Abstract
Nontransfusion-dependent thalassemia (NTDT) patients may develop iron overload and its associated complications despite receiving only occasional or no transfusions. The present 1-year, randomized, double-blind, placebo-controlled THALASSA (Assessment of Exjade in Nontransfusion-Dependent Thalassemia) trial assessed the efficacy and safety of deferasirox in iron-overloaded NTDT patients. A total of 166 patients were randomized in a 2:1:2:1 ratio to starting doses of 5 or 10 mg/kg/d of deferasirox or placebo. The means ± SD of the actual deferasirox doses received over the duration of the study in the 5 and 10 mg/kg/d starting dose cohorts were 5.7 ± 1.4 and 11.5 ± 2.9 mg/kg/d, respectively. At 1 year, the liver iron concentration (LIC) decreased significantly compared with placebo (least-squares mean [LSM] ± SEM, −2.33 ± 0.7 mg Fe/g dry weight [dw], P = .001, and −4.18 ± 0.69 mg Fe/g dw, P < .001) for the 5 and 10 mg/kg/d deferasirox groups, respectively (baseline values [means ± SD], 13.11 ± 7.29 and 14.56 ± 7.92 mg Fe/g dw, respectively). Similarly, serum ferritin decreased significantly compared with placebo by LSM −235 and −337 ng/mL for the deferasirox 5 and 10 mg/kg/d groups, respectively (P < .001). In the placebo patients, LIC and serum ferritin increased from baseline by 0.38 mg Fe/g dw and 115 ng/mL (LSM), respectively. The most common drug-related adverse events were nausea (n = 11; 6.6%), rash (n = 8; 4.8%), and diarrhea (n = 6; 3.6%). This is the first randomized study showing that iron chelation with deferasirox significantly reduces iron overload in NTDT patients with a frequency of overall adverse events similar to placebo.
Introduction
Unlike transfusion-dependent β-thalassemia major,1 patients with clinically milder forms of thalassemia, including β-thalassemia intermedia, α-thalassemia (mainly HbH disease), and HbE/β-thalassemia, require occasional or no blood transfusions. Despite limited transfusion needs, nontransfusion-dependent thalassemia (NTDT) patients may develop clinically relevant iron overload with serious sequelae, including liver and endocrine dysfunction.2 Furthermore, elevated liver iron concentration (LIC) has been associated with increased morbidity in patients with β-thalassemia intermedia.2 Iron overload in NTDT patients results primarily from increased intestinal iron absorption caused by ineffective erythropoiesis3 and may develop as early as 5 years of age.4 Iron overload usually becomes clinically relevant after 10 years,5 making iron loads in NTDT patients in their 30s and 40s comparable to those in transfusion-dependent β-thalassemia patients.3 For example, in a previous study of β-thalassemia major patients (mean age, 17 years), LIC was 11.1 mg Fe/g dry weight (dw), although this may reflect prior suboptimal chelation therapy.6 For the NTDT patients examined in the present study, (mean age, 32 years), the LIC was 12.1 mg Fe/g dw,7 despite few patients having received previous iron-chelation therapy. Therefore, effective monitoring and control of iron burden is important, although often underappreciated.
Iron-chelation therapy is the only option for decreasing iron burden, because clinical anemia contraindicates phlebotomy. Data on iron chelation in NTDT patients are currently restricted to small, uncontrolled studies and case reports.4,8-14 Deferasirox (Novartis Pharmaceuticals) is a once-daily oral iron chelator with demonstrated efficacy and a well-characterized safety profile in patients with chronically transfused anemias.15-20 Given the different pathophysiology of iron metabolism and the slower iron-loading rate in NTDT compared with transfusion-dependent β-thalassemia patients, it is important to establish the efficacy, safety, and optimal doses of deferasirox in this population. We report herein 1-year data from the phase 2, prospective, randomized, double-blind, placebo-controlled THALASSA (Assessment of Exjade in NonTransfusion-Dependent Thalassemia) trial assessing the efficacy and safety of deferasirox in NTDT patients with iron overload. This is the first randomized, placebo-controlled study evaluating iron chelation in NTDT.
Methods
Key inclusion and exclusion criteria
Male or female patients ≥ 10 years of age with NTDT and iron overload (R2-MRI–measured LIC ≥ 5 mg Fe/g dw) and serum ferritin > 300 ng/mL at screening based on 2 consecutive values ≥ 14 days apart were eligible. In addition, patients were required to have not received transfusions within 6 months or chelation therapy within 1 month before study entry. Patients with previous exposure to deferasirox or with anticipated regular transfusions were excluded; unplanned transfusions during the study were allowed. Exclusion criteria also included: HbS variants of thalassemia syndromes, active hepatitis B (positive hepatitis B surface antigen with negative hepatitis B surface Ab) or hepatitis C (positive hepatitis C virus Ab and detectable hepatitis C virus RNA with alanine aminotransferase [ALT] above the normal range), cirrhosis, levels of ALT > 5× the upper limit of normal (ULN), serum creatinine > ULN or creatinine clearance ≤ 60 mL/min on 2 measurements, or significant proteinuria (urine protein/urine creatinine ratio > 1.0 mg/mg) on 2 measurements. Patients (or parents/guardians) provided written, informed consent before enrollment.
