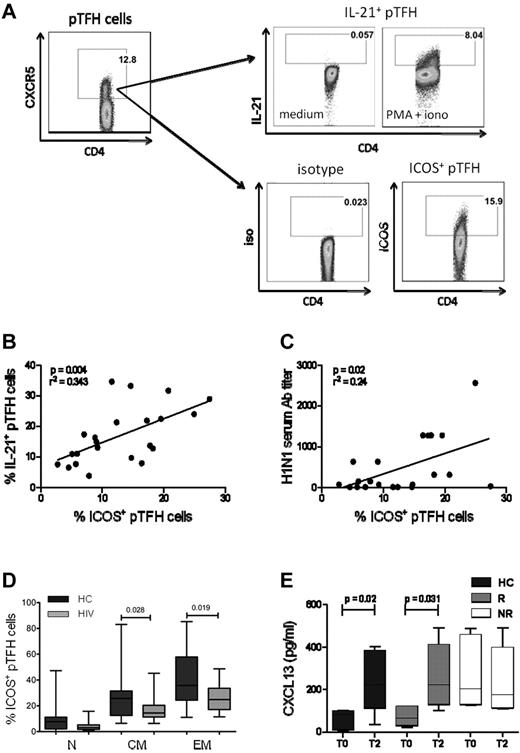
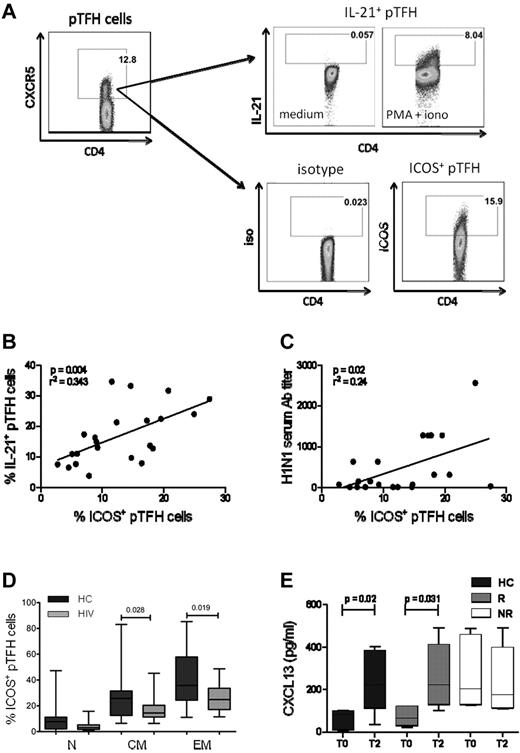
ICOS expression in pTFH cells is correlated with the production of IL-21 and H1N1 serum Ab titer
A distinguishing feature of GC TFH cells is the surface expression of ICOS.1,30 In the mouse model, ICOS is necessary for IL-21 production by TFH.31 Moreover, the ICOS-ICOS ligand interaction is essential for immunoglobulin secretion from human B cells in vitro.32 We investigated the relationship of ICOS expression with the pTFH phenotype and IL-21 secretion at T2. Figure 5A shows the gating strategy for detection of ICOS and PMA + ionomycin–stimulated IL-21 in CD4+CXCR5+ cells. The mean frequencies of ICOS and IL-21 expressing pTFH cells were 20.72% (range, 7.87%-33.6%) and 22.1% (range, 7.2%-41%), respectively, and were correlated positively with each other (Figure 5B, P = .04). In addition, frequencies of ICOS+ pTFH cells were also correlated with the H1N1 Ab titers at T2 (Figure 5C, P = .02). Like total pTFH, ICOS+ pTFH cells also had a predominant TCM phenotype in HCs and HIV-infected patients (Figure 5D).
ICOS expression in pTFH cells is correlated with IL-21 production and Ab response to H1N1/09 vaccine. (A) Representative dot plots showing the gating strategy for IL-21 and ICOS expression in pTFH cells, defined as CD4+CXCR5+ events, using appropriate isotype control Abs. Freshly isolated PBMCs at T2 were cultured in complete medium alone or in the presence of PMA, ionomycin, and brefeldin A for 5 hours and stained for intracellular IL-21. (B) Linear correlation of frequency of ICOS+ pTFH cells with IL-21–producing pTFH cells at T2 of 8 HCs, 7 HIV+ responders (R), and 7 HIV+ nonresponders (NR). (C) Linear correlation of frequency of ICOS+ pTFH cells with H1N1 serum Ab titer at T2 for 8 HCs, 7 HIV+ responders, and 7 HIV+ nonresponders. (D) Maturation subsets within ICOS+ pTFH cells. (E) Cryopreserved PBMCs were thawed, rested overnight, and cultured at 1 × 106 cells/mL for 5 days at 37°C with H1N1 vaccine Ag or medium as described. Supernatants were harvested and CXCL13 levels were determined by ELISA. Bar graphs show CXCL13 levels at T0 and T2 after vaccination in 5 HCs, 6 HIV+ responders, and 6 HIV+ nonresponders. Box plots represent median with 25th and 75th percentile borders. Error bars represent the 10th and 90th percentiles.
