Abstract
Platelet-associated (PA) IgG autoantibodies play an essential role in primary immune thrombocytopenia (ITP). However, little is known about the epitopes of these Abs. This study aimed to identify critical binding regions for PA anti-αIIbβ3 Abs. Because PA anti-αIIbβ3 Abs bound poorly to mouse αIIbβ3, we created human-mouse chimera constructs. We first examined 76 platelet eluates obtained from patients with primary ITP. Of these, 26 harbored PA anti-αIIbβ3 Abs (34%). Further analysis of 15 patients who provided sufficient materials showed that the epitopes of these Abs were mainly localized in the N-terminal half of the β-propeller domain in αIIb (L1-W235). We could identify 3 main recognition sites in the region; 2 eluates recognized a conformation formed by the W1:1-2 and W2:3-4 loops, 5 recognized W1:2-3, and 4 recognized W3:4-1. The remaining 4 eluates could not be defined by the binding sites. Within these regions, we identified residues critical for binding, including S29 and R32 in W1:1-2; G44 and P45 in W1:2-3; and P135, E136, and R139 in W2:3-4. Of 11 eluates whose recognition sites were identified, 5 clearly showed restricted κ/λ-chain usage. These results suggested that PA anti-αIIbβ3 Abs in primary ITP tended to recognize highly restricted regions of αIIb with clonality.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by thrombocytopenia that results from immune-mediated platelet destruction and reduced platelet production.1-4 In ITP, autoantibodies bound to platelets (platelet-associated antibodies; PA Abs) cause platelet destruction by Fcγ receptor–mediated phagocytosis.1 Furthermore, autoantibodies binding to megakaryocytes led to decreased maturation and cell death.5,6 Multiple targets of autoantibodies have been found in ITP. Among patients with chronic ITP, 43%-57% and 18%-50% harbored PA Abs that recognized the platelet membrane glycoprotein (GP) IIb/IIIa (integrin αIIbβ3) and the GPIb/IX/V complex, respectively.7-10
For more than 2 decades, efforts have been focused on identifying the target epitopes for PA Abs to improve our understanding of the pathogenesis of primary ITP and to pursue a therapeutic approach. We and others previously reported that, in chronic ITP, PA anti-αIIbβ3 Abs frequently bound to cation-dependent conformational antigens and did not react with αvβ3.11-13 Those data suggested that, in primary ITP, the target epitopes of anti-αIIbβ3 Abs may be localized mainly on αIIb; in contrast, in HIV-associated ITP, the target epitopes appeared to be localized to the 49-66 residues of β3.14 Moreover, we previously reported that, in one-third of patients with ITP (11 of 34), PA anti-αIIbβ3 Ab binding was markedly impaired against KO variant αIIbβ3, which had 2 amino acids inserted between residues 160 and 161 in the W3:4-1 loop of the β-propeller domain.15 However, target epitopes of most PA anti-αIIbβ3 Abs remain to be elucidated.
PA anti-αIIbβ3 Abs typically recognize conformational epitopes, rather than linear epitopes.16,17 Therefore, epitope mapping for PA Abs requires the retention of major conformations in αIIbβ3. Because we noticed that the PA anti-αIIbβ3 Abs from patients with ITP had markedly impaired reactivity to mouse αIIbβ3, we characterized target epitopes of PA anti-αIIbβ3 Abs by exploring their reactivity to the cells that expressed human-mouse chimeric αIIbβ3 in the present study. A thorough analysis of 15 eluates obtained from patients with primary ITP found that most of the PA anti-αIIbβ3 Abs recognized the N-terminal half of the β-propeller domain in αIIb. We identified 3 main Ab recognition sites in the region and residues that were critical for the binding of some Abs.
Methods
Patients
We first examined 76 eluates obtained from patients with primary ITP (21 men, 55 women). Diagnosis of primary ITP was based on a report from an international working group.18 We obtained written, informed consent for blood sampling necessary for this study from all patients, in accordance with the Declaration of Helsinki. This study was approved by the Osaka University Institutional Review Board. With the use of flow cytometry with 293T cells expressing αIIbβ3, we detected anti-αIIbβ3 autoantibodies in 26 of 76 (34%) platelet eluates (6 and 20 eluates were obtained from men and women, respectively). Of these 26 eluates, we further analyzed 15 patients who provided sufficient quantity of platelet eluates for this study. The characteristics of these 15 patients (PTs) are shown in Table 1. Although 5 eluates were obtained < 12 months after the ITP diagnosis, all (except PT 36) were classified as chronic ITP.18 PT 7 and PT 12 were studied in our previous report.15 The binding of PA anti-αIIbβ3 Ab of PT 12 was markedly impaired by the KO mutation in αIIb, whereas that of PT 7 was not affected by the mutation.
Platelet isolation and preparation of PA antibodies
Platelets and platelet eluates were prepared as previously described.19 In brief, washed platelets were obtained by differential centrifugation and adjusted to a concentration of 200 × 103/μL in PBS. PA Abs were eluted by vigorous mixing with an equal amount of diethyl ether. Platelet eluates were maintained at −80°C until use.
