Abstract
The paradigm for managing primary immune thrombocytopenia (ITP) in adults has changed with the advent of rituximab and thrombopoietin receptor agonists (TPO-RAs) as options for second-line therapy. Splenectomy continues to provide the highest cure rate (60%-70% at 5+ years). Nonetheless, splenectomy is invasive, irreversible, associated with postoperative complications, and its outcome is currently unpredictable, leading some physicians and patients toward postponement and use of alternative approaches. An important predicament is the lack of studies comparing second-line options to splenectomy and to each other. Furthermore, some adults will improve spontaneously within 1-2 years. Rituximab has been given to more than 1 million patients worldwide, is generally well tolerated, and its short-term toxicity is acceptable. In adults with ITP, 40% of patients are complete responders at one year and 20% remain responders at 3-5 years. Newer approaches to using rituximab are under study. TPO-RAs induce platelet counts > 50 000/μL in 60%-90% of adults with ITP, are well-tolerated, and show relatively little short-term toxicity. The fraction of TPO-RA–treated patients who will be treatment-free after 12-24 months of therapy is unknown but likely to be low. As each approach has advantages and disadvantages, treatment needs to be individualized, and patient participation in decision-making is paramount.
Introduction
Consult: A 34-year-old patient presents with a platelet count of 10 000/μL accompanied by petechiae, ecchymoses, and epistaxis. Workup leads to the diagnosis of immune thrombocytopenia (ITP). Treatment with prednisone is initiated with good clinical and platelet response, and the drug is reasonably well tolerated. However, the platelet count falls and bleeding symptoms recur when the dose is tapered below 10 mg daily. Prednisone is reinitiated at a high dose and tapered more slowly, but the platelet count again falls to < 20 000/μL when the dose reaches 10 mg daily. It is now 5 months since diagnosis. What is the appropriate therapy? Specifically, is splenectomy indicated or should it be reserved and other treatments tried first?
Splenectomy has been the standard second-line treatment for adults with ITP for decades and remains the option that provides the highest cure rate. Careful choice of patients, widespread adoption of a laparoscopic approach, perioperative thromboprophylaxis, and better approaches to prevent and mitigate sepsis have helped reduce morbidity, costs,1 and possibly mortality.2 Concurrently, important advances have been made in understanding the pathogenesis of ITP accompanied by development of novel treatments, including anti-CD20 antibodies3 and thrombopoietin receptor agonists (TPO-RAs).4,5 This has led to increased uncertainty as to when splenectomy is advisable as the standard next step. It has also become clear that some adults improve over time either spontaneously or as a result of treatment, leading some to recommend deferring surgery for one to several years.6 Moreover, increased clinical awareness and use of laboratory testing now identify concomitant illnesses in patients who otherwise meet diagnostic criteria for ITP (“cryptic” secondary ITP), including some (eg, hepatitis C and common variable immune deficiency [CVID]) that mitigate against splenectomy.
It is our distinct impression that information of variable validity widely available on the Internet has increased the number of patients who wish to avoid splenectomy. The introduction of new therapies and increased awareness of late remissions in ITP have resulted in physicians tending to avoid or defer splenectomy, which is increasingly viewed as the last resort, particularly in the United States and some European countries. This is evidenced by a decrease in the rate of splenectomy for ITP from 50%-60% in previously reported cohorts to 20%-25% more recently.7
Two recent reviews of treatment for ITP have been published.8,9 The International Consensus document used both published studies and the accumulated experience of 22 international experts, somewhat akin to the process used to develop the initial ASH guidelines, although with less formal integration of expert opinion (Table 1).8 In contrast, the revised ASH guidelines focused on evidence-based medicine, with half of the writing group having expertise in trial methodology and data analysis, and the remainder in ITP.9 Both groups considered splenectomy, and both recommended it as a second-line therapy. However, the International Consensus gave it equal place among a number of options, whereas the ASH guidelines gave splenectomy its highest grade of recommendation based on its curative effects and the extensive published experience. Additional guidelines that consider the role of splenectomy in second-line treatment are summarized in Table 1.
Comparison between various national and international guidelines in regard to splenectomy in the treatment of ITP
| Guidelines . | Year . | Language . | Publication site . | Timing, indication, and restrictions for splenectomy . | |||
|---|---|---|---|---|---|---|---|
| Timing . | Age, y . | Stage . | Indication for splenectomy . | ||||
| ASH | 2011 | English | Blood9 | Deferred > 6 mo | NG | Second-line therapy after steroids; grade of recommendation IB | |
| International consensus | 2010 | English | Blood8 | Deferred > 6-12 mo | NG | One of the second-line therapies without preference | |
| French | 2009 | French | www.has-sante.fr | Deferred > 12 mo | NG | Second-line after steroids, but TPO-mimetics or rituximab should be considered in some patients | Platelet count < 30 000/μL associated with bleeding |
| Norwegian | 2011 | Norwegian | www.legeforeningen.no/hematologi | Deferred > 6-12 mo | NG | Second-line after failure of steroid and/or rituximab | |
| German | 2010 | German | Onkologie88 | Deferred > 12 mo | NG | Second-line after steroids but TPO-mimetics or rituximab should be considered in some patients | Platelet count < 30 000/μL associated with bleeding |
| British | 2003 | English | British Journal of Haematology89 | NG | 65? | Second-line after failure of steroids | |
| Swedish | 2010 | Swedish | www.sfhem.se | NG | NG | Second-line after failure of steroid in the absence of contraindication | Platelet count < 10 or < 30 000/μL and presence of bleeding or high steroid requirement |
| Guidelines . | Year . | Language . | Publication site . | Timing, indication, and restrictions for splenectomy . | |||
|---|---|---|---|---|---|---|---|
| Timing . | Age, y . | Stage . | Indication for splenectomy . | ||||
| ASH | 2011 | English | Blood9 | Deferred > 6 mo | NG | Second-line therapy after steroids; grade of recommendation IB | |
| International consensus | 2010 | English | Blood8 | Deferred > 6-12 mo | NG | One of the second-line therapies without preference | |
| French | 2009 | French | www.has-sante.fr | Deferred > 12 mo | NG | Second-line after steroids, but TPO-mimetics or rituximab should be considered in some patients | Platelet count < 30 000/μL associated with bleeding |
| Norwegian | 2011 | Norwegian | www.legeforeningen.no/hematologi | Deferred > 6-12 mo | NG | Second-line after failure of steroid and/or rituximab | |
| German | 2010 | German | Onkologie88 | Deferred > 12 mo | NG | Second-line after steroids but TPO-mimetics or rituximab should be considered in some patients | Platelet count < 30 000/μL associated with bleeding |
| British | 2003 | English | British Journal of Haematology89 | NG | 65? | Second-line after failure of steroids | |
| Swedish | 2010 | Swedish | www.sfhem.se | NG | NG | Second-line after failure of steroid in the absence of contraindication | Platelet count < 10 or < 30 000/μL and presence of bleeding or high steroid requirement |
NG indicates not given.