ICOS expression in pTFH cells is correlated with IL-21 production and Ab response to H1N1/09 vaccine. (A) Representative dot plots showing the gating strategy for IL-21 and ICOS expression in pTFH cells, defined as CD4+CXCR5+ events, using appropriate isotype control Abs. Freshly isolated PBMCs at T2 were cultured in complete medium alone or in the presence of PMA, ionomycin, and brefeldin A for 5 hours and stained for intracellular IL-21. (B) Linear correlation of frequency of ICOS+ pTFH cells with IL-21–producing pTFH cells at T2 of 8 HCs, 7 HIV+ responders (R), and 7 HIV+ nonresponders (NR). (C) Linear correlation of frequency of ICOS+ pTFH cells with H1N1 serum Ab titer at T2 for 8 HCs, 7 HIV+ responders, and 7 HIV+ nonresponders. (D) Maturation subsets within ICOS+ pTFH cells. (E) Cryopreserved PBMCs were thawed, rested overnight, and cultured at 1 × 106 cells/mL for 5 days at 37°C with H1N1 vaccine Ag or medium as described. Supernatants were harvested and CXCL13 levels were determined by ELISA. Bar graphs show CXCL13 levels at T0 and T2 after vaccination in 5 HCs, 6 HIV+ responders, and 6 HIV+ nonresponders. Box plots represent median with 25th and 75th percentile borders. Error bars represent the 10th and 90th percentiles.
We also examined the CXCR5+ CD4 T-cell subset for expression of the other conventional TFH markers programmed death-1 (PD-1)33 and the transcription factor B-cell lymphoma 6 (Bcl6).34 Shown in supplemental Figure 3A are representative histograms of phenotypic characterization of pTFH and non-pTFH cells in PBMCs of HCs. PD-1 and Bcl6 were expressed at similar levels in pTFH and non-pTFH cells (supplemental Figure 3B). These findings are in agreement with previous studies9,35 showing that pTFH cells only partially express the markers of conventional GC TFH cells. Another important feature of TFH cells is the production of the chemokine CXCL13, the ligand for CXCR5 that promotes B-cell differentiation and immunoglobulin secretion.36 Because circulating TFH cells are capable of producing this chemokine,37 we investigated its expression on Ag-specific stimulation of pTFH cells. H1N1-induced production of CXCL13 peaked at day 5 in PBMC cultures of HCs (supplemental Figure 2B). Prevaccination levels of CXCL13 in 5-day culture supernatants of H1N1 Ag–stimulated PBMCs were higher in nonresponders compared with responders and HCs, but failed to increase 4 weeks after vaccination. In contrast, responders and HCs showed an increase in Ag-stimulated CXCL13 levels at T2 (Figure 5E).
Discussion
TFH cells are a subset of CD4 T cells that reside in GC and are involved in the initiation and maintenance of responses that generate memory B cells and long-lived plasma cells during an immune response.1 The circulating CXCR5+ CD4 memory T cells, designated here as pTFH cells, are similar to the GC TFH cells in their ability to promote B-cell differentiation and Ab secretion in vitro.9 In the present study, we show that in the unique setting of H1N1/09 influenza vaccination–induced Ab responses in chronic HIV-infected patients and HCs, the pTFH cells of vaccine responders were qualitatively superior than those of vaccine nonresponders despite being similar in prevaccination frequencies in the different response groups. Vaccine responders exhibited pTFH cell expansion in association with Ag-specific IL-21 secretion, and their purified pTFH cells had the ability to support Ag-specific Ab responses and B-cell differentiation in cocultures with autologous B cells. Our study shows for the first time that qualitative deficiency in this peripheral CD4 T-cell subset can signify immunodeficiency in virologically controlled HIV-infected patients with quantitative CD4 T-cell immune reconstitution. These data also suggest that analysis of pTFH cells could serve as a basis for developing future hypotheses toward understanding vaccine-induced immune responses and correlates of protection from seasonal influenza and other infections.