Construction of expression vectors
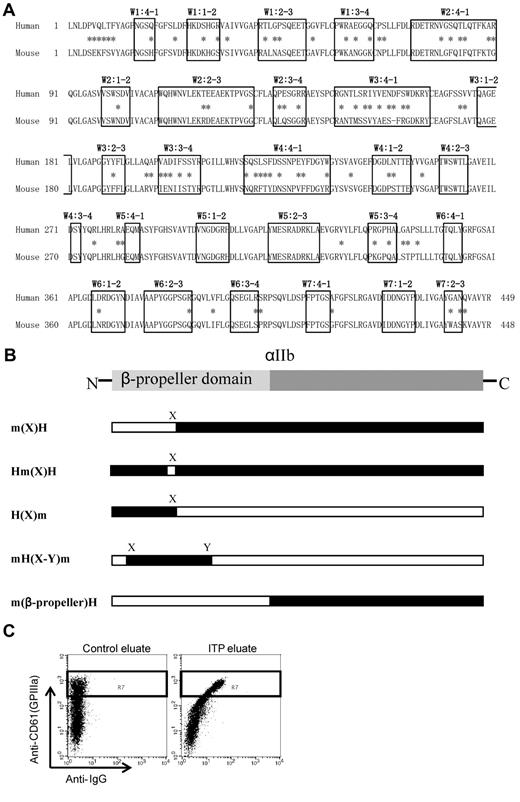
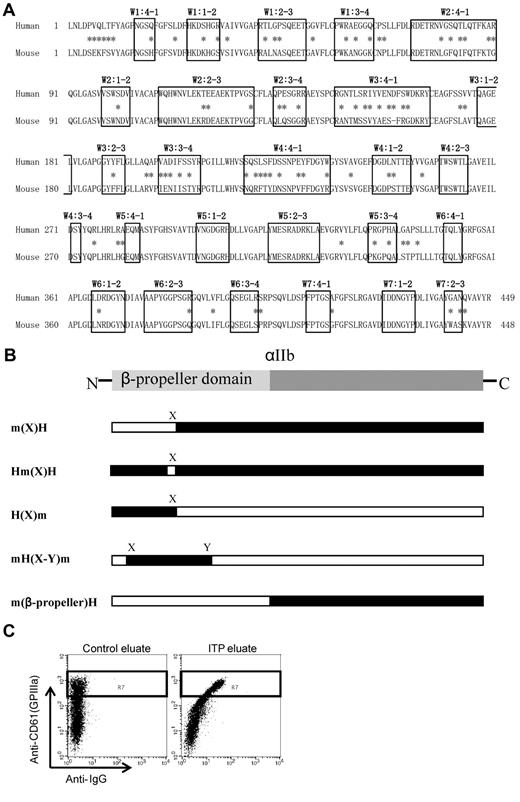
The N-terminal portion of αIIb, known as the β-propeller domain, contains 7 radially arranged β-sheets, termed “W” because of their topology. Each W structure has 4 anti-parallel β-strands and 4 connecting loops.20 Figure 1A shows the human and mouse sequence alignment of the β-propeller domains. The boxes indicate the small loop structures of each β-sheet domain, and the asterisks indicate amino acid differences between the human and mouse sequences. Human αIIb and β3 cDNAs cloned into the pcDNA3 vector were gifts from Dr Peter Newman and Dr Gilbert White (BloodCenter of Wisconsin), respectively. Mouse αIIb and β3 cDNAs were obtained by reverse transcribing platelet RNA from C57BL/6 mice, amplifying by PCR, and subcloning into the pcDNA3 expression vector. αIIb expression vectors with swapped human and mouse cassettes were constructed with the megaprimer PCR method21 or with a site-directed mutagenesis kit (Agilent Technologies). The entire mutated αIIb sequence was confirmed for each vector. The swapping mutants are shown in Figure 1B. The m(X)H expressed the human αIIb that carried the mouse sequences from the N-terminus to the X region; conversely, H(X)m expressed mouse αIIb that carried the human sequences from the N-terminus to the X region. Hm(X)H expressed human αIIb in which the only X region was swapped with the corresponding region from mouse αIIb. mH(X-Y)m expressed mouse αIIb with a large part of the N-terminal region (X to Y) swapped with the corresponding region from human αIIb. m(β-propeller)H expressed the entire β-propeller domain of mouse αIIb, attached to the (C-terminal) remainder of the protein from the human αIIb sequence. The human-mouse chimera plasmids are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Structure and alignment of the β-propeller domain of αIIb. (A) Human and mouse sequence alignment of the β-propeller domain. The boxes indicate the small loop structures of each β-sheet domain, and the asterisks indicate amino acid difference between human and mouse sequences. (B) Abbreviation of each human-mouse chimeric αIIb/human β3. m(X)H; human αIIb (black) carrying mouse sequences (white) from the N-terminus to the X region. Hm(X)H; human αIIb was exchanged with the only X region for the corresponding sequence of mouse αIIb. H(X)m; mouse αIIb carrying human sequences from the N-terminus to the X region. mH(X-Y)m; mouse αIIb was exchanged with the region of X to Y for the corresponding human αIIb. m(β-propeller)H; human αIIb was exchanged with the entire β-propeller domain of αIIb for the corresponding mouse αIIb. (C) Assessment of PA Abs binding to mutated αIIbβ3-expressing cells. Binding of PA Abs (horizontal) was analyzed in a subset of CD61 highly positive cells denoted by the rectangle (R7) in the dot blots.
Structure and alignment of the β-propeller domain of αIIb. (A) Human and mouse sequence alignment of the β-propeller domain. The boxes indicate the small loop structures of each β-sheet domain, and the asterisks indicate amino acid difference between human and mouse sequences. (B) Abbreviation of each human-mouse chimeric αIIb/human β3. m(X)H; human αIIb (black) carrying mouse sequences (white) from the N-terminus to the X region. Hm(X)H; human αIIb was exchanged with the only X region for the corresponding sequence of mouse αIIb. H(X)m; mouse αIIb carrying human sequences from the N-terminus to the X region. mH(X-Y)m; mouse αIIb was exchanged with the region of X to Y for the corresponding human αIIb. m(β-propeller)H; human αIIb was exchanged with the entire β-propeller domain of αIIb for the corresponding mouse αIIb. (C) Assessment of PA Abs binding to mutated αIIbβ3-expressing cells. Binding of PA Abs (horizontal) was analyzed in a subset of CD61 highly positive cells denoted by the rectangle (R7) in the dot blots.
Detection of PA anti-αIIbβ3 Ab binding
αIIb and β3 expression vectors were transiently transfected into 293T cells by lipofection (Lipofectamine2000; Invitrogen). Two days after transfection, the cells were detached from dishes with a solution of 0.02% ethylenediaminetetraacetic acid in PBS (Nakarai tesque); the cells were then washed once with PBS and resuspended at a concentration of 5 × 106/mL in Tyrode buffer with 1mM CaCl2 and 1mM MgCl2. Then, 50-μL aliquots of platelet eluates were incubated with equal amounts of cell suspension for 30 minutes on ice, followed by staining with Alexa Fluor 488–conjugated anti–human IgG (Invitrogen) and phycoerythrin-conjugated anti–human CD61 (Becton Dickinson [BD]). After washing, the cells were resuspended in Tyrode buffer that contained propidium iodine (1 μg/mL) to identify dead cells and then the cells were analyzed on a flow cytometer (FACScan; BD).
PA Ab binding (Alexa 488 staining) was analyzed in a subset of cells that were highly positive for CD61; this subset is denoted by the rectangle (R7) in the dot blots (Figure 1C). We confirmed that CD61+ cells in this region were exclusively complexed with transfected αIIb but not with endogenous αV by staining with anti-αIIbβ3–specific antibodies; an allophycocyanin (APC)–conjugated anti–human CD41a (clone HIP8; BD), an FITC-conjugated anti–human CD41 (clone P2; Beckman Coulter), and an FITC-conjugated anti–mouse CD41 (clone MWReg30; BD). PA Ab IgG binding 293T cells that expressed chimeric αIIb complexed with human β3 (chimera αIIbβ3) relative to the cells that expressed wild-type human αIIbβ3 (wt αIIbβ3) was calculated as follows: specific PA Ab binding to chimera αIIbβ3/specific PA Ab binding to wt αIIbβ3 × 100 (%).