This article on “How I treat ITP” investigates the question: What is the optimal second-line treatment for adults with ITP? Splenectomy is considered in the context of the 2 most commonly used, newer approaches to second-line therapy: rituximab and TPO-RAs. Because no comparative evidence-based recommendations can be made, our focus is to consider and balance the potential benefits and relative contraindications of each option to help inform individualized care.
What is ITP?
ITP is an autoimmune disease characterized by isolated thrombocytopenia (platelet count < 100 000/μL) resulting from accelerated clearance and destruction of antibody-coated platelets by tissue macrophages, predominantly in the spleen.10 Antiplatelet antibodies also target antigens on megakaryocytes and proplatelets, variably suppressing platelet production.11,12 Plasma TPO is generally normal or only minimally elevated, primarily because of accelerated clearance via megakaryocytes and platelets.13 Activated cytotoxic CD8+ T cells may contribute to thrombocytopenia in certain patients.14 A potential underlying etiology giving rise to secondary ITP, such as infection with hepatitis C, HIV, or Helicobacter pylori, and coexistence of systemic lupus erythematosus, antiphospholipid syndrome, or common variable immunodeficiency (hypogammaglobulinemia, CVID), is identified in approximately 20% of patients with immune thrombocytopenia.15 Discovering an underlying cause is important because it may impact the efficacy and safety of splenectomy and other approaches (as discussed in the following sections).16,17 Moreover, patients, such as in the case example, are often not thoroughly evaluated for secondary ITP, and an underlying etiology may not be apparent on presentation.
Who should be treated?
Treatment is generally confined to patients who are bleeding or are perceived to be at significant risk of bleeding. The risk of bleeding is multifactorial, and its assessment is complex. For the most part, bleeding is related to a decreased platelet count, increasing age, comorbidities (including their treatments), risk of trauma, and previous history of bleeding.18 Although a platelet count of < 30 000/μL is often used as a surrogate marker,4,5,9,19 lower (10-20 000/μL) or higher (50 000/μL) thresholds are pursued depending on the risk of bleeding, the presence of comorbidities, patient's lifestyle, and risk of trauma, all of which need to be weighed against the probable benefits and the risk of treatment-related side effects.20 Some therapies may improve health-related quality of life, especially fatigue, although this issue is infrequently considered or invoked as a reason to treat.21,22 Comparative outcome studies are lacking here as well.
When should splenectomy be considered among second-line therapies?
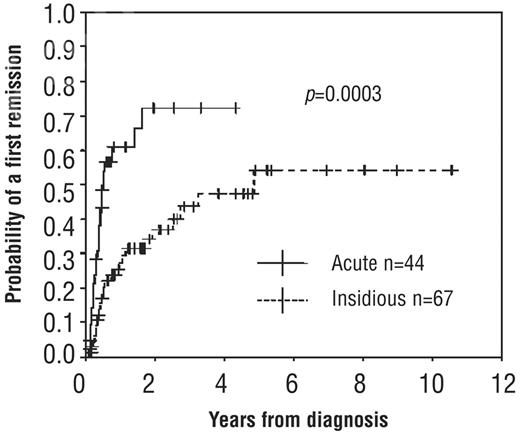
The frequency of complete remission (CR)23 after a course of first-line therapy with corticosteroids ranges from 10%-30% with daily oral prednisone24 to as much as 60%-80% with high-dose, pulsed dexamethasone (HDD).6,25,26 However, the latter remains to be confirmed in controlled trials, and currently available evidence does not establish the superiority of HDD.27 In the absence of such evidence, we do not routinely use multiple cycles of HDD to induce remission in patients who have failed a course of prednisone. Patients may develop hemostatic platelet counts by one year (Figure 1)6,28 or occasionally after many years of severe disease without additional treatment.29,30 Accordingly, the International Consensus statement suggests deferring splenectomy until the chronic phase (> 12 months), if possible, unless an adequate count cannot be maintained with medical therapy, adverse reactions to medical alternatives develop, or there is compelling patient preference (eg, because of lifestyle or employment).8
Probability of first CR according to the type of onset (insidious or acute). Kaplan-Meier curves show that remissions continued to occur with or without treatment with low-dose steroids between 6 months and 3 years and that remissions occurred earlier and at higher rates in patients with an acute onset of symptoms as opposed to those with an insidious onset. Splenectomized patients were censored at the time of splenectomy. © Ferrata Storti Foundation, Italy. Obtained and reprinted from Sailer et al6 with permission.
Probability of first CR according to the type of onset (insidious or acute). Kaplan-Meier curves show that remissions continued to occur with or without treatment with low-dose steroids between 6 months and 3 years and that remissions occurred earlier and at higher rates in patients with an acute onset of symptoms as opposed to those with an insidious onset. Splenectomized patients were censored at the time of splenectomy. © Ferrata Storti Foundation, Italy. Obtained and reprinted from Sailer et al6 with permission.