A major question that naturally arises is about the origin of these circulating CXCR5+ CD4 T cells. Data from ICOS-deficient humans suggest that these are post-GC reaction memory TFH cells.1,38 Whether these pTFH cells originate from cells that migrate from GCs or are committed extrafollicular helper cells is unknown. The high expression of CCR7 on pTFH cells suggests that they may traffick from the periphery to secondary lymphoid organs,9,35,39-41 possibly after Ag encounter. In their surface phenotype, the pTFH cells only partially resemble GC TFH. CXCR5 is the single molecule that sets the pTFH cells apart from other peripheral CD4 cells and is also universally expressed on GC TFH cells. In agreement with previous data,9 other classic GC TFH markers (ie, PD-1 and Bcl6) were expressed at a lower frequency in the pTFH cells.
HCs had 10%-15% CD4 cells expressing CXCR5 and had a predominantly central memory phenotype, confirming previous reports.8,9 Interest in defining various CD4 T-cell subsets in untreated and treated HIV infection stems from the progressive depletion of CD4 T cells that occurs in HIV infection.12 A prior report in cART-naive HIV-infected patients with CD4 < 200 cells/mm3 showed deficiencies of IL-21–secreting peripheral CD4 T cells with a progressive decline in CXCR5+ CD4 T cells.26 Our cohort consisted of cART-treated HIV-infected patients, most with controlled viremia and CD4 > 350 cells/mm3. In this study population, we did not observe a preferential loss of the CXCR5+ CD4 T-cell subpopulation in patients compared with HCs, and attribute the difference from the previous study to the quantitative normalization of peripheral CXCR5+ CD4 T cells due to antiretroviral therapy.
Patients with chronic HIV infection frequently manifest poor Ab responses to vaccines and had a reported 39% failure rate for seroconversion after a single dose of H1N1/09 vaccine during the H1N1 influenza outbreak.15 In the present cohort, half of the patients failed to mount a serologic response and were shown to have immunologic deficits that were discernible only after vaccination.19,20 Included in these deficits were a failure of expansion of memory B cells and poor up-regulation of plasma IL-21, one of the main cytokines produced by pTFH cells. These findings, together with the fact that IL-21 is the most critical γc-sharing cytokine for mediating B-cell proliferation, differentiation, and immunoglobulin production,42 were the major impetus for the present study. Interestingly, pTFH cell expansion occurred at T2 only in responders and HCs, but not in nonresponders. Ki67, the marker for cell proliferation,43 has been used to evaluate vaccine-induced specific T-cell responses in CD4 and CD8 T cells directly ex vivo or after in vitro Ag stimulation.44,45 The percentage of pTFH cells at week 4 after vaccination was correlated positively with serum H1N1 Ab titer and with IL-21 in culture supernatants of Ag-stimulated PBMCs, suggesting that the expansion of pTFH cells was an important component of the immune response to H1N1 influenza vaccine. Further support for this premise comes from the observed direct correlation of frequencies of pTFH cells with memory B cells in vaccinated subjects, suggesting that vaccination-induced expansion of pTFH cells is required for the generation of memory B cells. Moreover, direct evidence of qualitative impairment of Ag-specific pTFH cells was noted in their failure to adequately support autologous B cells in HIV-infected nonresponders, with failure of induction of the IgG response and generation of plasmablasts. IgG production was dependent on cognate interactions between T and B cells, because B cells cultured alone with H1N1 Ag did not produce IgG (data not shown). In this system, Ag-specific B cells could act as autologous APCs, because our cultures did not have any other APC present. Earlier studies have described the Ag-presenting properties of B cells in priming CD4+ T cells extensively.46-48 Because the B cells cultured alone did not show any IgG after Ag stimulation, this indicates that their differentiation was dependent on help from functional pTFH cells. The absence of IgG production in B cells cocultured with non-pTFH cells was not attributed to excessive B-cell death because the frequencies of viable B cells (ViViD−) at culture termination were not different between pTFH and non-pTFH cultures. These data are in agreement with existing evidence that pTFH cells are needed to maintain and regulate B-cell differentiation into plasma cells and memory B cells.9 The implications of these findings is that quantitative CD4 T-cell immune reconstitution after cART may be associated with underlying immunodeficiency that is uncovered after nonadjuvanted vaccines such as the H1N1/09 influenza vaccine used in our patients. A testable hypothesis generated from the present study is that Ag-specific pTFH cell expansion and function induced by vaccines are important correlates of a successful humoral immune response.