We always used ≥ 2 eluates obtained from healthy subjects as control in each experiment, and specific IgG binding was calculated by subtracting the mean fluorescence intensity (MFI) of IgG binding in control eluates from the MFI of IgG binding in patient eluates. Expressions of the chimera αIIbβ3 and wt αIIbβ3 cells were monitored by anti-CD61 binding, and the specific PA Ab binding was compensated by CD61 expression.
PA Ab binding to platelets was determined as previously described.22
Detection of light chain usage by PA anti-αIIbβ3 Abs
Platelet eluates were incubated with 293T cells that transiently expressed human αIIbβ3. This was followed by incubation with FITC-conjugated anti-κ, PE-conjugated anti-λ Abs (Simultest Anti-Kappa/Anti-Lambda; BD), and APC-conjugated anti-CD61 (Invitrogen). After washing, cells were resuspended in Tyrode buffer that contained propidium iodine. Next, they were analyzed by flow cytometry to evaluate anti-κ and anti-λ Ab binding to cells highly positive for CD61.
Results
Target epitopes of PA anti-αIIbβ3 Abs are mainly localized in the N-terminus of the β-propeller domain in αIIb
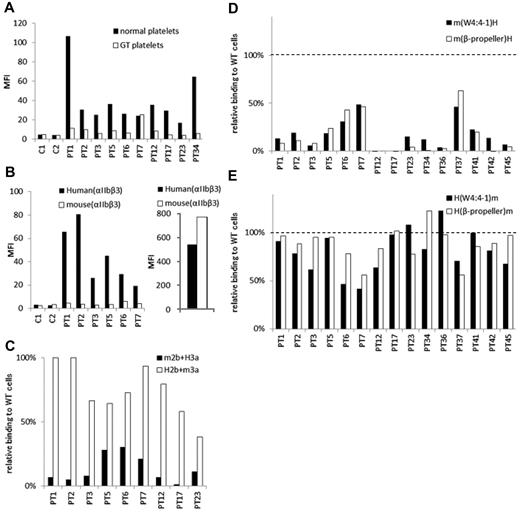
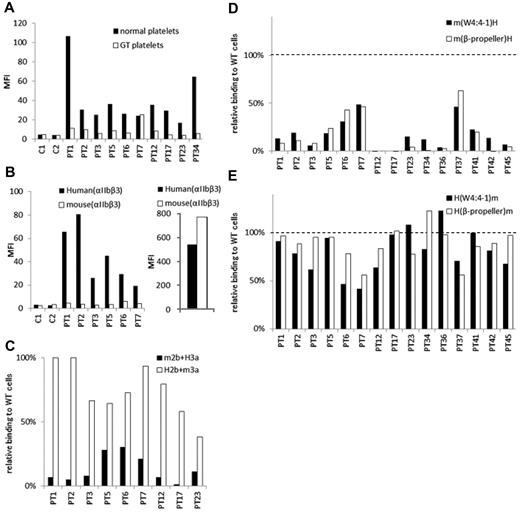
We examined PA Ab reactivity to normal platelets and platelets obtained from a patient with type I Glanzmann thrombasthenia (GT), whose platelets completely lack surface αIIbβ3 expression as we previously reported.23 In eluates from 9 of 10 patients with ITP, the PA Ab reactivity to GT platelets was markedly lower than their reactivity to normal platelets (Figure 2A). The marked reduction in the reactivity to GT platelets was also observed in other 3 eluates (supplemental Figure 1). These results indicated that most PA Abs in these eluates were directed to αIIbβ3. We then examined PA Ab reactivity to 293T cells that expressed mouse αIIbβ3. We found that, in the eluates examined, the PA Abs did not react with mouse αIIbβ3 (Figure 2B). To identify which subunit was essential for the binding, we examined the reactivity to mouse αIIb/human β3 (m2b/H3a) and human αIIb/mouse β3 (H2b/m3a). We found that the PA Abs reacted similarly to cells that expressed H2b/m3a and cells that expressed human αIIbβ3 (38.5%-100%). However, PA Ab reactivity to m2b/H3a was markedly reduced in all 9 eluates examined (1.3%-30.3%; Figure 2C). These results confirmed that the target epitopes of PA anti-αIIbβ3 Abs were mainly present in αIIb.
The epitopes of PA anti-αIIbβ3 Abs are mainly localized in the N-terminus of the β-propeller domain of αIIb. (A) Binding of PA Abs to normal (black) or αIIbβ3-deficient platelets (GT platelets; white). C1 and C2 show control eluates. (B) Binding of PA Abs to 293T cells that expressed human (black) or mouse αIIbβ3 (white; left). Expression levels of human and mouse αIIbβ3 were indicated in the right panel monitored by anti-CD61 Ab. (C) Relative binding of PA Abs to 293T cells that expressed mouse αIIb/human β3 (m2b/H3a; black) or human αIIb/mouse β3 (H2b/m3a; white) compared with human αIIbβ3-expressing cells. (D) Relative binding of PA Abs to 293T cells that expressed human αIIb replaced with whole β-propeller domain (white) or the N-terminus to W4:4-1 loop (black) of mouse αIIb with human β3. (E) Relative binding of PA Abs to 293T cells that expressed mouse αIIb replaced with whole β-propeller domain (white) or the N-terminus to W4:4-1 loop (black) of human αIIb with human β3. Shown were means of ≥ 2 independent experiments.
The epitopes of PA anti-αIIbβ3 Abs are mainly localized in the N-terminus of the β-propeller domain of αIIb. (A) Binding of PA Abs to normal (black) or αIIbβ3-deficient platelets (GT platelets; white). C1 and C2 show control eluates. (B) Binding of PA Abs to 293T cells that expressed human (black) or mouse αIIbβ3 (white; left). Expression levels of human and mouse αIIbβ3 were indicated in the right panel monitored by anti-CD61 Ab. (C) Relative binding of PA Abs to 293T cells that expressed mouse αIIb/human β3 (m2b/H3a; black) or human αIIb/mouse β3 (H2b/m3a; white) compared with human αIIbβ3-expressing cells. (D) Relative binding of PA Abs to 293T cells that expressed human αIIb replaced with whole β-propeller domain (white) or the N-terminus to W4:4-1 loop (black) of mouse αIIb with human β3. (E) Relative binding of PA Abs to 293T cells that expressed mouse αIIb replaced with whole β-propeller domain (white) or the N-terminus to W4:4-1 loop (black) of human αIIb with human β3. Shown were means of ≥ 2 independent experiments.
