Abstract
Ruxolitinib is JAK1/JAK2 inhibitor with established clinical benefit in myelofibrosis (MF). We analyzed long-term outcomes of 107 patients with intermediate-2 or high-risk MF receiving ruxolitinib at MD Anderson Cancer Center (MDACC) on phase 1/2 trial. After a median of 32 months of follow-up, 58 patients (54%) were still receiving ruxolitinib, with overall survival (OS) of 69%. The splenomegaly and symptom reductions achieved with ruxolitinib were sustained with long-term therapy. Therapy was well tolerated; discontinuation rates at 1, 2, and 3 years were 24%, 36%, and 46%, respectively. OS of 107 MDACC patients was significantly better (P = .005) than that of 310 matched (based on trial enrollment criteria) historical control patients, primarily because of highly significant difference in OS in the high-risk subgroup (P = .006). Furthermore, among MDACC patients, those with high-risk MF experienced the same OS as those with intermediate-2 risk. Patients with ≥ 50% reduction in splenomegaly had significantly prolonged survival versus those with < 25% reduction (P < .0001). Comparison of discontinuation rates and reasons for stopping the therapy to those reported for other 51 patients in the phase 1/2 trial, and 155 ruxolitinib-treated patients in phase 3 COMFORT-I study, suggest that continued therapy with ruxolitinib at optimal doses contributes to the benefits seen, including OS benefit.
Introduction
Myelofibrosis is a Philadelphia chromosome–negative myeloproliferative neoplasm defined by clinical and pathologic characteristics that include splenomegaly (often massive), debilitating symptoms (eg, fatigue, early satiety, weight loss, night sweats, fever, and pruritus), cytopenias, and progressive bone marrow fibrosis.1 This chronic and incapacitating disease shortens life expectancy and severely compromises quality of life.1 Survival is highly variable with median values ranging from 2 to 11 years,2 depending on the presence of defined prognostic factors.2,3
Until recently, there have been no effective medical treatment options for patients with myelofibrosis.4,5 Most pharmacotherapies have been palliative6 and their effect on spleen size and symptoms is minimal and generally transient.7,8 Splenectomy may be considered for myelofibrosis patients with substantial organ enlargement and refractory splenic symptoms who have failed medical therapy9 ; however, the surgical mortality and morbidity rates (9% and 31%, respectively) are significant and limit the use of this modality.10 For patients with symptomatic splenomegaly who are not good surgical candidates, splenic radiation may be an option, but palliation is often short-lived and at the cost of significant toxicities.9 Although allogeneic stem cell transplantation is the only treatment with curative potential,11 it is associated with significant morbidity and mortality11,12 and few patients are eligible.6 Therefore, there is a need for well-tolerated myelofibrosis therapies that demonstrate durable improvements in the manifestations of the disease that impact patient quality of life and improve overall survival.
The recent identification of mutations associated with the JAK-STAT pathway and the appreciation of the role of cytokines that signal through JAK1 and JAK2 in the pathogenesis of myeloproliferative neoplasms13-15 has resulted in new treatment strategies for these diseases. Ruxolitinib (formerly INCB018424) is a JAK1 and JAK2 inhibitor that has demonstrated clinical benefit in patients with intermediate-2 and high-risk myelofibrosis in a phase 1/2 trial (study INCB18424-251)16 and in 2 recently completed phase 3 randomized clinical trials (COMFORT-I and COMFORT-II).7,17 In these studies, ruxolitinib was well-tolerated and demonstrated early and sustained clinical benefits in terms of reductions in spleen size and improvements in debilitating myelofibrosis-related symptoms, which led to the approval of ruxolitinib by the US Food and Drug Administration for the treatment of patients with intermediate- or high-risk myelofibrosis.
Study INCB18424-251 was a phase 1/2 study of ruxolitinib that included 158 patients: 52% (17 of 33) of patients receiving ruxolitinib at a starting dose of 15 mg twice daily achieved a ≥ 50% reduction in palpable splenomegaly that lasted for at least 12 months. Patients also reported dramatic and rapid symptom improvement, weight gain, and improved ability to walk. These benefits of ruxolitinib treatment are clearly clinically meaningful to patients with myelofibrosis. There are, however, limited data on longer-term outcomes with ruxolitinib, including durability of spleen size reduction and symptom improvement and potential effects of ruxolitinib treatment on survival. These issues were explored in a published letter describing the 51 patients treated with ruxolitinib on study INCB18424-251 at Mayo Clinic Rochester (the other 107 patients were treated at the MD Anderson Cancer Center [MDACC]).18 For these 51 ruxolitinib-treated patients, the discontinuation rate was high and the survival rate showed no significant difference between the ruxolitinib recipients and a cohort of all-risk 410 recipients of standard treatment at the Mayo Clinic during the past decade. Herein we report the results of a long-term follow-up of 107 patients at MDACC who participated in study INCB18424-251. First, survival relative to a matched historical control group (as well as factors correlated with overall survival) was analyzed. Second, a comparison of our results to those seen in the 51 patients who participated in study INCB18424-251 from Mayo Clinic Rochester was done. Third, comparison was also done of outcomes between 107 patients treated at MDACC and those treated with ruxolitinib (N = 155) as a part of the COMFORT-I placebo controlled randomized study.