The management of patients who fail corticosteroids is challenging, as there have been no comparative trials of treatment options in this setting. The International Consensus report lists more than 10 second-line therapeutic options, including splenectomy, without indicating a preference.8 The revised ASH guidelines recommend splenectomy (grade 1B evidence) for patients who failed corticosteroid therapy while suggesting treatment with TPO-RAs and rituximab presplenectomy (grade 2C evidence).9 What follows is our analysis of the factors to be considered when choosing among the 3 most common options (Table 2; Figure 2). We recognize that other approaches, including watchful waiting, may be appropriate in many patients. In addition, other treatments widely used in some countries, such as dapsone and immunosuppressive agents,8 are not commonly used as second-line treatments in our practice.
Comparison between splenectomy, thrombopoietic agents, and rituximab
| Therapy . | Efficacy and response prediction . | Safety . | Contraindications . | Mode of application and follow-up . | Grade of ASH 2011 guidelines recommendation . |
|---|---|---|---|---|---|
| Splenectomy | Highest cure rate; short-term response 80% and long-term response 60%-70% at 5-10 y Response hard to predict | Surgery-related mortality and morbidity (bleeding, infections, thrombosis); lifetime risk of overwhelming infection | Patients with comorbid conditions that increase the risk of complications | Invasive procedure usually performed laparoscopically requires preoperative and postoperative preparation and care and regular vaccination | Well-established treatment for ITP |
| Possible AE: venous thrombosis, pulmonary hypertension, atherosclerosis, dementia | Relative: elderly patients over 60-70 because high rate of complication and lower response; patients with immunodeficiency and secondary ITP eg, CVID, hepatitis C, neutropenia, possibly SLE | ASH: 1B after failure of steroids | |||
| TPO-RA | |||||
| Romiplostim | A maintenance treatment; 60%-80% achieve platelet elevation; sustained response in 70%-90% in those entering long-term treatment studies | Headache, rebound thrombocytopenia, weekly injection | Pregnancy and lactation, MDS | Weekly subcutaneous injections; requires dose adjustment and regular CBC | Approved treatment for ITP |
| Possible AE: bone marrow reticulin fibrosis, arterial and venous thrombosis, risk of malignancy (if MDS) | Relative: past history of venous or arterial thrombosis | ASH: 2C after failure of steroids before splenectomy | |||
| Eltrombopag | A maintenance treatment; 60%-80% achieve platelet elevation; sustained response in 70%-90% in those entering long-term treatment studies | Headache, rebound thrombocytopenia, elevated liver enzymes | As with romiplostim | Daily ingestions; requires dose adjustment and regular CBC and liver tests | ASH: 2C after failure of steroids before splenectomy |
| Possible AE: bone marrow reticulin fibrosis, arterial and venous thrombosis; nausea, vomiting in small percent; cataracts (very infrequent if at all) | Requires monitoring of liver tests but used successfully in large studies of patients with liver disease secondary to hepatitis C | ||||
| Anti-CD20 | |||||
| Rituximab | May be curative treatment; initial response in 50%-60%; sustained response 3-5 y in 20%; retreatment gives the same pattern of response as observed after the first course in complete responders cannot predict response | Infusion-related side effects (chills, fever, dyspnea), neutropenia, | Active hepatitis B virus, known clinically significant allergy, including past serum sickness with anti-CD20, or antimouse antibody | Weekly intravenous infusions for 4 wks; CBC required depending on the response | Not approved for ITP, only off-label use |
| Possible AE: increased risk of infection and viral reactivation, hypogammaglobulinemia, serum sickness (especially in children), multifocal leukoencephalopathy (PML) | Pregnancy and lactation | ASH: 2C after failure of steroids |
| Therapy . | Efficacy and response prediction . | Safety . | Contraindications . | Mode of application and follow-up . | Grade of ASH 2011 guidelines recommendation . |
|---|---|---|---|---|---|
| Splenectomy | Highest cure rate; short-term response 80% and long-term response 60%-70% at 5-10 y Response hard to predict | Surgery-related mortality and morbidity (bleeding, infections, thrombosis); lifetime risk of overwhelming infection | Patients with comorbid conditions that increase the risk of complications | Invasive procedure usually performed laparoscopically requires preoperative and postoperative preparation and care and regular vaccination | Well-established treatment for ITP |
| Possible AE: venous thrombosis, pulmonary hypertension, atherosclerosis, dementia | Relative: elderly patients over 60-70 because high rate of complication and lower response; patients with immunodeficiency and secondary ITP eg, CVID, hepatitis C, neutropenia, possibly SLE | ASH: 1B after failure of steroids | |||
| TPO-RA | |||||
| Romiplostim | A maintenance treatment; 60%-80% achieve platelet elevation; sustained response in 70%-90% in those entering long-term treatment studies | Headache, rebound thrombocytopenia, weekly injection | Pregnancy and lactation, MDS | Weekly subcutaneous injections; requires dose adjustment and regular CBC | Approved treatment for ITP |
| Possible AE: bone marrow reticulin fibrosis, arterial and venous thrombosis, risk of malignancy (if MDS) | Relative: past history of venous or arterial thrombosis | ASH: 2C after failure of steroids before splenectomy | |||
| Eltrombopag | A maintenance treatment; 60%-80% achieve platelet elevation; sustained response in 70%-90% in those entering long-term treatment studies | Headache, rebound thrombocytopenia, elevated liver enzymes | As with romiplostim | Daily ingestions; requires dose adjustment and regular CBC and liver tests | ASH: 2C after failure of steroids before splenectomy |
| Possible AE: bone marrow reticulin fibrosis, arterial and venous thrombosis; nausea, vomiting in small percent; cataracts (very infrequent if at all) | Requires monitoring of liver tests but used successfully in large studies of patients with liver disease secondary to hepatitis C | ||||
| Anti-CD20 | |||||
| Rituximab | May be curative treatment; initial response in 50%-60%; sustained response 3-5 y in 20%; retreatment gives the same pattern of response as observed after the first course in complete responders cannot predict response | Infusion-related side effects (chills, fever, dyspnea), neutropenia, | Active hepatitis B virus, known clinically significant allergy, including past serum sickness with anti-CD20, or antimouse antibody | Weekly intravenous infusions for 4 wks; CBC required depending on the response | Not approved for ITP, only off-label use |
| Possible AE: increased risk of infection and viral reactivation, hypogammaglobulinemia, serum sickness (especially in children), multifocal leukoencephalopathy (PML) | Pregnancy and lactation | ASH: 2C after failure of steroids |
AE indicates adverse event; MDS, myelodysplastic syndrome; SLE, systemic lupus erythematosus; CBC, complete blood count; and PML, progressive multifocal leukoencephalopathy.