We next examined PA anti-αIIbβ3 Ab reactivity with several αIIb chimeras. All αIIb chimeras were expressed with the human β3 on 293T cells. The m(β-propeller)H and m(W4:4-1)H chimeras comprised the human αIIb with substitutions for the mouse β-propeller domain (L1-R449) and the N-terminal half of the β-propeller domain (L1-W235), respectively, because there are few amino acid differences between human and mouse in the C-terminal half of the β-propeller domain (Figure 1B). Compared with wt αIIbβ3, all 15 of the eluates showed markedly impaired binding to cells that expressed the m(β-propeller)H and those that expressed the m(W4:4-1)H chimera (Figure 2D). These results were confirmed by the antigen capture ELISA method in selected patients (supplemental Figure 2). In sharp contrast, most of the 15 eluates tested showed high PA Ab reactivity with the converse αIIb chimeras, H(β-propeller)m and H(W4:4-1)m, complexed with human β3. The PA Ab reactivity was comparable with that observed with wt human αIIbβ3 (Figure 2E). These results indicated that the epitopes of PA anti-αIIbβ3 Abs were mainly localized to the N-terminal half of the β-propeller domain (L1-W235) in αIIb.
Identification of 3 main recognition sites in the N-terminal half of the β-propeller domain in αIIb
Next, we aimed to identify critical target epitopes for PA anti-αIIbβ3 Abs in the N-terminal half of the β-propeller domain in αIIb. For this, we created human-mouse αIIb chimeras that had serial changes in which small sections of human αIIb were exchanged with the corresponding mouse sequences. The series extended from the N-terminus to the W4:4-1 loop (Table 2). We noticed that the reactivity of many PA Abs decreased markedly when a specific loop from human was exchanged for the corresponding mouse sequence. Consequently, we identified 3 groups (A, B, and C) on the basis of loops that appeared to be essential for PA Ab reactivity: the W1:1-2, W1:2-3, and W3:4-1 loops (Table 2). Then, we further characterized the autoantigenic epitopes in each group.
Group A: the W1:1-2 and W2:3-4 loops are essential for PA anti-αIIbβ3Ab binding.
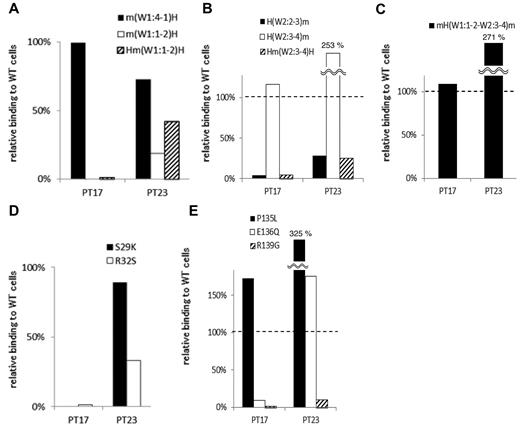
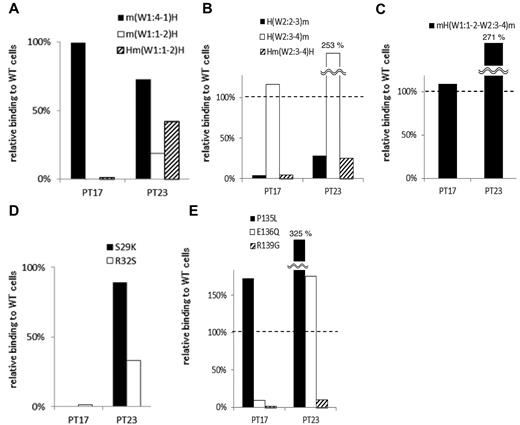
In 2 samples (from PTs 17 and 23), the PA Ab reactivity with m(W1:1-2)H, the human αIIb that carried the mouse sequence from the N-terminus to the W1:1-2 loop, was markedly impaired compared with PA Ab reactivity with m(W1:4-1)H (Table 2; Figure 3A). Moreover, these PA Abs showed impaired binding with Hm(W1:1-2)H, the human αIIb that carried only the mouse W1:1-2 loop (Figure 3A). These results suggested that the W1:1-2 loop was essential for PA Ab reactivity in these patients. Conversely, we tested whether we could restore PA Ab reactivity with the mouse αIIb sequence by replacing sections with a series of corresponding human sequences from the N-terminus. We found that PA Ab reactivity in these 2 samples was only restored when the mouse W2:3-4 loop was exchanged with the corresponding human sequence. This was confirmed by the observation that the PA Ab reactivity was markedly impaired for Hm(W2:3-4)H, the human αIIb that carried only the mouse W2:3-4 loop (Figure 3B). These results indicated that both the W1:1-2 and the W2:3-4 loops were essential for PA Ab reactivity. Moreover, the PA Ab reactivity with mH(W1:1-2–W2:3-4)m, the mouse αIIb that carried the human W1:1-2 to W2:3-4 sequence, was not reduced compared with the reactivity with the wt human αIIb. This supported our hypothesis that these PA Abs specifically recognized the W1:1-2 and W2:3-4 loops (Figure 3C).
Group A: the W1:1-2 and W2:3-4 loops are essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs in 2 patients (PTs 17 and 23) to m(W1:4-1)H (black), m(W1:1-2)H (white), and Hm(W1:1-2)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to H(W2:2-3)m (black), H(W2:3-4)m (white), and Hm(W2:3-4)H (shaded). (C) Relative binding of PA Abs to mH(W1:1-2–W2:3-4)m that the mouse αIIb carried the human W1:1-2 to W2:3-4 sequences. (D) Relative binding of PA Abs to S29K (black) and R32S (white) mutants. (E) Relative binding of PA Abs to P135L (black), E136Q (white) and R139G (shaded) mutants. Shown were means of ≥ 2 independent experiments.
Group A: the W1:1-2 and W2:3-4 loops are essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs in 2 patients (PTs 17 and 23) to m(W1:4-1)H (black), m(W1:1-2)H (white), and Hm(W1:1-2)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to H(W2:2-3)m (black), H(W2:3-4)m (white), and Hm(W2:3-4)H (shaded). (C) Relative binding of PA Abs to mH(W1:1-2–W2:3-4)m that the mouse αIIb carried the human W1:1-2 to W2:3-4 sequences. (D) Relative binding of PA Abs to S29K (black) and R32S (white) mutants. (E) Relative binding of PA Abs to P135L (black), E136Q (white) and R139G (shaded) mutants. Shown were means of ≥ 2 independent experiments.