Methods
Study design
The details of the INCB18424-251 study design (NCT00509899) have been previously published.16 This was an open-label, nonrandomized study of ruxolitinib that began enrollment in 2007. It was funded by the Incyte Corporation. Patients had primary myelofibrosis, post–polycythemia vera myelofibrosis, or post–essential thrombocythemia myelofibrosis (World Health Organization criteria 2008 revision)19 requiring therapy, and were either refractory or intolerant to prior therapy. Newly diagnosed patients with palpable splenomegaly of 10 cm or more below the left coastal margin were also eligible. Patients were at least 18 years of age, with an Eastern Cooperative Oncology Group performance status of ≤ 2 and intermediate- or high-risk disease by the Lille system20 (prognostic factors included hemoglobin < 10 g/dL and white cell count < 4 × 109/L or > 30 × 109/L). Other inclusion criteria were an absolute neutrophil count > 1.5 × 109/L, platelet count > 100 × 109/L, bilirubin ≤ 2.0 mg/dL, alanine aminotransferase ≤ 2.5× the upper limit of normal, and creatinine ≤ 2.5 mg/dL. The clinical study protocol was approved by the MDACC institutional review board, and the study was conducted in accordance with Good Clinical Practice guidelines per the International Conference on Harmonization. All patients provided written informed consent in accordance with the Declaration of Helsinki.
The objective of this analysis was to evaluate the long-term efficacy and safety of ruxolitinib in patients who were enrolled in study INCB18424-251 at the MDACC. This analysis also evaluated survival benefits compared with an historical control cohort and explored factors influencing survival of the ruxolitinib-treated patients. In addition, we compare our long-term experience with that seen in the Mayo Clinic Rochester cohort from study INCB18424-251, and that seen in patients treated with ruxolitinib as a part of the COMFORT-I placebo controlled randomized study.
Historical cohort
The historical control patients were identified from 3 large databases (MDACC, Houston, TX; University of Pavia, Pavia, Italy; and Hospital Niguarda cà Granda, Milan, Italy). Among patients in these 3 databases, 310 patients were identified that would have fulfilled enrollment criteria of study INCB18424-251; therefore, the control group of 310 patients was matched to 107 patients participating in the study INCB18424-251 based on the study eligibility criteria. Patients in the control group had a median year of initial diagnosis of 2002 (range 1978-2010), and a median start date for observation at the MDACC, University of Pavia, or Hospital Niguarda cà Granda of 2004 (range 1980-2010). There was no improvement in survival within the control group of patients based on the decade (or 5-year time periods) during which those patients were seen and followed (data not shown).
Statistical analysis
International Prognostic Scoring System (IPSS) risk category was assigned retrospectively, using the baseline data parameters to determine the risk level for each patient at the time of first observation at MDACC (ruxolitinib-treated patients) or for the historical controls, at MDACC, Hospital Niguarda cà Granda, or University of Pavia. The 5 risk factors for the IPSS are equally weighted (assigned 1 point each) and are: white blood cell count > 25 × 109/L, presence of constitutional symptoms, age > 65 years, hemoglobin < 10 g/dL, and peripheral blood blasts of 1% or more.2 High-risk patients are those with 3 or more points, intermediate-2–risk patients are those with 2 points, intermediate-1–risk patients have 1 point, and low-risk patients have 0 points. The Dynamic International Prognostic Scoring System (DIPSS) assigns a double weight to the presence of hemoglobin < 10 g/dL, and one point to the remaining 4 risk factors. High-risk patients have 5 or 6 points, intermediate-2–risk patients have 3 or 4 points, intermediate-1–risk patients have 1 or 2 points, and low-risk patients have 0 points.
All time-to-event analyses were conducted using the Kaplan-Meier method. For the duration of spleen-length response (palpation), the start point required a confirmed reduction of ≥ 50%, reflecting established response criteria (International Working Group for Myelofibrosis Research and Treatment).21 The starting point of the duration measurement was set as the date of first observed ≥ 50% reduction from baseline, which was subsequently confirmed, with at least a 12-week interval separating the consecutive observations of response. For purpose of our analysis, a loss of response was defined as the date of first observation of spleen-length reduction that was < 25% from baseline (although these patients still had smaller spleens than at the beginning of the therapy). Patients who did not lose their response were censored at the time of their last date of spleen-length measurement. For the duration of response by spleen volume (measured by magnetic resonance imaging [MRI] or computed tomography [CT]), duration was determined for patients who had at least one measurement of a ≥ 35% reduction from baseline at any time during the study. Patients who achieved this reduction at the last visit before data cutoff were included in the analysis with a response of 1 day. A loss of response was defined as the first date with a spleen-volume reduction that was < 10% relative to baseline.
The reverse Kaplan-Meier method was used to estimate follow-up time for patients.22 The log-rank test and a Cox proportional hazard model adjusted for differences in IPSS risk were used to compare overall survival between patients in study INCB18424-251 at the MDACC with the historical control group. Comparisons between ruxolitinib-treated patients and the historical controls were also conducted for the IPSS high-risk and intermediate-2–risk subgroups. The Cox proportional hazard model allowed us to estimate hazard ratios and 95% confidence intervals. Additional survival analyses using the log-rank test were conducted to compare subgroups of the patients in study INCB18424-251. These analyses were based on selected baseline characteristics including IPSS/DIPSS risk status, cytogenetic status, sex, age, hemoglobin, white blood cell count, palpable spleen length, and the postbaseline characteristic of confirmed reduction in palpable spleen length during ruxolitinib treatment.