Study design
THALASSA was a multinational, prospective, randomized, double-blind, placebo-controlled phase 2 study (registered at www.clinicaltrials.gov as NCT00873041). Patients were randomized in a 2:1:2:1 ratio to the following 4 treatment groups for the 52-week treatment period: (1) 5 mg/kg/d of deferasirox, (2) 5 mg/kg/d of matching placebo, (3) 10 mg/kg/d of deferasirox, or (4) 10 mg/kg/d of matching placebo. Doses were doubled at 24 weeks for patients with LIC > 7 mg Fe/g dw and LIC reduction < 15% from baseline. Dose adjustment recommendations were also provided based on continuous safety assessments. If serum ferritin was < 100 ng/mL or LIC was < 3 mg Fe/g dw at any visit, treatment was to be suspended until LIC increased to ≥ 5 mg Fe/g dw and serum ferritin to > 300 ng/mL. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study was approved by local ethics committees of all participating study sites. An independent data-monitoring committee reviewed safety data and advised on study continuation and/or changes to protocol.
Randomization and masking
After a 4-week screening phase, patients were block randomized using an interactive voice response system. After confirming that the patient fulfilled the inclusion/exclusion criteria, the investigator contacted the interactive voice response system to obtain a randomization number linking the patient to a treatment arm. Because blinding of dose was not possible, blinding was only applied to the treatment received. All persons were blinded to the treatment from the time of randomization until database lock.
Assessments
The primary efficacy end point was the absolute change in LIC from baseline at 52 weeks. Supportive analyses of the primary end point included assessment of the number and proportion of patients with a LIC decrease of ≥ 3 mg Fe/g dw, those with ≥ 30% reduction in LIC, and those with LIC ≤ 7, ≤ 5, and ≤ 3 mg Fe/g dw at 1 year. Secondary end points included the change in LIC from baseline at 24 weeks, change in serum ferritin from baseline at 52 weeks, and correlation of serum ferritin and LIC. Results are presented by starting dose group.
LIC was assessed at screening, at week 24, at week 52, when the serum ferritin stopping target was achieved, or at discontinuation for other reasons. LIC was measured using a validated R2-MRI technique (FerriScan), assuming reproducibility over the range of clinical conditions studied.21 Serum ferritin was measured during screening and every 4 weeks thereafter. Baseline serum ferritin was calculated as the average of all screening measurements on or before the day of randomization. Both LIC and serum ferritin were analyzed at a central laboratory. Patients came to the study site at all visits to perform the scheduled assessments.
Safety was evaluated through continuous monitoring and recording of adverse events (AEs) and serious AEs (SAEs), laboratory testing, and clinical evaluations. Compliance was estimated based on the cumulative dose taken based on the amount of study medication dispensed and the amount returned or on patient diaries, and was compared with the cumulative planned dose.
Statistical methods
Power calculations indicated that a total sample size of 52 patients for each deferasirox group and 26 patients for each placebo group (156 patients in total) were required to achieve a study power of 90% to demonstrate superiority of at least one deferasirox group compared with placebo for the primary end point. This was based on the assumption of a mean decrease of 3 mg Fe/g dw in deferasirox patients compared with placebo and a common standard deviation of 4 mg Fe/g dw.
The primary efficacy variable was the absolute change in LIC from baseline to week 52. If no LIC measurement was available at week 52, the last available postbaseline LIC measurement before week 52 was used. ANCOVA included the treatment as factor and baseline LIC as covariate. As a result, the primary efficacy results were presented adjusted for baseline. Multiplicity was addressed by controlling the 1-sided family-wise type I error rate to 0.025 with the Dunnett method22 and presenting 2-sided simultaneous 95% confidence intervals (95% CIs). Absolute change from baseline LIC at week 24 was analyzed in the same way as the primary efficacy variable.
For serum ferritin quarterly change from baseline, a mixed-effect model was fitted on the full analysis set with fixed-factors treatment, quarter, and treatment-by-quarter interaction. When the results were missing for a quarter, the results from the last available quarter were used for that patient. For the fourth quarter, each deferasirox group was compared with placebo using the Dunnett adjustment for multiple comparisons to the placebo control group (family-wise type I error rate, 0.025). The 2 deferasirox groups were compared with a 2-sided significance level of 5%.
The correlation of LIC versus serum ferritin at baseline was assessed as well as the correlation of relative change in LIC versus relative change in serum ferritin at week 24 and week 52.
The effect of dose increase was evaluated by summarizing the last LIC value after the week-24 LIC assessment and the last value before or at the week-24 LIC assessment, with descriptive statistics by treatment group and separately for patients with and without increase. Patient subgroup analyses were also performed.
Efficacy was assessed for the full analysis set (all randomized patients). Placebo groups were pooled and shown as one group for efficacy analyses. If there was no LIC measurement available at week 52, the last available postbaseline LIC measurement was carried forward. The absolute change in LIC at week 52 with last observation carried forward were analyzed using ANCOVA with treatment as factor and baseline LIC as covariate. Differences between deferasirox and placebo were compared using the Dunnett adjustment22 and a 1-sided significance level of P = .025 to establish that the mean decrease with deferasirox was greater than that with placebo. If both deferasirox arms were statistically superior to placebo, the 2 deferasirox groups were compared at a 2-sided significance level of P = .05. Correlation of baseline LIC and serum ferritin was assessed.
All patients who received at least 1 dose of study medication were included in the safety population. Safety data for the 2 placebo groups were analyzed separately to allow for potential effects of different excipient quantities in deferasirox formulations.