The W1:1-2 and W2:3-4 loops harbor 2 and 3 amino acid differences between human and mouse, respectively. Therefore, we replaced each of these amino acids in the human sequence with that in the mouse sequence. The S29K and R32S mutations in the W1:1-2 loop and the E136Q and R139G mutations in the W2:3-4 loop almost completely abolished the reactivity of PA Abs from PT 17. The PA Abs from PT 23 also showed markedly impaired reactivity with the R32S and R139G mutations (Figure 3D-E). These results indicated that the S29, R32, E136, and R139 residues in αIIb, particularly the 2 arginines, were critical for the binding of PA Abs in these patients.
Group B: the W1:2-3 loop is essential for PA anti-αIIbβ3Ab binding.
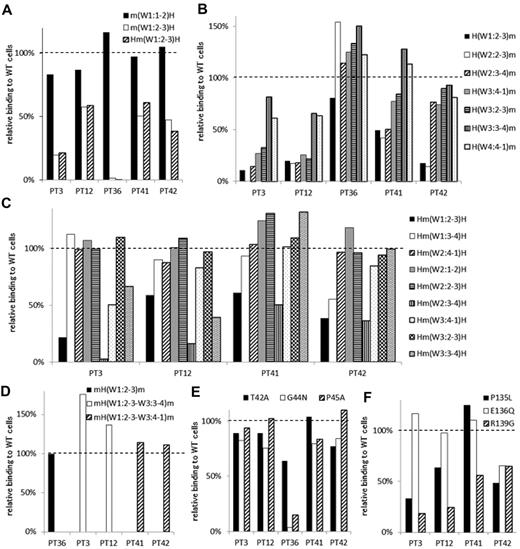
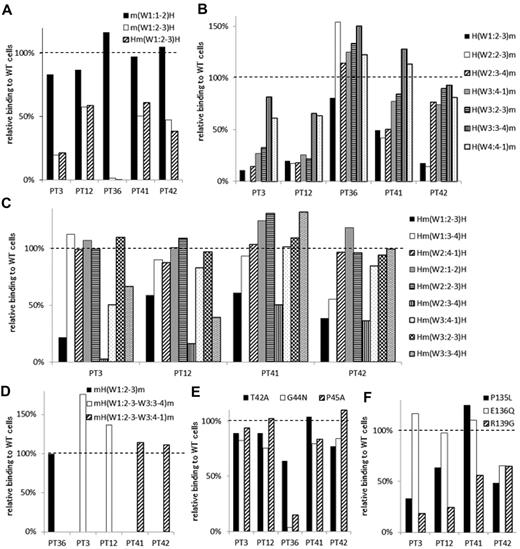
In 5 samples (from PTs 3, 12, 36, 41, and 42), the PA Ab reactivity was impaired with m(W1:2-3)H compared with m(W1:1-2)H (Table 2; Figure 4A). The sample from PT 36 showed complete loss of PA Ab reactivity with m(W1:2-3)H. The reactivity with Hm(W1:2-3)H was essentially the same as the reactivity with m(W1:2-3)H (Figure 4A). These results suggested that the W1:2-3 loop was essential for PA Ab reactivity in these samples. Again, we tested whether we could restore PA Ab reactivity with the mouse αIIb sequence by replacing sections with a series of corresponding human sequences from the N-terminus (Figure 4B). We found that the PA Ab reactivity in the sample from PT 36 was almost fully restored with H(W1:2-3)m, which had the human amino acid sequence from the N-terminus to the W1:2-3 loop. The sample reactivity from PT 36 with mH(W1:2-3)m was the same as that observed with the wt human αIIbβ3 sequence (Figure 4D). These results suggested that the W1:2-3 loop contained an epitope(s) for PA anti-αIIbβ3 Abs in the sample from PT 36.
Group B: the W1:2-3 loop is essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs in 5 patients (PTs 3, 12, 36, 41 and 42) to m(W1:1-2)H (black), m(W1:2-3)H (white), and Hm(W1:2-3)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to mouse αIIb replaced from the N-terminus to the indicated loops with the corresponding human sequences. (C) Relative binding of PA Abs to human αIIb replaced the indicated loop with the corresponding mouse sequences. (D) Relative binding of PA Abs to mH(W1:2-3)m (black), mH(W1:2-3–W3:3-4)m (white), and mH(W1:2-3–W3:4-1)m (shaded). (E) Relative binding of PA Abs to T42A (black), G44N (white), and P45A (shaded) mutants. (F) Relative binding of PA Abs to P135L (black), E136G (white), and R139G (shaded) mutants. Shown were means of ≥ 2 independent experiments.
Group B: the W1:2-3 loop is essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs in 5 patients (PTs 3, 12, 36, 41 and 42) to m(W1:1-2)H (black), m(W1:2-3)H (white), and Hm(W1:2-3)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to mouse αIIb replaced from the N-terminus to the indicated loops with the corresponding human sequences. (C) Relative binding of PA Abs to human αIIb replaced the indicated loop with the corresponding mouse sequences. (D) Relative binding of PA Abs to mH(W1:2-3)m (black), mH(W1:2-3–W3:3-4)m (white), and mH(W1:2-3–W3:4-1)m (shaded). (E) Relative binding of PA Abs to T42A (black), G44N (white), and P45A (shaded) mutants. (F) Relative binding of PA Abs to P135L (black), E136G (white), and R139G (shaded) mutants. Shown were means of ≥ 2 independent experiments.
In contrast, the PA Abs in the samples from PTs 3 and 12 showed restored reactivities with H(W3:3-4)m, and the samples from PT 41 and PT 42 showed restored reactivities with H(W3:4-1)m and H(W2:3-4)m, respectively (Figure 4B). These results suggested that loop(s) other than the W1:2-3 may be important for PA Ab reactivity in these patients. To localize the epitope(s) important for PA Ab reactivity in these patients, we constructed human αIIb expression vectors in which each surface loop from W1:2-3 to W3:3-4 was replaced with the corresponding mouse sequence (Figure 4C). We found that the PA Abs from these 4 patients showed impaired reactivity with Hm(W1:2-3)H and Hm(W2:3-4)H. This indicated that both the W1:2-3 and the W2:3-4 loops were involved in PA Ab binding efficiency. In addition, the W3:3-4 loop also appeared to be involved in PA Ab reactivity in samples from PTs 3 and 12 (Figure 4C). Furthermore, PA Ab binding was fully restored to levels observed with wt αIIb, when the samples from PTs 3 and 12 reacted with mH(W1:2-3-W3:3-4)m and the samples from PTs 41 and 42 reacted with mH(W1:2-3-W3:4-1)m (Figure 4D). These results suggested that the PA Abs from PTs 3 and 12 mainly recognized the W1:2-3, W2:3-4, and W3:3-4 loops and that the PA Abs from PTs 41 and 42 recognized the W1:2-3 and W2:3-4 loops.
