In this issue of Blood, Fuchs and colleagues provide evidence that circulating DNA and histones, presumably released from neutrophils, would be the second hit for development of thrombotic microangiopathies (TMAs), a group of life-threatening disorders characterized by thrombi in the microvasculature resulting in thrombocytopenia, microangiopathic hemolysis, and organ dysfunction.1
TMA includes thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). TTP is caused by a severe deficiency of von Willebrand factor cleaving protease, ADAMTS13, because of autoantibodies or genetic mutations. HUS is caused by infection with Shiga-toxin–producing Escherichia coli and is typically associated with bloody diarrhea. Atypical HUS, which has a link with defective complement regulation, is also present. Other conditions such as cancer, bone marrow transplantations, and lupus can present with features of TMA. Although patients with congenital TTP show severe ADAMTS13 deficiency, some patients may remain asymptomatic for many years.2 An infection often precedes acute TMA.3
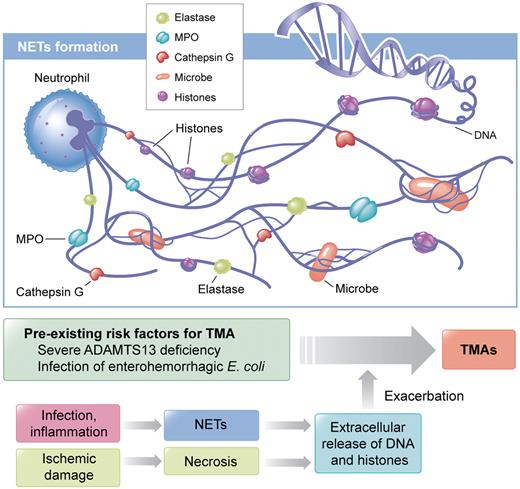
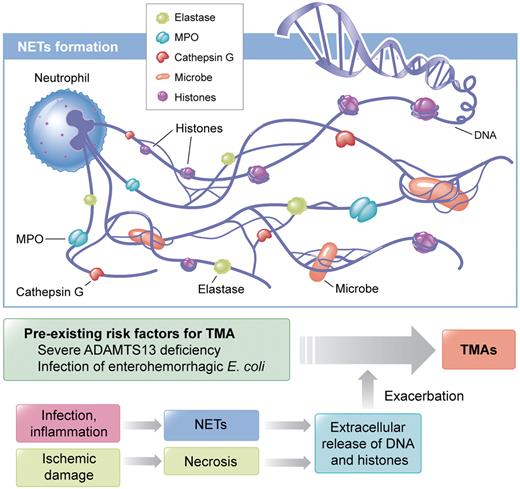
The innate immune response plays a crucial role for defense against invading microbes. Neutrophils, the most abundant leukocytes, are early responding cells that migrate in large numbers to sites of infection and release nuclear chromatin associated with nuclear histones and granular antimicrobial proteins after cell death to form neutrophil extracellular traps (NETs; see figure).4 Microbes bind to NETs and are subsequently killed by anti-microbial proteins. Extracellular DNA and histones have recently been shown to have prothrombotic characteristics.5,6 Histones cause thrombocytopenia, promote thrombosis, and contribute to organ damage and death.7,8 In most cases, in vivo studies demonstrating the prothrombotic characteristics of histones have been performed in the mouse or baboon model.
Neutrophils release nucleosomes, a complex of DNA and histones, in response to infection or inflammatory stimuli. Neutrophil extracellular traps (NETs) are composed of nucleosomes decorated with granular components including myeloperoxidase (MPO), neutrophil elastase, and cathepsin G.4 NETs bind and kill microbes.4 NETs also immobilize platelets10 and erythrocytes. Histones are known to stimulate thrombosis and to cause cytotoxicity in mice.5-7 Once patients with pre-existing risk factors for thrombotic microangiopathy (TMA) are infected, DNA and histones, in conjunction with granular proteins, are released and acute TMAs would be induced. Another possible origin of extracellular DNA and histones is necrotic tissue released after ischemic damage. Professional illustration by Kenneth X. Probst.
Neutrophils release nucleosomes, a complex of DNA and histones, in response to infection or inflammatory stimuli. Neutrophil extracellular traps (NETs) are composed of nucleosomes decorated with granular components including myeloperoxidase (MPO), neutrophil elastase, and cathepsin G.4 NETs bind and kill microbes.4 NETs also immobilize platelets10 and erythrocytes. Histones are known to stimulate thrombosis and to cause cytotoxicity in mice.5-7 Once patients with pre-existing risk factors for thrombotic microangiopathy (TMA) are infected, DNA and histones, in conjunction with granular proteins, are released and acute TMAs would be induced. Another possible origin of extracellular DNA and histones is necrotic tissue released after ischemic damage. Professional illustration by Kenneth X. Probst.
At Bern University Hospital and the University of Bern, plasma samples from TMA patients of different clinical categories have been collected and stored more than 10 years. Retrospectively, Fuchs and colleagues used these samples to investigate the possible risk factors to develop TMAs.1 First, they found elevated plasma levels of DNA, nucleosomes, lactate dehydrogenase (LDH), myeloperoxidase (MPO), and S100A8/A9 in acute TMA patients. LDH, a cytoplasmic enzyme, is a marker of tissue damage. MPO is abundantly stored in granules of neutrophils. S100A8/A9, present in the cytosol of neutrophils and monocytes, is a marker of inflammation. In clinical remission, plasma levels of DNA, LDH, MPO, and S100A8/A9 were decreased. Importantly, the great reduction of plasma levels of DNA and MPO was concomitant with the increase in platelet counts and plasma ADAMTS13 levels in acute TTP patients with remission, indicating correlation of DNA and MPO levels in disease state. Severe ADAMTS13 deficiency per se does not lead to an increase in plasma DNA or MPO levels, while DNA and MPO are elevated only during disease flare-up. These findings indicated that extracellular DNA and histone levels during acute TMA could increase the risk for developing TMAs and provide a second hit that triggers acute disease in patients at risk for TMA. Disease pathogenesis sometimes involves additional unknown genetic factors and/or environmental triggers. For example, mice lacking the ADAMTS13 gene are predisposed to acute TMA, but Shiga-toxin is needed to induce the acute disease.9
What is the triggering event for extracellular DNA and histones in TMA patients? Recent studies showed infection as the most commonly identified etiologic factor for TMA,3 and LPS can induce NET formation through platelet TLR4.10 Therefore, it is conceivable that release of DNA and histones from neutrophils is caused by a preceding infection. What is the origin of extracellular DNA and histones? Beside NETs, necrotic tissue after ischemic damage could be a source of these 2 compounds (see figure). What are the natures of DNA and histones? Circulating DNA is likely fragmented by endogenous DNases4 and histones are cleaved by activated protein C7 and/or other proteases. Therefore, tools that degrade and inactivate prothrombotic DNA and histones would seemingly be promising candidates for preventing TMAs and other thrombotic complications. Finally, this is a retrospective study; thus, whether nucleosomes, DNA, or histones contribute directly to the clinical manifestation of acute TMAs remains to be determined. Nevertheless, evidence that extracellular DNA and histones are elevated in patients with acute TMA and decreased in remission concomitant with the increase in platelet counts advances the understanding of the pathogenesis of acute TMAs.
Conflict-of-interest disclosure: T.M. is an inventor of the ADAMTS13 assay method, which is related to its patent. X.P.F. declares no competing financial interests. ■