Survival analyses were conducted at MDACC using Statistica Software. Duration of spleen response was conducted using SAS Version 9.1.3 and provided by Incyte Corporation on request from MDACC. We requested demographic data from the Mayo Clinic Rochester cohort in study INCB18424-251 and it was produced by Incyte Corporation using the clinical study database. We also requested discontinuation rates and reasons for discontinuation for MDACC and Mayo Clinic Rochester for study INCB18424-251 and for COMFORT-I; these data were produced by Incyte Corporation using simple cumulative discontinuation. Causes of discontinuation as assigned by the investigator during the conduct of the study were categorized by the defined headings under which the data were collected in the study case report forms. Discontinuation rates for patients in study INCB18424-251 at Mayo Clinic Rochester are as reported by Tefferi et al.18 Discontinuation rates for all study populations were also measured by Kaplan-Meier analysis by Incyte Corporation based on the respective study clinical databases, per our request; these rates were similar to those determined by simple cumulative discontinuation rates. Mean total daily dose for patients enrolled at MDACC and Mayo Clinic Rochester in study INCB18424-251, and the phase 3 study of ruxolitinib (COMFORT-I) was determined as a weekly average total daily dose based on clinical datasets at Incyte Corporation, per our request.
Results
Comparison to matched historical control
The baseline demographic characteristics of the patients enrolled at MDACC in study INCB18424-251 (n = 107) and the historical control population (n = 310) are summarized in Table 1. In general, the patients were well-matched. Among the ruxolitinib-treated patients, 63 (59%) were high-risk, 34 (32%) were intermediate-2 risk, and 10 (9%) were intermediate-1 risk according to IPSS. In the historical control group, 165 (53%) patients were high risk and 145 (47%) were intermediate-2 risk. In the historical control group (n = 310), 88% received one or more therapies for myelofibrosis during the follow-up period. Ruxolitinib-treated patients were somewhat younger than patients in the control group (51% of ruxolitinib-treated patients were older than 65 years versus 69% in the control group, although median age was similar) and had marginally higher mean hemoglobin (10.2 vs 9.7 g/dL). White blood cell count was higher (18.9 × 109/L vs 12.0 × 109/L), and median spleen size larger (19 cm vs 6 cm) among ruxolitinib-treated patients compared with control patients, although both populations had similar ranges of palpable spleen sizes. Male to female ratio, platelet count, and cytogenetic characteristics were similar between the 2 groups; however, cytogenetic information was not available for approximately one-third of the control population. The median follow-up time for survival was 32 months and 55 months for patients at MDACC and the control cohort, respectively.
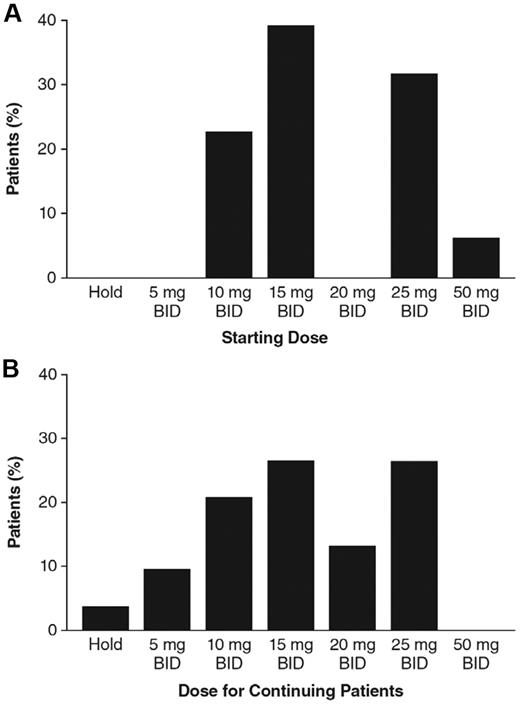
For the ruxolitinib-treated patients in study INCB18424-251, initial doses were based on protocol-stipulated assigned cohorts, and ranged from 10 mg twice daily (BID) to 50 mg BID and from 25 mg daily (QD) to 200 mg QD.16 Seventy-nine of the 107 patients (74%) from MDACC in study INCB18424-251 began the study using BID dosing regimens (range 10 mg-50 mg BID; Figure 1A). The remaining 28 patients began dosing at 50 mg QD (n = 19), 100 mg QD (n = 6), and 200 mg QD (n = 3).
Dose levels for patients enrolled at MDACC in study INCB18424-251. (A) Initial doses were assigned by the clinical study protocol amendment in operation at the time of enrollment. Seventy-nine of 107 patients began dosing with twice-daily regimens ranging from 10 mg BID to 50 mg BID; the proportion of all patients starting with BID doses at a given dose group is shown. (B) Fifty-eight patients remained on study at the time of data analysis; the proportion of patients receiving the indicated BID doses is shown.
Dose levels for patients enrolled at MDACC in study INCB18424-251. (A) Initial doses were assigned by the clinical study protocol amendment in operation at the time of enrollment. Seventy-nine of 107 patients began dosing with twice-daily regimens ranging from 10 mg BID to 50 mg BID; the proportion of all patients starting with BID doses at a given dose group is shown. (B) Fifty-eight patients remained on study at the time of data analysis; the proportion of patients receiving the indicated BID doses is shown.