In the present study, we observed an up-regulation of H1N1 Ag–stimulated IL-21 secretion in PBMC culture supernatants at T2 compared with prevaccination levels in responders but not in nonresponders. The observed correlation between the frequency of TFH- and H1N1 Ag–induced IL-21 in PBMC culture supernatants favors but does not prove pTFH as an important source of IL-21, because other cellular sources of IL-21 have been described previously.49,50 In this context, we observed that most IL-21–producing T cells were indeed pTFH and that the IL-21–secreting pTFH cells did not cosecrete IL-17 or IFNγ after PMA + ionomycin stimulation (unpublished observations). This finding is in agreement with previous report indicating that IL-21–producing CD4 T cells have a stable phenotype and are distinct from Th17 cells.51 Whether the IL-21 responses characterize protective immunity to influenza and if the findings reported here are applicable for other vaccines aimed at generating humoral immune responses needs to be investigated.
The requirement for ICOS in TFH-cell development and persistence during the T- to B-cell interaction has been demonstrated elegantly in mice and in humans.38,52,53 In patients with ICOS deficiency, reduced numbers of circulating CXCR5+ CD4 T cells were associated with severely disturbed GC formation.38 Because in the present study we observed a positive correlation between the percentage of ICOS+ pTFH cells and the frequency of IL-21+ pTFH cells in vaccinated HIV-infected subjects, we propose that low ICOS expression is associated with low production of IL-21 and results in a lack of an appropriate B-cell response to H1N1/09 influenza vaccine. The chemokine CXCL13, the ligand for CXCR5, is produced in high quantities by TFH cells and is expressed abundantly in the GC light zone,36 where it facilitates the interaction between TFH and B cells, which also express CXCR5 highly. Together the CXCL13 and CXCR5 molecules are critical for normal organization of B-cell follicles in secondary lymphoid tissues.7,36 pTFH cells also express high levels of CXCL13 and induce differentiation of autologous B cells to plasma cell and immunoglobulin secretion in vitro.37 Increased plasma levels of CXCL13 have been reported in HIV infection.54 Interestingly, in the present study, CXCL13 levels in PBMC culture supernatants were higher in nonresponders than in responders and HCs prevaccination, but failed to show an Ag-stimulated increase at 4 weeks after vaccination. The higher prevaccination levels of CXCL13 in nonresponders could reflect a failure of utilization by pTFH or B cells or, more likely, may be a component of generalized immune activation. An inability to increase the levels of this chemokine after vaccination could be a causative factor leading to impaired GC reaction with failure of development of Ab-secreting B cells, but this possibility needs further investigation.
Our study had certain limitations. Our overall sample size was small and the number of H1N1/09–vaccinated patients available for study was limited. However, it is remarkable that this patient group, without an obvious preselection bias, was evenly divided between responders and nonresponders. The novel findings reported herein related to pTFH cells in the context of H1N1/09 vaccination complement our previous reports of immunologic differences distinguishing responders and nonresponders.19,20 The coculture experiments of purified pTFH and B cells provided additional evidence that the nonresponsiveness to an H1N1 vaccine–induced Ab response in nonresponders is associated with impairment of Ag-specific pTFH cell expansion and function. Studies in larger cohorts will help to determine whether the relative ease of analyzing pTFH cells is useful in clinical studies of pTFH cells as correlates of vaccine-induced immunity, particularly protective Ab responses. To understand pTFH cell biology, global gene-expression profiling55 and the role of dendritic cells56 are clearly important. The results of the present study provide new insights and a rationale for future analyses of human pTFH cells to examine immune cell interactions that determine Ab responses to influenza vaccine and possibly to HIV vaccines in HIV-infected patients and in other conditions with perturbed Ab responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the laboratory sciences core of the Developmental Center for AIDS Research (A1073961) and Sylvester Comprehensive Cancer Center for facilitating the flow cytometry studies and the patients for participation in this study.
This study was supported by the National Institutes of Health (grant A1077501 to S.P.) and by a specialty laboratory grant from the International Maternal, Pediatric and Adolescent AIDS Clinical Trials Group (IMPAACT). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health (AI068632).
National Institutes of Health
Authorship
Contribution: S. Pallikkuth, A.P., S.Y.S., and V.K.G. performed the experiments; S. Pallikkuth, A.P., and S.Y.S. analyzed the data; M.F. and R.P. edited the manuscript; M.F. recruited the patients; R.P. provided guidance on flow cytometry analysis and design; and S. Pahwa designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Savita Pahwa, MD, Professor, Microbiology and Immunology, University of Miami Miller School of Medicine, 1580 NW 10th Ave, BCRI 712, Miami, FL 33136; e-mail: spahwa@med.miami.edu.
References
Author notes
S.P., A.P., and S.Y.S. contributed equally to this work.