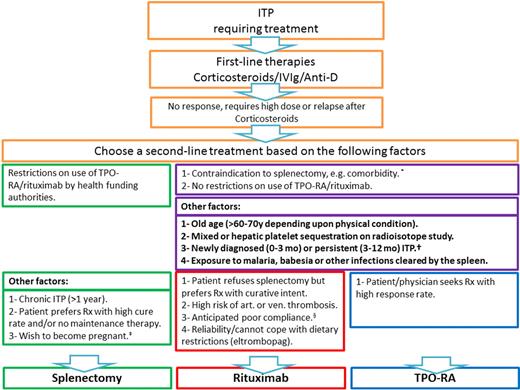
Suggested treatment algorithm for ITP. *These are overall factors that go for or against splenectomy without distinguishing between TPO-RA and rituximab. †Based on recommendations to defer splenectomy for 1 year, if possible. ‡Alternative option is rituximab, and wait for 12 months before conception. §Anticipated poor compliance is also applicable to splenectomy, although post-splenectomy management (eg, repeat vaccination, management of febrile illness, and follow-up regarding platelet count) would probably also be at risk.
Suggested treatment algorithm for ITP. *These are overall factors that go for or against splenectomy without distinguishing between TPO-RA and rituximab. †Based on recommendations to defer splenectomy for 1 year, if possible. ‡Alternative option is rituximab, and wait for 12 months before conception. §Anticipated poor compliance is also applicable to splenectomy, although post-splenectomy management (eg, repeat vaccination, management of febrile illness, and follow-up regarding platelet count) would probably also be at risk.
Splenectomy
Splenectomy has been used for decades as the primary option in patients who require additional treatment after a course of corticosteroids.
Pros
Splenectomy is “curative.”
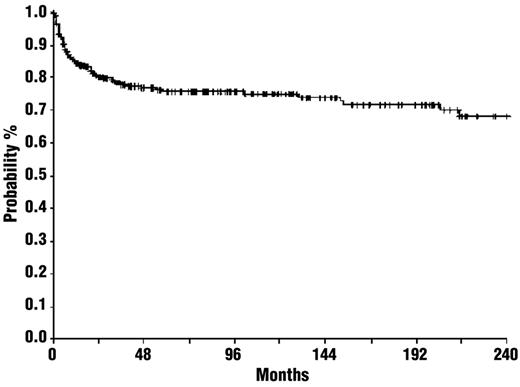
Splenectomy “cures” ITP by removing both the primary site of platelet destruction and an important site of antiplatelet antibody production in an uncertain but large proportion of patients. Platelet counts rise rapidly in 85% of patients. Relapses are encountered, especially in the first 2 years after surgery, but 60%-65% of patients remain in clinical remission 5-10 years after splenectomy, an outcome unmatched by any other therapy (Figure 3).2,31,32 A systematic review of 135 case-series published between 1966 and 2004 revealed a complete response rate of 66%, with a median duration of follow-up of 28 months (range, 1-153 months).2 A recent systematic review of 23 articles and 1223 patients after laparoscopic splenectomy recorded a success rate of 72% at 5 years.33 Responding patients require relatively little follow-up (eg, yearly platelet counts and repeated vaccinations), except in pregnant women who are at risk for having a fetus with thrombocytopenia.
Probability of thrombocytopenia-free survival after splenectomy. The estimated relapse-free survival for all patients during plateaus at 75% after 48 months. © Ferrata Storti Foundation, Italy. Obtained and reprinted from Vianelli et al31 with permission.
Probability of thrombocytopenia-free survival after splenectomy. The estimated relapse-free survival for all patients during plateaus at 75% after 48 months. © Ferrata Storti Foundation, Italy. Obtained and reprinted from Vianelli et al31 with permission.
Splenectomy failure does not jeopardize response to most medical treatments.
It has been thought for some time that some patients who fail splenectomy may be managed with lower doses of corticosteroids than were required before surgery.19 However, strong evidence is lacking. TPO-RAs and rituximab are equally effective in splenectomized and nonsplenectomized patients.34,35 The only therapies known to be less effective after splenectomy are intravenous anti-D immunoglobulin36 and probably dapsone.
Advantages of laparoscopic splenectomy.
Laparoscopic splenectomy37 is widely used as the preferred approach because it is less traumatic, engenders less postoperative pain, and is associated with fewer wound infections and other complications leading to shorter hospital stays, more rapid convalescence, and more rapid return to work, all of which contribute to lower costs.38,39 Cosmesis is improved compared with the open approach. Laparoscopy does not increase the frequency of missing accessory spleens.40 Consequently, laparoscopic splenectomy has been embraced as the “gold standard”1,41 but should only be performed by experienced operators.42 The conversion rate to open laparotomy ranges from 5%-15%.38,42 As with open splenectomy, it is important to elevate platelet counts preoperatively to > 20 000/μL to avoid longer hospital stay, blood transfusions, and other complications.43
Splenectomy has a well-characterized safety profile and generally preventable complications.
Perioperative complications of splenectomy include bleeding, infection, and thrombosis. Long-term complications include overwhelming sepsis by encapsulated bacteria and vascular/thrombotic events. Most complications are infrequent and either preventable or treatable (see “Splenectomy: Cons”). Surgical expertise combined with proper patient selection and preparation is key to reducing complication rates and involves: excluding patients with serious comorbidities for general surgery and “older” patients (age cut-offs for those in otherwise excellent health is debated), optimizing platelet counts perioperatively, and judicious use of antibiotics and thromboprophylaxis.43 We recommend pneumococcal, meningococcal, and Haemophilus influenzae vaccination before splenectomy and periodically every 5 years or according to titers.1,44
Splenectomy reduces cost.