At the time of data analysis, 58 (54%) of the 107 patients were still receiving ruxolitinib therapy. Discontinuation rates at 1, 2, and 3 years were 24%, 36%, and 46%, respectively. Causes of discontinuation as assigned by the investigator, categorized by the defined headings under which the data were collected in the study case report forms, are listed in Table 2. At the time of the analysis, approximately 80% of continuing patients were receiving doses of 10 mg BID to 25 mg BID (Figure 1B), 2 patients were on a temporary hold, and 5 patients were receiving QD doses.
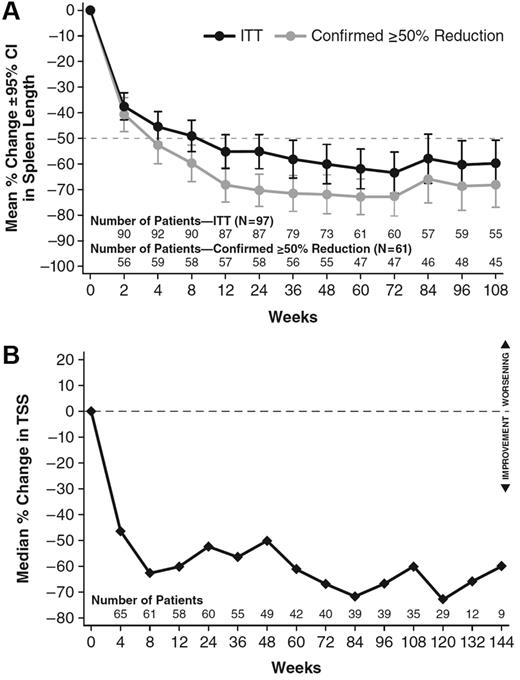
Reduction in palpable spleen length during ruxolitinib treatment was marked and durable. As reported previously,16 ruxolitinib treatment, particularly at doses of 15 mg BID to 25 mg BID, was associated with significant clinical response, as assessed by a ≥ 50% reduction in palpable spleen length. Figure 2A illustrates the mean percent change from baseline in spleen length as measured by palpation for all patients enrolled at MDACC with palpable spleen at baseline (97 of 107 patients) and for that subset of patients with enlarged spleen who exhibited a ≥ 50% decrease in palpable spleen length (61 of 97 patients). Confirming the earlier results,16 there was a rapid and marked reduction in spleen size for the majority of ruxolitinib-treated patients (74 of 97, or 76%) that appeared durable.
Effect of ruxolitinib on spleen size and myelofibrosis symptoms for patients enrolled at MDACC in study INCB18424-251. (A) Spleen length was measured at each study visit by manual palpation. Mean change from baseline (± SEM) for all 97 patients with palpable spleen at baseline and the 61 patients who demonstrated a ≥ 50% reduction in spleen length, confirmed 12 weeks later, is shown. (B) The modified Myelofibrosis Symptom Assessment Form (MFSAF) was used at serial clinic visits in 71 patients enrolled at MDACC, and a composite score corresponding to the symptoms of abdominal pain and discomfort, itching, night sweats, and bone/muscle pain was determined. The figure depicts the median percentage of change in this total symptom score over time.
Effect of ruxolitinib on spleen size and myelofibrosis symptoms for patients enrolled at MDACC in study INCB18424-251. (A) Spleen length was measured at each study visit by manual palpation. Mean change from baseline (± SEM) for all 97 patients with palpable spleen at baseline and the 61 patients who demonstrated a ≥ 50% reduction in spleen length, confirmed 12 weeks later, is shown. (B) The modified Myelofibrosis Symptom Assessment Form (MFSAF) was used at serial clinic visits in 71 patients enrolled at MDACC, and a composite score corresponding to the symptoms of abdominal pain and discomfort, itching, night sweats, and bone/muscle pain was determined. The figure depicts the median percentage of change in this total symptom score over time.
The duration of spleen response was formally explored using Kaplan-Meier analysis for patients with a confirmed ≥ 50% reduction in palpable spleen length (61 of 97 patients with palpable spleen at baseline; Figure 3A). Median duration of response was estimated to be 166 weeks. A subset of 25 patients in the trial had serial imaging by MRI (or CT in one patient) that was assessed by an independent central reader to objectively determine volumetric changes in spleen size in parallel with clinical determination of spleen length changes.16 As previously reported, these patients had a median reduction in spleen volume of 33% and a median reduction in palpable spleen length of 52% consistent with the association of a 50% reduction in spleen length by palpation and a ≥ 35% reduction in spleen volume as measured by imaging.16 Durability of response as assessed by imaging for patients who achieved a ≥ 35% reduction in spleen volume is shown in Figure 3B. The median duration of response was 93 weeks. Thus, both by palpation and imaging, the median duration of meaningful spleen size reduction was approximately 2 years from the onset of the response.