There are 3 amino acid differences between human and mouse in each W1:2-3 and W2:3-4 loop. These are the T42A, G44N, and P45A in the W1:2-3 loop and P135L, E136Q, and R139G in the W2:3-4 loop. Again, we examined the effects of substituting these single amino acids on PA Ab reactivity. We found that the PA Ab reactivity in the sample from PT 36 remained markedly impaired with either the G44N or the P45A mutation in the W1:2-3 loop. However, no mutation in the W1:2-3 loop significantly impaired the PA Ab reactivities in samples from the other 4 patients (Figure 4E). In contrast, the R139G mutation in the W2:3-4 loop impaired reactivity in the samples from all 4 of these patients. In addition, samples from PTs 3 and 12 showed a similarly impaired pattern in the PA Ab reactivities with the P135L, E136Q, and R139G mutations (Figure 4F).
Group C: the W3:4-1 loop is essential for PA anti-αIIbβ3 Ab binding.
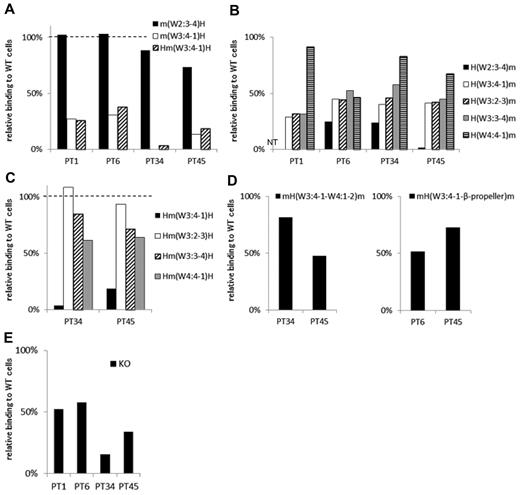
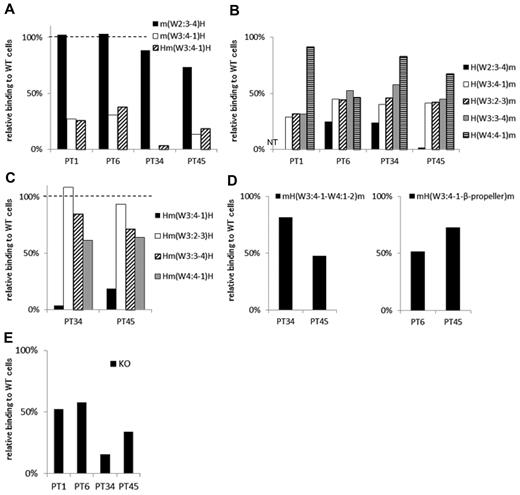
In 4 samples (from PTs 1, 6, 34, and 45) the PA Ab reactivity was markedly impaired with m(W3:4-1)H compared with m(W2:3-4)H. In addition, the PA Ab reactivity was similarly impaired with Hm(W3:4-1)H (Table 2; Figure 5A). These results suggested that the W3:4-1 loop was essential for PA Ab reactivity in these samples. Interestingly, in samples from PTs 1, 34, and 45, the PA Abs showed nearly fully restored reactivity with H(W4:4-1)m (Figure 5B). This suggested that the W4:4-1 loop may also affect PA Ab reactivity. In fact, in PTs 34 and 45, the PA Ab reactivity was markedly impaired with Hm(W3:4-1)H and moderately impaired with Hm(W4:4-1)H (Figure 5C). The sample from PT 1 was not tested because of insufficient sample. The sample from PT 34 showed PA Ab reactivity with mH(W3:4-1–W4:1-2)m that was nearly comparable with its reactivity with wt αIIb (Figure 5D). This supported the notion that both W3:4-1 and W4:4-1 loops were important for PA Ab reactivity in this patient. In contrast, in the sample from PT 45, PA Ab reactivity was not restored with mH(W3:4-1–W4:1-2)m (Figure 5D). Finally, we tested the sample from PT 45 with the mouse αIIb that carried the human sequence from W3:4-1 to the C-terminal of the β-propeller domain, mH(W3:4-1–β-propeller)m. We found that the sample from PT 45 showed reactivity with the mH(W3:4-1–β-propeller)m comparable with reactivity with the wt αIIb. This suggested that the C-terminal half of the β-propeller domain may also contribute to PA Ab binding in the sample from PT 45 (Figure 5D). In the sample from PT 6, PA Ab reactivity was not fully restored with H(W4:4-1)m but was almost fully restored with H(β-propeller)m (Figures 5B and 2E). However, the PA Ab reactivity was impaired with mH(W3:4-1–β-propeller)m (Figure 5D). These results suggested that the N-terminus and the C-terminal portion of the β-propeller domain were important for the PA Ab reactivity in the sample from PT 6.
Group C: the W3:4-1 loop is essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs to m(W2:3-4)H (black), m(W3:4-1)H (white), and Hm(W3:4-1)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to mouse αIIb replaced from the N-terminus to the indicated loops with the human corresponding sequences. (C) Relative binding of PA Abs to human αIIb replaced the indicated loop with the mouse corresponding sequences. (D) Relative binding of PA Abs to mH(W3:4-1–W4:1-2)m and mH(W3:4-1–β-propeller)m that the mouse αIIb carried the human sequences from W3:4-1 to the C-terminal of the β-propeller domain. (E) Relative binding of PA Abs to 2 amino acids insertion mutant in W3:4-1 loop (KO variant αIIbβ3). Shown were means of ≥ 2 independent experiments.
Group C: the W3:4-1 loop is essential for PA anti-αIIbβ3 Ab binding. (A) Relative binding of PA Abs to m(W2:3-4)H (black), m(W3:4-1)H (white), and Hm(W3:4-1)H (shaded) compared with wt αIIbβ3. (B) Relative binding of PA Abs to mouse αIIb replaced from the N-terminus to the indicated loops with the human corresponding sequences. (C) Relative binding of PA Abs to human αIIb replaced the indicated loop with the mouse corresponding sequences. (D) Relative binding of PA Abs to mH(W3:4-1–W4:1-2)m and mH(W3:4-1–β-propeller)m that the mouse αIIb carried the human sequences from W3:4-1 to the C-terminal of the β-propeller domain. (E) Relative binding of PA Abs to 2 amino acids insertion mutant in W3:4-1 loop (KO variant αIIbβ3). Shown were means of ≥ 2 independent experiments.