Role of funding source
The study was sponsored by Novartis Pharma AG and designed by the sponsor in close collaboration with a study steering committee composed of expert hematologists in the field of thalassemia. The sponsor conducted the statistical analysis. Authors had full access to the data and participated actively in interpreting data and writing and critically reviewing the article with the assistance of a medical writer funded by the sponsor. The corresponding author had final responsibility for the manuscript content and decision to submit for publication.
Results
Patient disposition
The study was conducted between November 24, 2008 and June 22, 2011. A total of 339 patients were screened and 166 were randomized to receive treatment (Figure 1). Overall, 89.2% of patients (n = 148) completed 1 year of the study.
Patient disposition. *Other: (n = 2) > 20 transfusions/lifetime (note: patients were excluded before the protocol was amended to remove this criterion); (all n = 1): (i) iron chelation therapy < 6 months of study start (note: the protocol was amended to exclude patients with iron chelation therapy < 1 month of study start; however, this patient was excluded before this protocol amendment): (ii) concomitant hydroxyurea; (iii) concomitant autoimmune hemolytic anemia with severe anemia; (iv) ALT > 3× ULN (note: the protocol was amended to exclude patients with ALT > 5× ULN; however, this patient was excluded before this protocol amendment); (v) invalid creatinine results; (vi) safety concern; and (vii) rescreened by mistake.
Patient disposition. *Other: (n = 2) > 20 transfusions/lifetime (note: patients were excluded before the protocol was amended to remove this criterion); (all n = 1): (i) iron chelation therapy < 6 months of study start (note: the protocol was amended to exclude patients with iron chelation therapy < 1 month of study start; however, this patient was excluded before this protocol amendment): (ii) concomitant hydroxyurea; (iii) concomitant autoimmune hemolytic anemia with severe anemia; (iv) ALT > 3× ULN (note: the protocol was amended to exclude patients with ALT > 5× ULN; however, this patient was excluded before this protocol amendment); (v) invalid creatinine results; (vi) safety concern; and (vii) rescreened by mistake.
Baseline demographics and clinical characteristics
Baseline demographics and clinical characteristics were similar across groups (Table 1). The baseline LIC was slightly higher in the placebo group than in the deferasirox 5 and 10 mg/kg/d starting dose cohorts.
Demographic, disease, and baseline patient characteristics
| Variable . | Deferasirox 5 mg/kg/d (n = 55) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 5 and 10 mg/kg/d (n = 56) . |
|---|---|---|---|
| Disease, n (%) | |||
| β-thalassemia intermedia | 32 (58.2) | 30 (54.5) | 33 (58.9) |
| α-thalassemia* | 5 (9.1) | 9 (16.4) | 8 (14.3) |
| HbE/β-thalassemia | 18 (32.7) | 16 (29.1) | 15 (26.8) |
| Mean age, y ± SD | 33.1 ± 12.3 | 31.7 ± 11.7 | 31.4 ± 12.2 |
| Median age, y (range) | 33 (10-60) | 31 (12-69) | 32 (10-59) |
| Pediatric patients, n (%)† | 6 (10.9) | 7 (12.7) | 8 (14.3) |
| Male: female ratio, n | 29:26 | 29:26 | 31:25 |
| Splenectomy, n (%) | 29 (52.7) | 31 (56.4) | 28 (50.0) |
| Prior transfusions received, n (%)‡ | 49 (89.1) | 50 (90.9) | 46 (82.1) |
| Previous chelation, n (%) | 8 (14.5) | 16 (29.1) | 20 (35.7) |
| Deferoxamine | 7 (12.7) | 15 (27.3) | 17 (30.4) |
| Deferiprone | 0 (0) | 1 (1.8) | 3 (5.4) |
| Deferoxamine + deferiprone | 1 (1.8) | 0 (0) | 0 (0) |
| Mean LIC, mg Fe/g dw ± SD§ | 13.11 ± 7.29 | 14.56 ± 7.92 | 15.94 ± 10.85 |
| Median LIC, mg Fe/g dw (range) | 11.7 (2.6-38.6) | 11.7 (5.0-32.8) | 13.0 (5.0-49.1) |
| LIC category, n (%)¶ | |||
| < 7 mg Fe/g dw | 10 (18.2) | 8 (14.5) | 13 (23.2) |
| 7-15 mg Fe/g dw | 31 (56.4) | 26 (47.3) | 20 (35.7) |
| > 15 mg Fe/g dw | 14 (25.5) | 21 (38.2) | 22 (39.3) |
| Mean serum ferritin, ng/mL ± SD | 1141 ± 805 | 1174 ± 684 | 1305 ± 1017 |
| Median serum ferritin, ng/mL (range) | 988 (370-5609) | 1015 (342-4224) | 994 (304-6419) |
| Serum ferritin category, n (%) | |||
| < 500 ng/mL | 5 (9.1) | 4 (7.3) | 8 (14.3) |