Duration of spleen size reduction measured by palpable spleen length, or MRI/CT-imaged volume for patients enrolled at MDACC in study INCB18424-251. (A) The duration of a ≥ 50% reduction in palpable spleen length was estimated using the Kaplan-Meier method based on the 61 patients who exhibited a confirmed ≥ 50% reduction in palpable spleen length. The horizontal axis represents the time from onset of response. Patients could begin responding at any time; therefore, “weeks from onset” does not correspond to weeks in the study. Twenty-eight of 61 patients lost the response (date of first observation of spleen length reduction < 25% from baseline) before the analysis date. The median duration of response was estimated to be 166 weeks. (B) The duration of a ≥ 35% reduction in spleen volume was estimated using the Kaplan-Meier method based on the 17 patients who had a ≥ 35% reduction from baseline. The horizontal axis represents the time from onset of response. Patients could begin responding at any time; therefore, “weeks from onset” does not correspond to weeks in the study. Seven of 17 patients lost the response (defined by the first date with < 10% reduction from baseline) before the analysis date. The median duration of response was estimated to be 93 weeks, but note this estimate was based on few events in a small population.
Duration of spleen size reduction measured by palpable spleen length, or MRI/CT-imaged volume for patients enrolled at MDACC in study INCB18424-251. (A) The duration of a ≥ 50% reduction in palpable spleen length was estimated using the Kaplan-Meier method based on the 61 patients who exhibited a confirmed ≥ 50% reduction in palpable spleen length. The horizontal axis represents the time from onset of response. Patients could begin responding at any time; therefore, “weeks from onset” does not correspond to weeks in the study. Twenty-eight of 61 patients lost the response (date of first observation of spleen length reduction < 25% from baseline) before the analysis date. The median duration of response was estimated to be 166 weeks. (B) The duration of a ≥ 35% reduction in spleen volume was estimated using the Kaplan-Meier method based on the 17 patients who had a ≥ 35% reduction from baseline. The horizontal axis represents the time from onset of response. Patients could begin responding at any time; therefore, “weeks from onset” does not correspond to weeks in the study. Seven of 17 patients lost the response (defined by the first date with < 10% reduction from baseline) before the analysis date. The median duration of response was estimated to be 93 weeks, but note this estimate was based on few events in a small population.
Myelofibrosis-related symptoms were assessed by serial completion of the modified Myelofibrosis Symptom Assessment Form (MFSAF) beginning with the 33rd patient enrolled at MDACC. As reported previously, symptom severity was decreased in the majority of patients for up to 1 year.16,23 Serial use of the MFSAF has continued in the study, and Figure 2B illustrates that a total symptom score, defined as the composite of scores for night sweats, itching, abdominal pain and discomfort, and bone/muscle pain continues to show a median reduction of approximately 60% for more than 2 years of treatment. Four patients with symptom data did not have palpable spleen at baseline. Three of the 4 had a 50% or greater improvement in symptom score during treatment, indicating that patients without an enlarged spleen can also experience benefits of ruxolitinib therapy.
Survival
There were 33 deaths in the ruxolitinib group after a median follow-up of 32 months, for an overall survival rate of 69%. Fourteen of the deaths occurred while on therapy or within 30 days of discontinuation, and 19 were off study. None of the deaths were considered by the investigator to be therapy related. Causes of death on study were myocardial infarction or cardiac arrest (n = 4), multiorgan failure (n = 3), disease progression or myelofibrosis (n = 2), sepsis (n = 1), pneumonia (n = 1), and brain aneurysm, pancreatic mass with liver metastasis, abdominal aortic aneurysm (n = 1 each). There were 187 deaths in the control group. Overall survival was significantly greater among patients treated with ruxolitinib compared with historical controls in an analysis adjusted for IPSS risk status (hazard ratio [HR] = 0.58; 95% confidence interval [CI], 0.39-0.85, P = .005; Figure 4A). In comparison to historical controls, the difference in overall survival was highly significant between the high-risk patient subgroups: 1-, 2- and, 3-year survival rates were 95%, 83%, and 63% for the ruxolitinib-treated group and 81%, 58%, and 35% for the control group (HR = 0.50, 95% CI, 0.31-0.81; P = .006; Figure 4B). Although the hazard ratio for the intermediate-2–risk control and ruxolitinib-treated groups favored ruxolitinib, the result was not statistically significant (HR = 0.85; 95% CI, 0.43-1.71; P = .71; Figure 4C). Notably, high-risk patients (per either IPSS or DIPSS) treated with ruxolitinib had a similar survival to intermediate-2 patients treated with ruxolitinib, with 1-, 2-, and 3-year survival rates of 95%, 83%, and 63% for high-risk patients and 97%, 79%, and 70% for intermediate-2–risk patients (HR = 1.36; 95% CI, 0.64-2.89; P = .43; Figure 4D). This subgroup analysis is independent of the historical control, and is anchored by the validated IPSS scoring system.
Kaplan-Meier comparison of overall survival between patients at MDACC in study INCB18424-251 and the historical control population. (A) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients, with HR, 95% CI, and P value, adjusted for baseline IPSS risk status. There were 33 deaths in the ruxolitinib-treated group (N = 107) and 187 deaths in the control group (N = 310). (B) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients designated as high risk according to the IPSS. There were 21 deaths in the high-risk ruxolitinib-treated group (N = 63) and 111 deaths in the control group (N = 165). (C) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients designated as intermediate-2 according to the IPSS. There were 10 deaths in the intermediate-2 risk ruxolitinib-treated group (N = 34) and 76 deaths in the control group (N = 145). (D) Kaplan-Meier plot of overall survival for ruxolitinib-treated patients at MDACC in study INCB18424-251 designated as high risk and intermediate-2 according to the IPSS. *Hazard ratio >1 favors intermediate-2 risk group.