There are many amino acid differences between human and mouse in the W3:4-1 and W4:4-1 loops (Figure 1A). Therefore, it was difficult to identify critical residues in these regions. However, we previously studied a KO variant of αIIbβ3 with a 2-amino acid (Arg-Thr) insertion between F160 and S161 in the W3:4-1 loop. In that study, we found that this insertion affected PA Ab reactivity as well as ligand binding capacity.15 In the present study, samples from all 4 patients of group C showed impaired PA Ab reactivity with this mutation (KO; Figure 5E).
Samples from the remaining 4 patients (PTs 2, 5, 7, and 37) could not be classified in the 3 groups described. However, all 4 had PA anti-αIIbβ3 Abs that mainly recognized the N-terminal half of the β-propeller domain of αIIb. In these patients, we did not detect any unique characteristics that might identify an epitope on any specific loop(s).
Light chain–restricted usage of PA anti-αIIbβ3 Abs
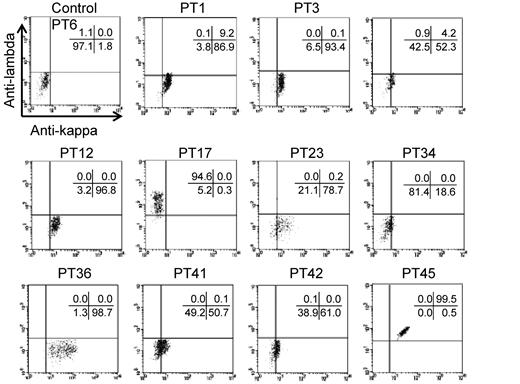
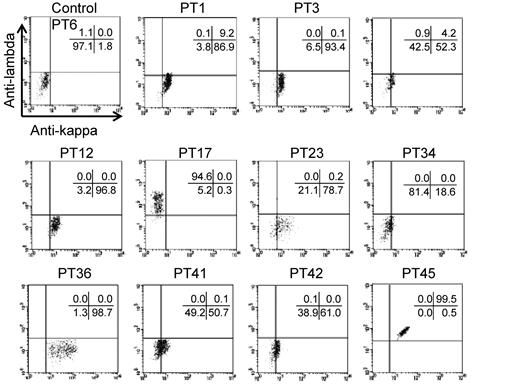
Our findings indicated that autoantigenic epitopes may be located on highly restricted regions of αIIb, which also suggested that many PA anti-αIIbβ3 Abs might exhibit clonality. Therefore, we next determined whether PA Abs exhibited restricted κ/λ light chain usage in the 11 eluates that had been classified in 1 of the 3 groups described in the previous paragraphs. We determined that samples from PTs 1, 3, 12, and 36 clearly showed restricted κ-chain usage, and the sample from PT 17 showed restricted λ-chain usage. In contrast, the PA Abs in samples from PT 45 were polyclonal. The samples from the remaining 5 patients also showed κ-chain preference, although the positivity was weak (Figure 6). These results suggested that the PA anti-αIIbβ3 Abs were clonal in many patients with primary ITP.
Light-chain usage of PA anti-αIIbβ3 Abs. Platelet eluates were reacted with wt αIIbβ3-expressing 293T cells, followed by the incubation with FITC–anti-κ, PE–anti-λ, and APC–anti-CD61 Abs. Anti-κ (horizontal) and anti-λ (vertical) Abs bindings were analyzed in a subset of cells that were highly positive for CD61. Representative results of ≥ 2 independent experiments are shown.
Light-chain usage of PA anti-αIIbβ3 Abs. Platelet eluates were reacted with wt αIIbβ3-expressing 293T cells, followed by the incubation with FITC–anti-κ, PE–anti-λ, and APC–anti-CD61 Abs. Anti-κ (horizontal) and anti-λ (vertical) Abs bindings were analyzed in a subset of cells that were highly positive for CD61. Representative results of ≥ 2 independent experiments are shown.
Discussion
Previous reports have found the importance of the β-propeller domain in αIIb, particularly the W3:4-1 loop, for PA anti-αIIbβ3Ab binding in patients with chronic ITP.13,15 In this study, we found that samples from 15 patients with primary ITP harbored PA anti-αIIbβ3 Abs that mainly recognized the N-terminal half of the β-propeller domain (L1-W235) of αIIb. A systematic examination with human-mouse αIIb chimeras found 3 main recognition sites: (1) a conformational epitope composed of W1:1-2 and W2:3-4 loops, (2) a region containing the W1:2-3 loop, and (3) a region containing the W3:4-1 loop. We further identified some single residues in these loops that were critical for PA Ab reactivity. Moreover, PA anti-αIIbβ3 Abs in many patients showed restricted κ/λ light chain usage. Our findings indicated that major epitopes of PA anti-αIIbβ3 Abs were localized in highly restricted regions in the β-propeller domain of αIIb and that PA anti-αIIbβ3 Abs may be monoclonal or oligoclonal in many patients with ITP.
It was surprising that PA anti-αIIbβ3 Abs did not bind to mouse αIIbβ3. αIIb and β3 showed 82% and 85% nucleotide sequence homology, respectively, between human and mouse. However, one report suggested that anti–human platelet Abs produced from splenocytes obtained from patients with ITP exhibited low cross-reactivity with mouse platelets.24 Another report showed that an anti–human αIIb antibody generated in mice exhibited significantly diminished binding to ITP platelets compared with normal platelets.25 Those results suggested that the reactivity of PA anti-αIIbβ3 Abs may be affected by subtle conformational differences between human and mouse αIIbβ3.