| 500-1000 ng/mL | 24 (43.6) | 23 (41.8) | 20 (35.7) |
| 1000-2500 ng/mL | 23 (41.8) | 26 (47.3) | 23 (41.1) |
| > 2500 ng/mL | 3 (5.5) | 2 (3.6) | 5 (8.9) |
| Variable . | Deferasirox 5 mg/kg/d (n = 55) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 5 and 10 mg/kg/d (n = 56) . |
|---|---|---|---|
| Disease, n (%) | |||
| β-thalassemia intermedia | 32 (58.2) | 30 (54.5) | 33 (58.9) |
| α-thalassemia* | 5 (9.1) | 9 (16.4) | 8 (14.3) |
| HbE/β-thalassemia | 18 (32.7) | 16 (29.1) | 15 (26.8) |
| Mean age, y ± SD | 33.1 ± 12.3 | 31.7 ± 11.7 | 31.4 ± 12.2 |
| Median age, y (range) | 33 (10-60) | 31 (12-69) | 32 (10-59) |
| Pediatric patients, n (%)† | 6 (10.9) | 7 (12.7) | 8 (14.3) |
| Male: female ratio, n | 29:26 | 29:26 | 31:25 |
| Splenectomy, n (%) | 29 (52.7) | 31 (56.4) | 28 (50.0) |
| Prior transfusions received, n (%)‡ | 49 (89.1) | 50 (90.9) | 46 (82.1) |
| Previous chelation, n (%) | 8 (14.5) | 16 (29.1) | 20 (35.7) |
| Deferoxamine | 7 (12.7) | 15 (27.3) | 17 (30.4) |
| Deferiprone | 0 (0) | 1 (1.8) | 3 (5.4) |
| Deferoxamine + deferiprone | 1 (1.8) | 0 (0) | 0 (0) |
| Mean LIC, mg Fe/g dw ± SD§ | 13.11 ± 7.29 | 14.56 ± 7.92 | 15.94 ± 10.85 |
| Median LIC, mg Fe/g dw (range) | 11.7 (2.6-38.6) | 11.7 (5.0-32.8) | 13.0 (5.0-49.1) |
| LIC category, n (%)¶ | |||
| < 7 mg Fe/g dw | 10 (18.2) | 8 (14.5) | 13 (23.2) |
| 7-15 mg Fe/g dw | 31 (56.4) | 26 (47.3) | 20 (35.7) |
| > 15 mg Fe/g dw | 14 (25.5) | 21 (38.2) | 22 (39.3) |
| Mean serum ferritin, ng/mL ± SD | 1141 ± 805 | 1174 ± 684 | 1305 ± 1017 |
| Median serum ferritin, ng/mL (range) | 988 (370-5609) | 1015 (342-4224) | 994 (304-6419) |
| Serum ferritin category, n (%) | |||
| < 500 ng/mL | 5 (9.1) | 4 (7.3) | 8 (14.3) |
| 500-1000 ng/mL | 24 (43.6) | 23 (41.8) | 20 (35.7) |
| 1000-2500 ng/mL | 23 (41.8) | 26 (47.3) | 23 (41.1) |
| > 2500 ng/mL | 3 (5.5) | 2 (3.6) | 5 (8.9) |
HbH disease (n = 8), HbH Constant Spring (n = 6), genotype not determined (n = 6), CSEA Bart's (n = 1), and Hb Agrino (n = 1).
Pediatric subjects were < 18 years of age.
Patients did not receive any transfusion in the 6 months prior to study entry.
95% of the ULN for LIC is < 1.8 mg Fe/g dw.21
One patient was missing from the placebo group.
Exposure to treatment and compliance
The means ± SD of actual deferasirox doses received over the study (taking into consideration dose adjustments permitted from week 24) in the 5 and 10 mg/kg/d starting dose cohorts were 5.7 ± 1.4 and 11.5 ± 2.9 mg/kg/d, respectively. The median durations of treatment were 12.2 (range, 0-15) and 12.1 (range, 2-14) months, respectively. Dose was increased at week 24 in 81 (48.4%) patients overall. These dose increases occurred in 26 (47.3%) patients receiving deferasirox 5 mg/kg/d and in 25 (45.5%) patients receiving deferasirox 10 mg/kg/d as starting doses. Fifteen (56.3%) patients in each of the placebo 5 and 10 mg/kg/d groups also had dose increases. Doses were interrupted at least once in 36 (65.5%), 42 (76.4%), and 36 (64.3%) patients in the deferasirox 5 mg/kg/d, deferasirox 10 mg/kg/d, and placebo groups, respectively. Dose was reduced in 12 (21.8%), 11 (20.0%), and 8 (14.3%) patients in the deferasirox 5 mg/kg/d, deferasirox 10 mg/kg/d, and placebo groups, respectively. Dose reductions were due to AEs in 12 of 12 patients treated with deferasirox 5 mg/kg/d, in 9 of 11 patients treated with deferasirox 10 mg/kg/d, and in 7 of 8 placebo patients. Other reasons for dose reductions were dosing error, increased serum creatinine, and low LIC (n = 1 for each) in the deferasirox 10 mg/kg/d group and laboratory test abnormality (n = 1) in the placebo group. Medication compliance was high, with 94.8% of deferasirox-treated patients and 95.7% of placebo-treated patients taking the planned study dose.
Twenty-one patients required at least one blood transfusion during the study, including 7 (12.7%) patients receiving deferasirox 5 mg/kg/d, 8 (14.5%) patients receiving deferasirox 10 mg/kg/d, and 6 (10.7%) patients receiving placebo. Of these 21 patients, 10 received 1 transfusion, 7 received 2 transfusions, 2 received 3 transfusions, and 2 received 4 transfusions.