Before TPO-RA, the annual cost of treating severe chronic ITP was estimated to be $40 000,45 although comprehensive cost-effectiveness studies are lacking in adults. However, the cost of TPO-RAs ranges from $2500-$4000 per month, and each 4-infusion course of rituximab costs $10 000-$50 000, whereas the estimated total procedural cost of splenectomy is not more than $20 000. These cost estimates apply only to patients who respond. The societal cost of failed splenectomy includes the cost of surgical complications and subsequent therapy.
Pregnancy.
Women with ITP who contemplate pregnancy may opt for splenectomy because most options for management of the maternal platelet count, including TPO-RA and rituximab, may not be safe for the fetus.46,47 However, women in remission after splenectomy may relapse during pregnancy, and the fetus/neonate may develop thrombocytopenia, even if the splenectomized mother remains in remission.48
Cons
Removal of a “healthy” organ.
Splenectomy is irreversible and consequently leads to the loss of its multiple hematologic and immunologic functions (eg, elimination of abnormal blood cells, cell particles, and organisms from the blood and production of antibodies to blood-borne antigens).49
Unpredictability of response.
The response to splenectomy cannot be predicted using readily available clinical criteria (eg, previous response to steroids or intravenous immunoglobulin), other than “older” age, which is ill-defined.2 In a retrospective review of 111In-labeled autologous platelet sequestration studies, the complete response rate after splenectomy was 87% (median, 3.8 years follow-up) in patients having predominantly splenic sequestration as opposed to 35% in those with “mixed” or hepatic sequestration (odds ratio = 5.39; 95% confidence interval [CI], 1.3-21.6).50 Confirmatory studies are needed. Major limitations include restricted availability and technical difficulty.51
Post-splenectomy mortality and morbidity.
Splenectomy is an invasive procedure associated with near-term complications primarily related to general anesthesia and surgery and long-term complications from loss of splenic functions.
Overall mortality.
The 30-day mortality and complication rates after laparoscopic splenectomy (0.2% and 9.6%) are reported to be lower than after open splenectomy (1% and 12.9%).2 A Danish cohort study compared 3812 patients splenectomized for various indications between 1996 and 2005, 8310 nonsplenectomized patients with matched underlying conditions, and 38 120 controls. Ninety-day mortality was elevated in the splenectomized cohort as a whole and in the subcohort with ITP (n = 269; adjusted relative risk [RR] = 33.6; 95% CI, 7.9-143) compared with the general population. However, for patients with ITP, the difference in mortality during the first year after splenectomy was not significant compared with the nonsplenectomized cohort. Interestingly, after one year, the RR of death in the splenectomized ITP patients was significantly lower than in the matched ITP controls treated otherwise (RR = 0.4; 95% CI, 0.2-0.7).52 However, the assessment period preceded the introduction of TPO-RAs and extensive use of rituximab; therefore, data in the nonsplenectomized ITP cohort were derived from an era when patients typically underwent prolonged exposure to high doses of corticosteroids and other immunosuppressives not widely used and/or no longer considered safe or appropriate in this setting.
Risk of infection.
Since 1952, it has been evident that asplenic subjects are at increased risk of life-threatening infections.53 The Danish cohort study identified a 14-fold higher RR of sepsis in splenectomized ITP patients (n = 269) during the first year after splenectomy and a 4-fold higher RR after 1 year compared with the general population. Importantly, compared with nonsplenectomized ITP patients, a higher rate of sepsis was observed only during the first 90 days.53 Interestingly, enteric organisms were the predominant cause of early and late postsplenectomy bacteremia; encapsulated bacteria, such as pneumococci, meningococci, and Haemophilus influenzae, were rarely encountered in the splenectomized cohort, perhaps a result of widespread vaccination and early intervention with antibiotics. No sepsis-related mortality was reported in 2 Italian studies that included 612 splenectomized ITP patients.31,54
Repeated patient education is vital because the rarity of sepsis predisposes to noncompliance. Med-alert bracelets may be helpful. Guidelines for preventing sepsis and/or improving the outcome, when it occurs, vary but may include: (1) repeat vaccination every 5-10 years or when antibody titers (especially to pneumococcus) fall; (2) measurement of body temperature at the onset of any illness and urgent emergency room evaluation for a fever of 101°F or higher with initiation of intravenous antibiotics, such as ceftriaxone (absent allergy); and (3) consideration of penicillin 250-500 mg twice a day for life; however, the incidence of pneumococcal penicillin resistance currently approaches 30%. Exposure to infections with known worse outcomes in splenectomized patients (eg, malaria and babesiosis) should be avoided if at all possible. Table 3 summarizes preoperative and postoperative recommendations to minimize post-splenectomy infectious complications.