Kaplan-Meier comparison of overall survival between patients at MDACC in study INCB18424-251 and the historical control population. (A) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients, with HR, 95% CI, and P value, adjusted for baseline IPSS risk status. There were 33 deaths in the ruxolitinib-treated group (N = 107) and 187 deaths in the control group (N = 310). (B) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients designated as high risk according to the IPSS. There were 21 deaths in the high-risk ruxolitinib-treated group (N = 63) and 111 deaths in the control group (N = 165). (C) Kaplan-Meier plot of overall survival for ruxolitinib-treated and historical control patients designated as intermediate-2 according to the IPSS. There were 10 deaths in the intermediate-2 risk ruxolitinib-treated group (N = 34) and 76 deaths in the control group (N = 145). (D) Kaplan-Meier plot of overall survival for ruxolitinib-treated patients at MDACC in study INCB18424-251 designated as high risk and intermediate-2 according to the IPSS. *Hazard ratio >1 favors intermediate-2 risk group.
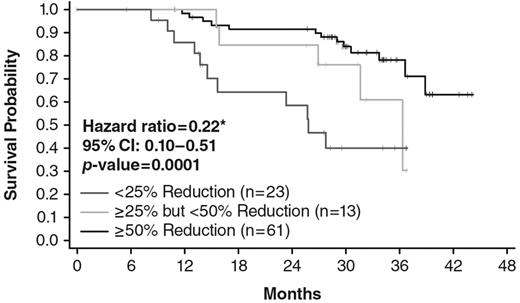
Additional exploratory analyses of factors associated with survival differences were conducted in ruxolitinib-treated patients. Patients were grouped according to the observed spleen response on therapy, and the overall survival of the groups compared. Patients who experienced a confirmed ≥ 50% reduction in palpable spleen size (n = 61) had significantly prolonged survival compared with the minority of patients (n = 23) with a < 25% reduction in spleen from baseline (HR = 0.223; 95% CI, 0.097-0.512; P = .0001; Figure 5). Patients who exhibited intermediate reductions in splenomegaly showed an intermediate survival profile (Figure 5). Other evaluable baseline characteristics such as sex, high white blood cell count (> 25 × 109/L), cytogenetic abnormalities, or anemia (hemoglobin < 10 g/dL) did not have a statistically significant impact on overall survival during treatment with ruxolitinib.
Kaplan-Meier analysis of overall survival (in months) of patients enrolled at MDACC in study INCB18424-251 by degree of spleen length reduction. Patients were analyzed in 3 groups: patients who exhibited confirmed response of ≥ 50% reduction of palpable spleen length (n = 61), patients who exhibited confirmed response of > 25% but < 50% reduction in palpable spleen length (n = 13), and patients who exhibited confirmed < 25% reduction in palpable spleen length (n = 23). *Comparison of < 25% reduction to ≥ 50% reduction.
Kaplan-Meier analysis of overall survival (in months) of patients enrolled at MDACC in study INCB18424-251 by degree of spleen length reduction. Patients were analyzed in 3 groups: patients who exhibited confirmed response of ≥ 50% reduction of palpable spleen length (n = 61), patients who exhibited confirmed response of > 25% but < 50% reduction in palpable spleen length (n = 13), and patients who exhibited confirmed < 25% reduction in palpable spleen length (n = 23). *Comparison of < 25% reduction to ≥ 50% reduction.
Myelofibrosis transformed to acute myeloid leukemia in 9 (8.4%) of the 107 patients in the ruxolitinib group; 5 (4.7%) while on therapy or within 30 days of discontinuation and 4 off study, for a transformation rate of 0.036 per patient-year. Among the 310 patients in the historical group there were 32 (32 of 310; 10.3%) transformations, for a transformation rate of 0.038 per patient-year.
Comparison to other patients enrolled in INCB18424-251 and COMFORT-I
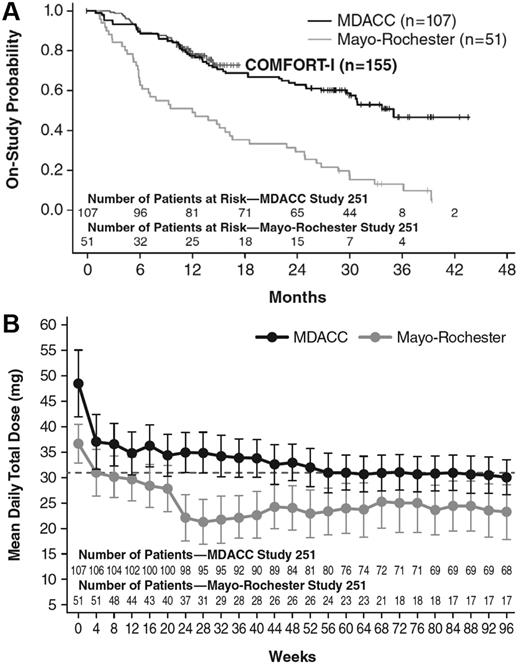
The baseline demographic characteristics of the patients enrolled at Mayo Clinic Rochester in study INCB18424-251 (n = 51) are summarized in Table 1. Patients were well matched in terms of age, hemoglobin, and palpable spleen length at baseline. Discontinuation rates reported were 51%, 72%, and 89% for 1, 2, and 3 years, respectively.18 These are markedly different from those observed at MDACC (24%, 36%, and 46% for 1, 2, and 3 years, respectively; Table 2). Even at 6 months, discontinuations at the Mayo Clinic Rochester were much higher than observed at the MDACC or those seen in 155 patients randomized to ruxolitinib as a part of the COMFORT-I study (Figure 6A). In addition, although the starting dose at the 2 clinical sites was governed by the dosing cohort assignments (and, for example, all patients who began at the lowest dose [25 mg QD] and none who began at the highest dose [50 mg BID] were enrolled at the Mayo Clinic Rochester), dose adjustments were allowed per protocol, up to a maximum of 25 mg BID. However, the mean ruxolitinib dose over time at MDACC was much higher than that for patients enrolled at the Mayo Clinic Rochester and was similar to the mean total daily dose of 31 mg in COMFORT-I ruxolitinib-treated patients (Figure 6B).