Effect of deferasirox on LIC
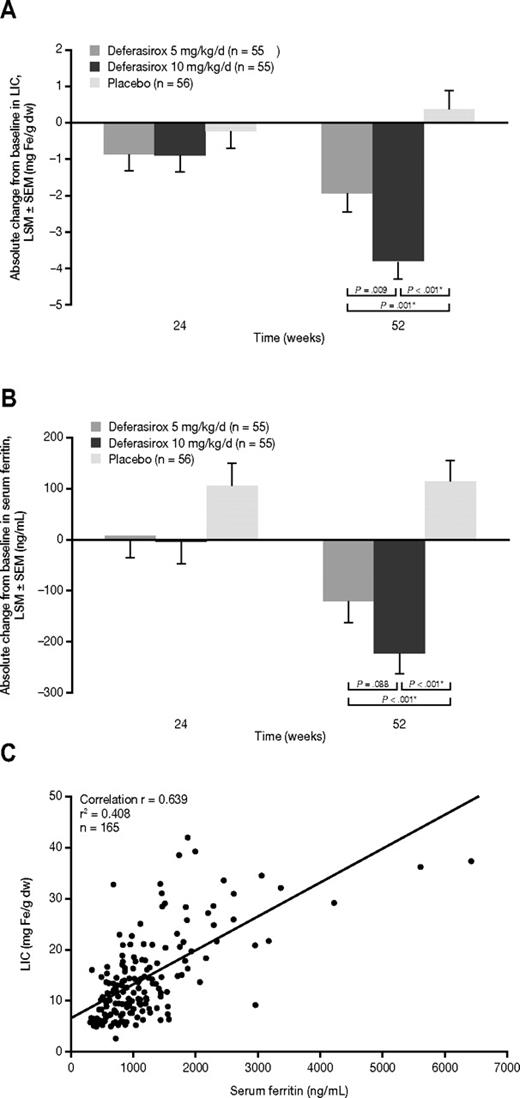
LIC decreased significantly from baseline to week 52 by an LSM ± SEM of −1.95 ± 0.50 mg Fe/g dw (95% CI, −2.94 to −0.96) in the deferasirox 5 mg/kg/d starting dose cohort and by −3.80 ± 0.48 mg Fe/g dw (95% CI, −4.76 to −2.85) in the deferasirox 10 mg/kg/d starting dose cohort (Figure 2A). There was a significantly greater decrease in LSM ± SEM LIC of −1.85 ± 0.70 mg Fe/g dw in the deferasirox 10 mg/kg/d versus 5 mg/kg/d cohort (95% CI, −3.22 to −0.48; P = .009). Decreases in LIC were significantly greater with deferasirox compared with placebo in the deferasirox 5 mg/kg/d (−2.33 ± 0.70 mg Fe/g dw; P = .001) and deferasirox 10 mg/kg/d cohorts (−4.18 ± 0.69 mg Fe/g dw; P < .001). In placebo patients, LIC increased from baseline by 0.38 ± 0.49 mg Fe/g dw (95% CI, −0.59 to 1.34). Reduction in LIC with both doses of deferasirox was observed from week 24 (Figure 2A and Table 2). Mean ± SD LIC at week 24 in patients receiving dose increases in the deferasirox 5 mg/kg/d and 10 mg/kg/d starting dose cohorts was 16.2 ± 8.9 and 18.0 ± 9.6 mg Fe/g dw, respectively. Mean ± SD actual deferasirox dose over the 52-week treatment period in patients receiving dose increases was 6.8 ± 1.0 and 14.1 ± 2.0 mg/kg/d, respectively. There was a trend for greater reduction in LIC in patients with higher baseline LIC. A decrease in LIC of ≥ 3 mg Fe/g dw at last assessment was observed in 18 (32.7%) patients in the deferasirox 5 mg/kg/d cohort, 31 (56.4%) patients in the 10 mg/kg/d cohort, and 6 (10.7%) patients in the placebo group. A ≥ 30% reduction in LIC at last assessment was observed in 14 (25.5%) patients in the deferasirox 5 mg/kg/d group, 27 (49.1%) patients in the deferasirox 10 mg/kg/d group, and 1 (1.8%) patient in the placebo group (Table 2). A shift table for LIC category change is presented in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Overall, a greater proportion of patients moved to a lower iron burden range by week 52 in the deferasirox 10 mg/kg/d group (n = 26; 47.3%) compared with the deferasirox 5 mg/kg/d group (n = 20; 36.4%) or the placebo group (n = 7; 12.5%). Of these patients, 13 (23.6%) achieved LIC < 5 mg Fe/g dw in the deferasirox 10 mg/kg/d group compared with only 8 (14.5%) in the 5 mg/kg/d group and 2 (3.6%) in the placebo group.
LIC and serum ferritin measurements. (A) Absolute change ± SEM in LIC over time. (B) Absolute change ± SEM in serum ferritin over time. (C) Relationship between LIC and serum ferritin at baseline. *P value adjusted with the Dunnett method. For serum ferritin, the quarterly average was used and the last available quarter was carried forward.
LIC and serum ferritin measurements. (A) Absolute change ± SEM in LIC over time. (B) Absolute change ± SEM in serum ferritin over time. (C) Relationship between LIC and serum ferritin at baseline. *P value adjusted with the Dunnett method. For serum ferritin, the quarterly average was used and the last available quarter was carried forward.