Preoperative and postoperative measures to reduce and/or prevent complications after splenectomy
| Before or after splenectomy . | Measures . |
|---|---|
| Before splenectomy | |
| Patient education regarding risk of overwhelming sepsis | Early administration of oral antibiotic therapy that covers S pneumoniae and H influenzae in case of fever (amoxicillin-clavulanate, cefuroxime axetil, or levofloxacin) AND immediate travel to a hospital for assessment and intravenous antibiotics |
| Vaccination | Vaccination against S pneumoniae, meningococcus, and H influenzae type b, ideally at least 14 days before scheduled splenectomy |
| Elevation of platelet count | Elevation of platelets to > 50 × 109/L by steroids or IVIg or another treatment |
| After splenectomy | |
| Antibiotic prophylaxis | Postoperative antibiotics prophylaxis until the risk of infection is abated |
| Thromboprophylaxis | Early mobilization, good hydration, and early initiation of prophylactic anticoagulants once hemostasis is ensured if any risk of thrombosis |
| Discontinuation of other treatments | Gradual tapering of steroids, discontinuation of TPO-RAs (provided that counts are good) |
| Revaccination | Vaccination against S pneumoniae every 5 years and annual flu vaccine |
| Regular follow-up | Responding patients require platelet count every 3 months for 1 year and no less than annually thereafter patients need to be reminded of precautions. Pregnancy requires reevaluation |
| Before or after splenectomy . | Measures . |
|---|---|
| Before splenectomy | |
| Patient education regarding risk of overwhelming sepsis | Early administration of oral antibiotic therapy that covers S pneumoniae and H influenzae in case of fever (amoxicillin-clavulanate, cefuroxime axetil, or levofloxacin) AND immediate travel to a hospital for assessment and intravenous antibiotics |
| Vaccination | Vaccination against S pneumoniae, meningococcus, and H influenzae type b, ideally at least 14 days before scheduled splenectomy |
| Elevation of platelet count | Elevation of platelets to > 50 × 109/L by steroids or IVIg or another treatment |
| After splenectomy | |
| Antibiotic prophylaxis | Postoperative antibiotics prophylaxis until the risk of infection is abated |
| Thromboprophylaxis | Early mobilization, good hydration, and early initiation of prophylactic anticoagulants once hemostasis is ensured if any risk of thrombosis |
| Discontinuation of other treatments | Gradual tapering of steroids, discontinuation of TPO-RAs (provided that counts are good) |
| Revaccination | Vaccination against S pneumoniae every 5 years and annual flu vaccine |
| Regular follow-up | Responding patients require platelet count every 3 months for 1 year and no less than annually thereafter patients need to be reminded of precautions. Pregnancy requires reevaluation |
IVIg indicates intravenous immunoglobulin.
Vascular complications.
Splenectomy may increase morbidity from venous thromboembolism (VT) or atherosclerosis. Increased procoagulant-derived microparticles,55 platelet activation, disturbance and activation of the endothelium, altered lipid profiles, and persistent thrombocytosis have been implicated in hypercoagulability in ITP.44
VT is reported to occur in up to 10% of patients with hematologic diseases undergoing splenectomy.2,38,42,56 The risk of VT is highest in the first year after splenectomy, but even thereafter the rate of VT in splenectomized ITP was 2.7-fold (95% CI, 1.1-6.3) higher than in age-matched controls.57 However, these studies did not include a nonsplenectomized ITP cohort. In a more recent French study of 275 patients given thromboprophylaxis who underwent laparoscopic splenectomies for various hematologic disorders (76% with ITP), only 1% developed VT.42 Portal vein thrombosis is a known early complication of open or laparoscopic splenectomy. Systematic screening for portal vein thrombosis with Doppler ultrasound or CT scan revealed a high incidence (8%-37%), but symptomatic cases are rare (< 2%),57,58 making the value of routine postoperative surveillance unproven. Secondary pulmonary arterial hypertension has been reported after splenectomy in patients with hemolytic anemia (eg, spherocytosis and thalassemia), but only anecdotally after splenectomy for ITP.59 There is insufficient information to know whether splenectomy predisposes to other reported vascular complications, (eg, multi-infarct dementia and acute coronary syndrome).60
Summary.
Splenectomy offers the greatest opportunity for cure, and societal costs appear favorable. There is little evidence to suggest increased long-term mortality and morbidity compared with medical treatment options if patients are managed appropriately, especially with regard to selection, preoperative and perioperative management, laparoscopy by an experienced surgeon, and prophylaxis of and attention to sepsis and thrombosis.
On the other hand, data comparing splenectomized and nonsplenectomized ITP patients, which suggest little difference in adverse outcomes (eg, sepsis), were acquired in patients with extensive steroid exposure before the current era of TPO-RA and rituximab. Furthermore, the long-term consequences of splenectomy remain ill-defined. Delaying the decision for 12 months or more appears to lessen the need for surgery in a substantial proportion of patients. If this approach is adopted more widely, it may affect subsequent analyses because restricting splenectomy to third-line “salvage” therapy will probably reduce not only the need but also the response rate.
Primary alternative therapeutic options, including factors determining the choice of treatment
Rituximab
Pros.
Rituximab is appealing because of its curative potential and relative safety and because hematologists are familiar with its use in treating lymphoma. Four once-weekly intravenous infusions at 375 mg/m2 induce CR in 44% of patients.61 In 1 study, splenectomy was deferred by 2 years in 40% of the patients who were treated with rituximab.62 Patients with CRs generally persist at least 1 year; those with PRs usually relapse within 6 months.3 In adults, the CR rate falls to approximately 20% by 2-5 years after a single 4-infusion course.62,63 More durable remissions might be induced by adding 1-3 cycles of HDD or using maintenance rituximab.64,65 First infusion reactions induced by clearance of anti-CD20–coated B cells by macrophages and direct cytotoxicity to B cells is generally preventable by slowing the rate of infusion and preadministration of intravenous methylprednisolone (100-1000 mg), intravenous diphenhydramine (25-50 mg), and acetaminophen.
Cons.
Toxicities primarily involve first infusion reactions, serum sickness, which is more common (5%-10%) in children, and a series of rare complications, including fulminant hepatitis B and progressive multifocal leukoencephalopathy (primarily in the setting of profound immunodeficiency, such as when rituximab is used along with intensive chemotherapy), delayed neutropenia (more common when rituximab is combined with chemotherapy), hypogammaglobulinemia, and diverse idiosyncratic reactions.61,66 Persistent perturbations in the T- and B-cell repertoires and impaired response to specific antigens have been reported in non-ITP populations and in animal models,67 but their clinical significance is uncertain. Immunizations, such as pneumovax, should be given before rituximab, as response is reduced for 4-6 months until B cells return. This may be especially important if the patient subsequently requires splenectomy. Although safety issues exist, more than one million patients have been treated for a variety of malignant and autoimmune indications since the 1990s with limited long-term serious toxicity documented to date.
Additional considerations.