Comparison of discontinuation rates and mean ruxolitinib dosing over time in study INCB18424-251. (A) Kaplan-Meier estimates of discontinuation rates in patients enrolled from MDACC and Mayo Clinic Rochester. At 6 months, discontinuations at the Mayo Clinic Rochester were much higher than those observed at the MDACC Center. (B) Mean total daily dose of ruxolitinib at the MDACC was much higher than that for patients enrolled at the Mayo Clinic Rochester and was similar to the mean total daily dose of ruxolitinib in COMFORT-I (31 mg).
Comparison of discontinuation rates and mean ruxolitinib dosing over time in study INCB18424-251. (A) Kaplan-Meier estimates of discontinuation rates in patients enrolled from MDACC and Mayo Clinic Rochester. At 6 months, discontinuations at the Mayo Clinic Rochester were much higher than those observed at the MDACC Center. (B) Mean total daily dose of ruxolitinib at the MDACC was much higher than that for patients enrolled at the Mayo Clinic Rochester and was similar to the mean total daily dose of ruxolitinib in COMFORT-I (31 mg).
Discussion
These data represent a comprehensive long-term patient follow-up of ruxolitinib therapy. The results suggest that ruxolitinib therapy has a potential to improve survival in patients with myelofibrosis. Of the 107 patients treated at our center, over half were still receiving therapy after a median 32-month follow-up. There was a low rate of discontinuation because of toxicity or progressive disease, suggesting that long-term therapy was well tolerated. Clinically significant benefits in spleen size reduction were observed for the majority of patients: 61 (63%) of 97 patients had at least a 50% reduction in palpable spleen size and 74 (76%) of 97 had at least a 25% reduction in palpable spleen size. Spleen reductions (measured by palpation or MRI) were sustained over time, with a median duration of response of approximately 2 years from onset of response. Similarly, myelofibrosis-related symptoms, which can be debilitating, were improved in the majority of patients in whom symptoms were assessed, and this improvement was also durable. Moreover, patients without an enlarged spleen benefited from ruxolitinib treatment, as evidenced by symptom improvement response.
In the absence of a concurrently randomized control group, this study used a historical cohort of patients as a control for the survival analysis. Well-known caveats related to use of historical controls, including selection bias and time bias, were considered and efforts were made to reduce and understand the potential bias in the analyses.19-21 To limit selection bias, we selected patients from established databases based on the enrollment criteria used for study INCB18424-251. Comparisons of demographics and baseline characteristics indicate that the historical controls used in this study were generally representative of the patients included in study INCB18424-251. There were, however, subtle differences in risk factors between the groups that have the potential to impact overall survival: patients in the ruxolitinib trial had higher leukocyte count and spleen size, while patients in the control arm were older and had slightly lower hemoglobin. The net effect of these differences cannot be readily determined. Among our control group of patients the time period of patient observation did not matter, as there was no improvement in their survival over time, thus significantly decreasing potential time bias. An International Working Group project reviewing the outcome of patients with intermediate-2 or high-risk myelofibrosis over the last several decades, also found that outcome of patients had not changed over time.23
Mindful of the caveats associated with historical comparisons, we observed an overall survival benefit for ruxolitinib-treated patients that was robust and statistically significant in an analysis that was adjusted for differences in baseline IPSS risk status, which incorporates age, baseline hemoglobin, white blood cell count, constitutional symptoms, and blast count. Based on subgroup analyses, the result seems to be driven primarily by patients categorized as high risk by the IPSS scale. Importantly, independent of historical control data, comparison of the 1-, 2-, and 3-year survival rates for ruxolitinib-treated patients categorized as high risk versus intermediate-2 risk suggests that ruxolitinib treatment abrogates the prognostic significance of high-risk status determined by IPSS or DIPSS, 2 independently validated prognostic scoring systems. Therefore, both by comparison to matched historical controls as well as by a comparison to a concurrent internal control group with expected survival determined using validated prognostic scoring systems, the data support a survival advantage for patients treated with ruxolitinib.