LIC and serum ferritin responses over 1 year with deferasirox versus placebo
| . | Deferasirox 5 mg/kg/d (n = 55) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 5 and 10 mg/kg/d (n = 56) . |
|---|---|---|---|
| LSM change from baseline in LIC, mg Fe/g dw ± SEM (95% CI)* | |||
| 24 wks | −0.87 ± 0.45 (−1.76 to 0.01) | −0.90 ± 0.45 (−1.79 to −0.01) | −0.24 ± 0.44 (−1.10 to 0.63) |
| 52 wks | −1.95 ± 0.50 (−2.94 to −0.96) | −3.80 ± 0.48 (−4.76 to −2.85) | 0.38 ± 0.49 (−0.59 to 1.34) |
| Median change from baseline in serum ferritin, ng/mL (min, max) | |||
| 12 wks | 33 (−531, 688) | −9 (−290, 759) | 27 (−296, 1250) |
| 24 wks | −21 (−520, 1056) | −64 (−833, 1562) | 33 (−620, 1415) |
| 36 wks | −54 (−671, 592) | −103 (−1041, 883) | 87 (−233, 1050) |
| 52 wks | −102 (−839, 376) | −202 (−1407, 988) | 81 (−344, 835) |
| Decrease of ≥ 3 mg Fe/g dw between baseline and wk 52 (LOCF), n (%) | 18 (32.7) | 31 (56.4) | 6 (10.7) |
| Comparison with placebo† | |||
| Estimated odds ratio‡ | 6.7 | 16.3 | |
| 95% CI | 2.1 to 21.1 | 5.2 to 50.8 | |
| Decrease of ≥ 30% in LIC between baseline and wk 52 (LOCF), n (%) | 14 (25.5) | 27 (49.1) | 1 (1.8) |
| . | Deferasirox 5 mg/kg/d (n = 55) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 5 and 10 mg/kg/d (n = 56) . |
|---|---|---|---|
| LSM change from baseline in LIC, mg Fe/g dw ± SEM (95% CI)* | |||
| 24 wks | −0.87 ± 0.45 (−1.76 to 0.01) | −0.90 ± 0.45 (−1.79 to −0.01) | −0.24 ± 0.44 (−1.10 to 0.63) |
| 52 wks | −1.95 ± 0.50 (−2.94 to −0.96) | −3.80 ± 0.48 (−4.76 to −2.85) | 0.38 ± 0.49 (−0.59 to 1.34) |
| Median change from baseline in serum ferritin, ng/mL (min, max) | |||
| 12 wks | 33 (−531, 688) | −9 (−290, 759) | 27 (−296, 1250) |
| 24 wks | −21 (−520, 1056) | −64 (−833, 1562) | 33 (−620, 1415) |
| 36 wks | −54 (−671, 592) | −103 (−1041, 883) | 87 (−233, 1050) |
| 52 wks | −102 (−839, 376) | −202 (−1407, 988) | 81 (−344, 835) |
| Decrease of ≥ 3 mg Fe/g dw between baseline and wk 52 (LOCF), n (%) | 18 (32.7) | 31 (56.4) | 6 (10.7) |
| Comparison with placebo† | |||
| Estimated odds ratio‡ | 6.7 | 16.3 | |
| 95% CI | 2.1 to 21.1 | 5.2 to 50.8 | |
| Decrease of ≥ 30% in LIC between baseline and wk 52 (LOCF), n (%) | 14 (25.5) | 27 (49.1) | 1 (1.8) |
LOCF indicates last observation carried forward.
Derived from an ANCOVA model with treatment as factor and baseline LIC as covariate.
Estimates were obtained from a logistical regression model with treatment regimen and baseline LIC as explanatory variables.
Ratios greater than 1 favor deferasirox.
Effect of deferasirox on serum ferritin
At week 52, serum ferritin decreased significantly from baseline by an LSM of −121 ng/mL (95% CI, −203 to −38; median, −99 ng/mL) in the deferasirox 5 mg/kg/d group and by −222 ng/mL (95% CI, −304 to −140; median, −190 ng/mL) in the deferasirox 10 mg/kg/d group (Figure 2B). Serum ferritin levels increased by 115 ng/mL (median, 78 ng/mL) in the placebo group. Decreases in serum ferritin were significantly greater with deferasirox compared with placebo: −235 ng/mL (P < .001) and −337 ng/mL (P < .001) in the deferasirox 5 mg/kg/d and 10 mg/kg/d groups, respectively. At week 24, serum ferritin levels were reduced from baseline for both deferasirox cohorts (Figure 2B and Table 2). The correlation (R) between baseline LIC and serum ferritin was 0.639 (Figure 2C).
Safety
Overall AEs were reported in 130 (78.3%) patients, including 42 (76.4%) receiving deferasirox 5 mg/kg/d, 43 (78.2%) receiving deferasirox 10 mg/kg/d, and 45 (80.4%) receiving placebo. Investigator-assessed drug-related AEs were reported in 40 (24.1%) patients, most of which were of mild to moderate severity and resolved without discontinuing treatment. The most common drug-related AEs were nausea, rash, and diarrhea (Table 3).