Because large randomized studies have not been completed, rituximab has not received FDA or European Medicines Agency approval for ITP, although it is currently reimbursed for this purpose in many countries. Currently, the drug is administered intravenously, although subcutaneous administration has been performed with 2 other anti-CD20 antibodies, 1 in ITP.68 The longest follow-up for which data are more than anecdotal is 3-5 years compared with 5-20 years for splenectomy. Dosing is empiric. Infusions of lower doses (eg, 100 mg) showed comparable but delayed short-term benefit.69,70 There is no mechanism or treatment effect that can be used to individualize dose or frequency of administration, other than depletion of peripheral blood B cells. Repeat administration has been used effectively in a few patients.71 Anecdotal information, which suggests that rituximab administered closer to the time of diagnosis is more effective, needs to be formally demonstrated. Outcomes may be improved by the use in combination with HDD. The single reported study combining one cycle of HDD followed by rituximab in previously untreated ITP patients yielded a combined partial response and CR rate of 63% at 6 months; of those, 15%-25% relapsed by 20 months.72,73 In a pilot study of 42 previously treated patients, a combination of rituximab with 3 cycles of HDD yielded a CR plus partial response of 71% and lasting responses in 57%.64
Summary.
Rituximab has been used widely and appears to be relatively safe to date. It provides long-term CR comparable with any therapy other than splenectomy. However, the long-term outcome after 4 standard-dose infusions is somewhat disappointing. Additional benefits from coadministering dexamethasone and maintenance therapy appear promising and are under investigation
TPO-RAs
TPO-RAs are the only second-line therapy validated by randomized, controlled trials; however, thus far, the comparator arm was placebo.4,5,22,34,35,74 Two TPO-RAs, romiplostim and eltrombopag, are approved for use in more than 80 countries. In the United States, romiplostim is administered as subcutaneous weekly injections, which, at the time of this writing, must be administered by a healthcare provider. Eltrombopag is an oral agent given daily that must be taken at least 2 hours apart from ingestion of food and 4 hours apart from calcium-containing products (eg, dairy) and supplemental iron.
Pros.
The reported response rates to TPO-RAs range from 59%-88%22,34,35 ; responses are generally sustained as long as treatment continues.22,75 The short-term safety and tolerability of both TPO-RAs have been carefully documented, and both were monitored closely for 3 years in the United States, as part of post-marketing surveillance by the FDA. In one long-term study, 5% of the patients discontinued therapy because of side effects.75 Only a few patients lose responsiveness once a stable platelet count has been attained.75 In responders, doses can be individualized to attain hemostasis; and in many patients, the dose can be increased temporarily to raise platelet counts further in advance of elective surgery. Many patients are able to discontinue or reduce the dose of concomitant ITP treatments, such as corticosteroids.22,34,74,75 Decreased bleeding, less need for other (rescue) treatments, and increased health-related quality of life have been seen in responders.22,34,74,75 A number of patients, after months to years of treatment, appear able to discontinue treatment and maintain an adequate platelet count.76
Cons.
The major disadvantage of these agents compared with splenectomy and rituximab is the indefinite duration of treatment. As TPO-RAs are potentially life-long treatments, compliance may be a real issue. The current requirement for a healthcare professional to administer romiplostim and the alimentary requirements to ensure effective dosing of eltrombopag are cumbersome. TPO-RAs require at least 1-2 weeks to take effect (in responders) and therefore play at most a supplementary role in managing urgent conditions. Patients may experience mild to moderate headache, myalgias, and gastrointestinal disturbances.46 Eltrombopag carries a black-box warning for hepatotoxicity. Neither should be used during pregnancy or lactation. “Rebound” thrombocytopenia occurs infrequently after abrupt discontinuation of TPO-RAs, which can be prevented by tapering the drugs and/or using rescue treatment, if needed.22 An increase in bone marrow reticulin has been observed in several patients.75,77,78 However, current evidence does not indicate progressive bone marrow fibrosis with prolonged exposure.77 TPO-RAs have not been shown to induce malignancy or myelodysplasia in patients with ITP thus far,77 but they are not indicated in patients with myelodysplasia because of the potential risk of acute myeloid leukemia (romiplostim carries a warning). Although it seems these agents have acceptable short- and intermediate-term safety profiles, long-term safety data beyond 5 years are limited.
Additional considerations.
Relapse occurs in a large majority of patients when treatment is interrupted.74,75,79 An increase in regulatory T cells (Tregs) is seen, and a few patients appear to maintain adequate platelet counts off therapy.80 In contrast to splenectomy and rituximab, TPO-RAs have only been in use for 7 years in trials and 3 years in general practice; hence, experience in patients who were not eligible for inclusion on protocols is limited.81 It is difficult to discern whether there is an increase in the rate of VT compared with other successful interventions; it is important to take the underlying risk of arterial or venous thrombosis into consideration in patients with ITP and to consider the use of aspirin in those at risk once the platelet count has entered a safe range (eg, ≥ 50 000/μL). Additional detailed analyses of toxicity have been reported in primary studies4,5,22,34,35,74,75 and in reviews.82 These agents are not approved for use presplenectomy in Europe unless surgery is contraindicated.
Summary.
TPO-RAs are highly effective before and after splenectomy, rituximab, and other agents. Toxicity appears limited, although surveys and studies are ongoing involving patients treated in the United States and elsewhere to better define complication rates (especially thrombosis and marrow fibrosis) in larger numbers of patients followed for longer times. There is no evidence at this time that there is any major difference in efficacy or toxicity between the 2 agents, although eltrombopag requires monitoring of liver function tests every 1-2 months, which may occasionally lead to at least temporary discontinuation, if abnormal. Anecdotal evidence suggests that infrequently one agent will be effective when the other is not.
Factors that influence the choice of treatment
Patient and physician preference
The patient is a critical protagonist in the decision to undergo or defer splenectomy. Increasing awareness of medical alternatives, input of patient advocacy groups, impact of the internet, inability to predict with certainty the response to a surgical procedure,83 and rare but dramatic adverse outcomes have changed the landscape with respect to the patient's perspective. In turn, this decreases the breadth of experience of hematologists and surgeons with the procedure and its relative long-term advantages. The recent demonstrations that a substantial fraction of patients may not require treatment beyond 1-2 years after diagnosis further lessen physician enthusiasm for splenectomy as initial second-line management. If, however, response becomes predictable based on sequestration of labeled platelets and such testing becomes more widely available, then there may be a resurgence in the use of splenectomy.