The most important factor that correlated with survival in ruxolitinib-treated patients was the degree of the spleen size reduction. Previously, we have shown that symptoms such as fatigue, abdominal pain and discomfort, appetite, and ability to move around were improved in ruxolitinib-treated patients, with improvement tracking with the degree of splenomegaly reduction.24 In that study, improvements in signs and symptoms of myelofibrosis were seen in patients who had at least a 25% reduction of spleen size as measured by palpation or MRI. Degree of spleen reduction has not been previously identified as a factor that is associated with prolonged survival, although this may be because no prior therapy has been able to produce meaningful and durable reductions in spleen size. In this study, achieving at least a 50% reduction in palpable spleen length was associated with significantly prolonged survival compared with a less than 25% reduction. In agreement with the positive impact of more modest splenomegaly improvements on symptoms, patients who experienced spleen size reductions between 25% and 50% relative to baseline exhibited overall survival intermediate to the groups of patients with greater or lesser spleen volume reductions. Although this analysis cannot establish a causal relationship between spleen size reduction and survival, such a relationship would not be entirely surprising. Splenomegaly is a hallmark of myelofibrosis and the complications associated with the progression of splenomegaly, such as progressive disability and cachexia, have been identified as primary reasons for limited survival among patients with myelofibrosis.25 These findings lend support to spleen reduction as a meaningful end point in clinical trials in patients with myelofibrosis.
The benefits observed in this analysis are consistent with 2 recently completed phase 3 clinical studies in patients with myelofibrosis. In COMFORT-I, a double-blind, placebo-controlled study, 87% and 52% of patients in the ruxolitinib and placebo groups, respectively, were still on therapy at the time of analysis; median follow-up was 32 weeks.17 A total of 41.9% of ruxolitinib-treated patients achieved a ≥ 35% reduction in spleen volume (measured by MRI or CT) at week 24, versus 0.7% receiving placebo.17 Moreover, the majority of patients treated with ruxolitinib experienced improvements in debilitating symptoms such as night sweats, early satiety, and pruritus, with 45.9% experiencing ≥ 50% reduction in total symptom score. In contrast, both splenomegaly and symptoms worsened over time in patients receiving placebo. The findings from MDACC for study INCB18424-251 are also consistent with those from COMFORT-II, in which ruxolitinib treatment resulted in reductions in spleen volume and improvements in quality of life, while those treated with best available therapy had progressive increases in spleen volume and deterioration in quality of life.7 The available follow-up period for participants in COMFORT-I and COMFORT-II for overall survival is shorter than for study INCB18424-251. Study-specific follow-up measures for COMFORT-I allow continued collection of survival data even for patients who discontinue from the study. Although almost all patients originally randomized to placebo have now been crossed over to receive ruxolitinib, the latest outcome update of the study noted a survival advantage for the patients who started therapy on the ruxolitinib arm versus those that were randomized to the placebo arm; 13 ruxolitinib and 24 placebo patients died during the study or during extended follow-up (median follow-up of 52 and 51 weeks, respectively), representing a hazard ratio (95% CI) of 0.499 (0.254, 0.98; P = .0395).17 Overall survival analysis in COMFORT-II is confounded by significant crossover from the best available therapy comparator arm to ruxolitinib and, more importantly, by limited survival follow-up for patients who discontinued participation in the study. Since this trial has a 2:1 randomization scheme, these factors are further exacerbated by a smaller comparator (73 patients). Thus, comparative analyses of overall survival are likely to be difficult to interpret. Collectively, these data support the value of randomized trials specifically designed and powered to evaluate survival benefits with therapy in myelofibrosis.
Although the outcomes described in this analysis mirror the results seen in the phase 3 trials of ruxolitinib, the results are in contrast to those seen in the study INCB18424-251 cohort from Mayo Clinic Rochester. Given the overall similarities in baseline characteristics, but the differences in discontinuation rates and mean total ruxolitinib daily dose observed between the 2 cohorts, we surmise that shorter duration of ruxolitinib therapy and treatment with lower doses may, in large part, explain the outcomes observed and the failure to distinguish a survival difference from the historical control population at the Mayo Clinic Rochester. Thus, these data in comparison to data from the MDACC cohort support the observation that continuing therapy with ruxolitinib at optimal doses contributes to the benefits seen with ruxolitinib including an overall survival benefit.
In summary, our findings demonstrate that long-term ruxolitinib therapy is associated with improved outcomes in patients with myelofibrosis, and that ruxolitinib treatment has the potential to change the clinical course of this disease. The spleen size and symptom reductions achieved with ruxolitinib were sustained with long-term therapy. Furthermore, ruxolitinib treatment was well tolerated as demonstrated by the observed discontinuation rate and reasons for discontinuation. Based on these analyses, there also appears to be a survival advantage for patients who were treated with ruxolitinib, although findings from such comparisons with historical control groups should be interpreted with caution. Further follow-up of this cohort of patients as well as those on the 2 phase 3 trials may provide more data to assess disease-modifying effects of treatment with ruxolitinib. Ultimately, randomized trials specifically designed and powered to evaluate survival benefits with ruxolitinib therapy in myelofibrosis would be welcomed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Studies performed at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, and University of Pavia were supported by grant 1005 from AIRC (Associazione Italiana per la Ricerca sul Cancro) “Special Program Molecular Clinical Oncology 5x1000” to AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative; www.progettoagimm.it).
Authorship
Contribution: S.V. and F.P. conceived the project, collected and analyzed the data, and wrote the manuscript; and all coauthors contributed to the acquisition of patient data, and reviewed, commented on, and approved the final text of the manuscript.
Conflict-of-interest disclosure: S.V., M.C., and F.P. have received research funding from the Incyte Corporation for the conduct of clinical studies. The remaining authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, MD, PhD, Leukemia Department, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 418, Houston, TX 77030; e-mail: sverstov@mdanderson.org.