Most common (≥ 3 patients in total) investigator-assessed drug-related AEs
| AE, n (%) . | Deferasirox 5 mg/kg/d (n = 55) . | Placebo 5 mg/kg/d (n = 28) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 10 mg/kg/d (n = 28) . | Total (n = 166) . |
|---|---|---|---|---|---|
| Nausea | 3 (5.5) | 1 (3.6) | 4 (7.3) | 3 (10.7) | 11 (6.6) |
| Skin rash | 2 (3.6) | 0 | 5 (9.1) | 1 (3.6) | 8 (4.8) |
| Diarrhea | 0 | 0 | 5 (9.1) | 1 (3.6) | 6 (3.6) |
| Headache | 2 (3.6) | 0 | 1 (1.8) | 2 (7.1) | 5 (3.0) |
| Upper abdominal pain | 2 (3.6) | 0 | 1 (1.8) | 0 | 3 (1.8) |
| Abdominal pain | 1 (1.8) | 1 (3.6) | 1 (1.8) | 0 | 3 (1.8) |
| AE, n (%) . | Deferasirox 5 mg/kg/d (n = 55) . | Placebo 5 mg/kg/d (n = 28) . | Deferasirox 10 mg/kg/d (n = 55) . | Placebo 10 mg/kg/d (n = 28) . | Total (n = 166) . |
|---|---|---|---|---|---|
| Nausea | 3 (5.5) | 1 (3.6) | 4 (7.3) | 3 (10.7) | 11 (6.6) |
| Skin rash | 2 (3.6) | 0 | 5 (9.1) | 1 (3.6) | 8 (4.8) |
| Diarrhea | 0 | 0 | 5 (9.1) | 1 (3.6) | 6 (3.6) |
| Headache | 2 (3.6) | 0 | 1 (1.8) | 2 (7.1) | 5 (3.0) |
| Upper abdominal pain | 2 (3.6) | 0 | 1 (1.8) | 0 | 3 (1.8) |
| Abdominal pain | 1 (1.8) | 1 (3.6) | 1 (1.8) | 0 | 3 (1.8) |
Six investigator-assessed drug-related SAEs were reported in 4 patients receiving deferasirox: abdominal pain, pyrexia, hepatotoxicity (not confirmed by a central laboratory), cellulitis, pruritus, and rash (n = 1 each). No drug-related SAEs were reported with placebo. No deaths occurred during the study in any group. Eight patients experienced AEs resulting in study discontinuation, including 3 patients in the deferasirox 5 mg/kg/d cohort (ie, fractured pelvis, anemia, and increased urine protein level); 3 patients in the deferasirox 10 mg/kg/d cohort (ie, pregnancy [n = 2], rash, and pruritus [n = 1]), and 2 patients in the placebo 10 mg/kg/d (ie, optic neuritis and severe anemia).
A cataract was recorded in a male patient (age, 46 years) randomized to deferasirox 5 mg/kg/d. This patient entered the study with a cataract and therefore it was unlikely to have been related to the study drug. One incidence of neurosensory deafness was reported in a patient receiving placebo 10 mg/kg/d and proteinuria was reported in 1 patient receiving deferasirox 5 mg/kg/d. The overall number of AEs and SAEs reported before and after dose increases was comparable within each treatment group.
Three (5.5%) patients in the deferasirox 10 mg/kg/d cohort had 2 consecutive serum creatinine increases of > 33% above baseline and above ULN, which occurred in the second half of the study. Serum creatinine returned to normal without dose interruption or reduction in one patient receiving 20 mg/kg/d when the event occurred, who achieved LIC of 1.3 mg Fe/g dw at the end of the study. In the remaining 2 patients, serum creatinine returned to normal after drug interruption (1 patient received 20 mg/kg/d and 1 received 10 mg/kg/d); both patients achieved reduction in LIC and serum ferritin levels. Of these 3 patients, 1 also experienced 2 consecutive creatinine clearances < 60 mL/min during the study; 1 additional patient receiving deferasirox 5 mg/kg/d did also.
No patients receiving deferasirox demonstrated an increase in ALT > 5× ULN and > 2× baseline, although there was one incidence in a patient receiving placebo 10 mg/kg/d. There were no progressive changes in mean serum creatinine, creatinine clearance, ALT, or the urinary protein/creatinine ratio (supplemental Figure 1).
Discussion
This is the first randomized, placebo-controlled trial evaluating iron chelation in NTDT patients with iron overload due mainly to increased intestinal iron absorption. These data provide the largest set of efficacy and safety data for iron-chelation therapy in NTDT patients to date, confirming preliminary findings in small studies and case reports.4,8-14 Despite no or only sporadic transfusions in these NTDT patients, high baseline LIC and serum ferritin levels confirm significant iron burden requiring iron chelation. In patients receiving placebo treatment, LIC and serum ferritin increased by 0.38 mg Fe/g dw (95% CI, −0.59 to 1.34) and 115 ng/mL, respectively, over 1 year; only 6 (10.7%) patients required transfusions in this group during this time. This is equivalent to a body iron increase of 0.011 mg/kg/d based on the formula published by Angelucci et al.23
Compared with placebo, deferasirox at starting doses of 5 and 10 mg/kg/d, with dose escalations up to 20 mg/kg/d in patients with high levels of iron overload, reduced LIC and serum ferritin levels significantly. Experience with deferoxamine has demonstrated that to minimize drug toxicity, lower doses should be used when body iron levels are low.24 This principle was built into the design of our study: considerably lower doses were used compared with those used in transfusion-dependent β-thalassemia patients (20-40 mg/kg/d). The use of 20 mg/kg/d in the present study was limited and further data will be obtained from the extension study. Reductions in LIC and serum ferritin were greater in patients receiving starting doses of deferasirox 10 versus 5 mg/kg/d. A LIC decrease ≥ 3 mg Fe/g dw was one of the success criteria used in a previous phase 3 study of deferasirox versus deferoxamine6 and a 30% decrease over 1 year was agreed to be clinically relevant by the study steering committee. After 1 year, over half (56.4%) of patients receiving deferasirox 10 mg/kg/d and almost a third (32.7%) of patients receiving deferasirox 5 mg/kg/d achieved reductions in LIC of ≥ 3 mg Fe/g dw. The apparent response observed in a small number of placebo patients may be attributed to variability in MRI assessments.21 A greater proportion of patients achieved LIC < 7, LIC < 5, and LIC < 3 mg Fe/g dw in the deferasirox 10 mg/kg/d cohort compared with the 5 mg/kg/d cohort or placebo. Because patients started with significantly elevated LIC, it is to be expected that more patients would achieve therapeutic targets with continued treatment in the extension. Dose was increased in 46.4% of deferasirox-treated patients after 24 weeks, highlighting the importance of dose adjustments for achieving therapeutic goals.