Comorbidities
Comorbidities (eg, serious cardiopulmonary disorders) increase the risk of general anesthesia and post-surgical complications. A higher rate of surgical complications and a lower response rate is reported in most studies of patients older than 45-70 years of age84-86 and in those with various secondary forms of ITP.15,87 Care must be taken when considering options for patients with a history or serious risk of thrombosis.
Restrictions imposed by health funding authorities
National health services in many European countries restrict the use of TPO-RA and rituximab. After application for permission, the cost may only be covered in splenectomized patients or if splenectomy is contraindicated, but the situation is liberalizing rapidly in some countries.
Deciding which option to pursue
Before considering treatment options, it is important to exclude inherited thrombocytopenias and secondary ITP that might not have been evident on presentation. A search should be made for conditions that may contribute to or coexist with persistent thrombocytopenia, including infections, such as HIV and hepatitis C (even if initially excluded), H pylori and possibly cytomegalovirus, thyroid disorders, immunodeficiency, low-grade lymphoid neoplasms or other lymphoproliferative disorders, and systemic autoimmune conditions. The lack of clinical findings (eg, no history of infection) does not exclude CVID, which might nonetheless be clinically significant in the post-splenectomy setting and which is more amenable to treatment with rituximab.17 If a substantial, albeit transient, response has not been achieved by prior ITP treatments, a bone marrow examination should be performed to exclude other conditions, particularly myelodysplasia.
The patient's prior history, anticipated course, and relevant pros and cons of each option are discussed in a personalized way with each patient and participating family members. The patient's goals, fears, individual capacities (eg, memory, frailty), family support, proximity to medical care, and likely tolerance of each approach are considered in addition to outcomes. The appropriate therapy for 2 persons with essentially identical clinical presentations, duration of disease, and response to other therapies may differ based on these additional medical and psychosocial factors.
At one end of the spectrum, splenectomy has the highest cure rate and may therefore be the preferred option for younger patients, who have the best response and lowest complication rates, engage in physically challenging sports or professions, are potentially noncompliant with protracted daily treatment, and do not wish to continue to “deal with” their ITP. Recommendations would be influenced by the results of platelet isotopic distribution studies (if confirmed and if available).51
At the other end of the spectrum, we try to avoid splenectomy in patients over 65-70 years of age (depending on their physical condition) not only because of higher complication rates but also because of lower response rates. The same considerations apply to the very frail, those with significant surgical comorbidities, history or risk of thrombosis, those with obligatory exposure to malaria or babesia, or those who have secondary ITP. Questions may be raised about post-splenectomy infection in teachers, veterinarians, healthcare providers, travelers to certain areas, or others with increased exposure to infectious conditions.
It is far more difficult to make recommendations for or against splenectomy to the majority of patients with ITP who do not meet these criteria and have no contraindications to surgery. Although splenectomy is recommended after the failure of steroids, there is an argument to be made for waiting one year or more after diagnosis before proceeding. We generally follow the recommendations of the International Consensus8 and often lean toward either rituximab (lower response rate, modest likelihood of cure) or TPO-RA (higher response rate, less likelihood of cure) after considering the many factors aforementioned based on personal clinical experience and patient preference. This difficulty is exemplified by the responses of each of the authors to how they would advise the patient presented in the case report.
Consult
We would proceed by excluding causes of secondary ITP and by assessing the risk of bleeding. This patient's course to date is consistent with the initial diagnosis of ITP, which is best substantiated by the response to prednisone and the absence of new evidence of an underlying cause of secondary ITP. Each of the 4 authors recommend therapeutic intervention as opposed to “wait and watch” because the patient had experienced bleeding with a platelet count < 20 000/μL. The probability of responding to low-dose or alternate-day corticosteroids was considered as remote. All authors proceeded to second-line treatment. Three different approaches were adopted. These were: (1) 4 weekly infusions of rituximab at conventional doses (W.G. and B.G.), (2) 4 weekly infusions of rituximab combined with 3 cycles of HDD (J.B.B.), and (3) a TPO-RA for a limited period (ie, 6 months; D.B.C.). None of the authors considered splenectomy as initial next step in this patient with “persistent” disease. TPO-RA was recommended as the next option if rituximab-based therapy fails, and rituximab would be considered if treatment with a TPO-RA failed or the patient wanted an option that might lead to cure. All authors would consider splenectomy if an adequate response was not achieved by 1 year.
In conclusion, the last decade has seen the introduction of new exciting suitable second-line medical approaches to manage adults with ITP. Each approach has unique benefits, limitations, and risks, and none has been subjected to head-to-head comparisons. Nor have quality of life or long-term comparative effectiveness analyses been performed to inform decisions. It is unlikely that any comparative studies will hold interest for the pharmaceutical industry. Even consortia of major academic centers will have difficulty pursuing these studies because of the relatively low frequency of disease, cost and reimbursement issues, and the duration of studies needed to address critical concerns. Therefore, optimal treatment will continue to involve a personalized approach to therapy that combines the art of medicine with the science through close collaboration between patients and healthcare providers for the foreseeable future.
Authorship
Contribution: W.G., B.G., D.B.C., and J.B.B. wrote and edited the article.
Conflict-of-interest disclosure: W.G. has received research grants from Roche and Amgen, and consultancy and lecture honoraria from Amgen and Glaxo Smith Kline (GSK). B.G. has participated in advisory boards and symposia for Amgen, Roche, GSK, and LFB and has received research funding from Roche. D.B.C. is an ad hoc member of the medical advisory board for Amgen, GSK, and Eisai. J.B.B. currently receives clinical research support from the following companies: Amgen, Cangene, GSK, Genzyme, IgG of America, Immunomedics, Ligand, Eisai Inc, Shionogi, and Sysmex. His family owns stock in Amgen and GSK. He has participated in advisory boards for Amgen, GSK, Ligand, Shionogi, Symphogen, and Eisai. He had a 1-day consult with Portola.
Correspondence: James B. Bussel, Department of Pediatric Hematology/Oncology, Weill Cornell Medical College, New York, NY 10065; e-mail: jbussel@med.cornell.edu.