Despite increased knowledge of iron metabolism in NTDT patients25 and the known association between LIC and morbidity,2 clinical guidelines on managing iron overload are lacking. For patients with β-thalassemia intermedia, it has been suggested that iron chelation should be initiated if LIC exceeds 7 mg Fe/g dw.26-30 However, recent data in patients with β-thalassemia intermedia indicated that LIC ≥ 7 and ≥ 6 mg Fe/g dw are associated with an increased risk of vascular and endocrine/bone morbidity, respectively.2 Our present data indicate that chelation in NTDT patients may be initiated earlier, when LIC exceeds 5 mg Fe/g dw, without evidence of tolerability issues, with a treatment goal of preventing the accumulation of iron to toxic levels.
The present study is the first to report the safety of deferasirox compared with placebo. AEs were manageable, even when some patients achieved LIC < 5 mg Fe/g dw toward the end of study, with overall AE incidence comparable between deferasirox and placebo. The incidence of ALT abnormalities was low and renal abnormalities were reversible, which is consistent with previous experience with deferasirox. Investigator-assessed drug-related AEs were mainly gastrointestinal, as has been demonstrated in deferasirox-treated patients with transfusion-dependent anemias15 ; however, the incidence was similar in the deferasirox-treated and placebo-treated groups. Although the majority of dose reductions were the result of AEs, the incidence of drug-related AEs was approximately half of that reported over 1 year in patients with transfusion-dependent anemias.15 Interestingly, the incidences of nausea and headache that were attributed to study drug by the investigators were higher in the placebo 10 mg/kg/d group compared with the placebo 5 mg/kg/d group, perhaps suggesting an effect of excipients in the formulation. These differences warrant further investigation.
Approximately one-third of screened patients were excluded from the present study because they did not meet the iron overload inclusion criteria (LIC at baseline ≥ 5 mg Fe/g dw). These criteria were agreed upon by the steering committee to take into account the risks of overchelation and safety issues observed in transfusion-dependent β-thalassemia patients. Our results show that NTDT patients may be chelated at starting doses of 5 and 10 mg/kg/d to lower LIC without increasing the risk of renal problems.
In conclusion, many NTDT patients may have high iron burdens that require iron-chelation therapy. In the present study, deferasirox at starting doses of 5 and 10 mg/kg/d, with dose escalations up to 20 mg/kg/d in patients with higher levels of iron overload, significantly reduced iron overload in NTDT patients compared with placebo and had a similar frequency of overall AEs.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rebecca Helson, PhD, for medical editoral assistance. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. The authors acknowledge Wei Deng (Novartis Pharmaceuticals) for statistical support.
This study was funded by Novartis Pharma AG.
Authorship
Contribution: A.T.T., J.P., V.V., A.K., S.C., P.S., N.S., R.G., Z.K., and M.D.C. served as the investigators on this trial and enrolled patients, contributed to data interpretation, and reviewed and provided comments on the manuscript; A.T.T., J.P., V.V., A.K., and M.D.C. served as study steering committee members and oversaw the conduct of the trial from study design to analysis plan and data interpretation; T.L., J.R., and D.H. assisted in developing the trial protocol, coordinated the execution of the trial, and contributed to the analysis, interpretation, and reporting of the trial data; Y.Z. served as the trial statistician; and all authors approved the final manuscript.
Conflict-of-interest disclosure: A.T.T. has received research funding and honoraria from Novartis Pharmaceuticals. J.P. has received research funding from and serves on the advisory committee and speakers' bureau for Novartis Pharmaceuticals. V.V. has received research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPO-L-ONE, Thailand (FerroKin Biosciences and National Research University, Thailand). A.K. has received honoraria and research funding from and participates in the speakers' bureau for Novartis Pharmaceuticals. S.C., P.S., and N.S. have received research funding from Novartis Pharmaceuticals. R.G. and Z.K. have received research grants and speakers' honoraria from Novartis Pharmaceuticals. T.L., J.R., Y.Z., and D.H. are full-time employees of Novartis Pharmaceuticals. M.D.C. has participated in the speakers' bureau for Novartis Pharmaceuticals.
For a full list of participating investigators and their affiliations, please see the supplemental Appendix.
Correspondence: Ali T. Taher, American University of Beirut Medical Center, Department of Internal Medicine, Beirut, Lebanon; e-mail: ataher@aub.edu.lb.