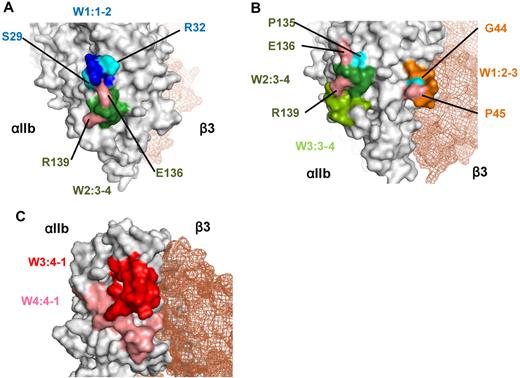
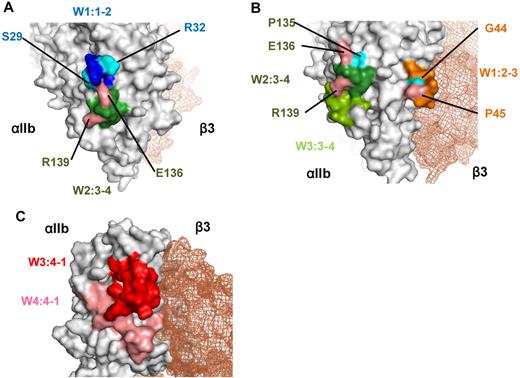
Two patients of group A had PA Abs that recognized the W1:1-2 and W2:3-4 loops. Although these 2 loops are separated by ∼ 100 amino acids in the primary αIIb sequence, the crystal structure showed that the W1:1-2 loop was close to the W2:3-4 loop on the lower face of the β-propeller domain (Figure 7A). Furthermore, the binding of PA Abs from PT 17 to wt αIIbβ3 could not be inhibited by linear peptides that corresponded to W1:1-2, W2:3-4, or a mixture of these peptides; this also suggested that the PA Abs recognized a conformational epitope composed of these 2 loops (data not shown). Moreover, the reactivity of Abs was highly affected by single amino acid substitutions (S29K, R32S, E136Q, and R139G) in the W1:1-2 and W2:3-4 loops (Figure 3D-E). Because arginine (R), lysine (K), and glutamic acid (E) are charged amino acids, these substitutions may affect ionic bonds between αIIb and the complementarity determining regions of the PA Abs.
Crystal structure of the recognition sites for the PA Abs. (A) Crystal structure of the recognition sites of group A made by PyMOL Version 1.4 software (DeLano Scientific LLC). W1:1-2 (blue) and W2:3-4 (green) loops and 4 critical residues (S29, R32, E136, R139) for the binding of PA Abs were indicated. (B) Crystal structure of the recognition sites of group B. W1:2-3 loop (orange) is located in the upper surface of αIIbβ3 interface, and W2:3-4 and W3:3-4 loops (green) are in the lower surface. Five critical residues (G44, P45, P135, E136, R139) for the binding of PA Abs were also indicated. (C) Crystal structure of the recognition sites of group C. W3:4-1 (red) and W4:4-1 (pink) loops were indicated. Both loops are located in the upper surface of αIIbβ3 interface, which is near the ligand binding site.
Crystal structure of the recognition sites for the PA Abs. (A) Crystal structure of the recognition sites of group A made by PyMOL Version 1.4 software (DeLano Scientific LLC). W1:1-2 (blue) and W2:3-4 (green) loops and 4 critical residues (S29, R32, E136, R139) for the binding of PA Abs were indicated. (B) Crystal structure of the recognition sites of group B. W1:2-3 loop (orange) is located in the upper surface of αIIbβ3 interface, and W2:3-4 and W3:3-4 loops (green) are in the lower surface. Five critical residues (G44, P45, P135, E136, R139) for the binding of PA Abs were also indicated. (C) Crystal structure of the recognition sites of group C. W3:4-1 (red) and W4:4-1 (pink) loops were indicated. Both loops are located in the upper surface of αIIbβ3 interface, which is near the ligand binding site.
We found that the W1:2-3 loop was critical for the reactivity of PA Abs in 5 patients of group B. However, when epitopes of the mouse αIIb were swapped with the corresponding human N-terminus sequences, recovery of PA Ab binding was heterogeneous among the patient samples (Figure 4B). In particular, the PA Abs from PT 36 appeared to exclusively recognize the W1:2-3 loop, and we further found that the PA Ab reactivity was markedly impaired with a single G44N or P45A substitution in the loop (Figure 4E). Because asparagine (N) has an amino group and proline (P) has a cyclic structure, the presence or absence of these amino acids may have highly affected hydrogen bonding between the Abs in PT 36 and αIIb, and/or they may have disrupted the conformation of the W1:2-3 loop. In addition, swapping human and mouse loop sequences showed that W2:3-4 loop was also important for the reactivity of PA Abs in the eluates of the remaining 4 patients. Finally, the W3:3-4 loop appeared to contribute to PA Ab binding in the eluates from 2 patients, PTs 3 and 12 (Figure 4C). Interestingly, the R139G mutation in the W2:3-4 loop had a profound effect on PA Ab binding in the eluates of the 4 patients in group B and the 2 patients in group A (Figures 3E and 4F). This indicated that R139 may be a critical epitope for many PA Abs. The crystal structure of αIIb showed that the W1:2-3 loop was located on the upper face, and the W2:3-4 and W3:3-4 loops were on the lower face of the β-propeller domain (Figure 7B). Although we could not rule out the possibility that PA Abs may be polyclonal and recognize different epitopes, we hypothesized that PA Abs recognize conformational epitope(s) composed of these multiple loops, based on our findings that PA Ab binding was markedly impaired with a single loop substitution. The crystal structure showed that the W1:2-3 and W2:3-4/W3:3-4 loops were located ∼ 30 Å apart; this circumscribes an area consistent with the typical contact areas between protein antigens and their cognate Abs.26,27 The 3-dimensional structure suggested that these loops formed a relatively flat surface (Figure 7B), also consistent with the observation that Abs typically interact with protein antigens on relatively flat complementarity determining regions.28
In the 4 patients of group C, we confirmed our previous finding that the W3:4-1 loop was one of the main target epitopes for PA anti-αIIbβ3 Abs. The PA Abs from these 4 eluates showed impaired reactivity with the KO variant αIIbβ3.20,29 We also showed that the W4:4-1 loop was important for the binding of 3 of 4 sample eluates. Again, the 3-dimensional structure (Figure 7C) indicated that the W3:4-1 loop was immediately adjacent to the W4:4-1 loop on the upper face of the β-propeller domain; this suggested that the PA Abs recognized an epitope composed of these 2 loops. Moreover, the C-terminal half of the β-propeller domain was important for efficient binding of the PA Abs in the samples from PTs 6 and 45. This suggested that the C-terminal region might maintain the proper conformation of the W3:4-1 loop region for the binding of PA Abs.
Our findings that many PA anti-αIIbβ3 Abs in patients with ITP recognized restricted regions of the β-propeller domain in αIIb have some interesting implications. The cause of primary ITP remains obscure; however, it has been suggested that molecular mimicry may trigger an immune response against platelet antigens in some secondary forms of ITP.14,30 A recent study reported that many bacterial proteins contained the human integrin-type β-propeller domain31 ; this suggested that conformational mimicry between these bacterial proteins and the β-propeller domain in αIIb might be involved in the production of PA anti-αIIbβ3 Abs. Consistent with other reports,32,33 we observed that many of the PA anti-αIIbβ3 Abs exhibited restricted κ/λ light chain usage, which further suggested that PA Abs might arise from an antigen-derived clonal expansion rather than from polyclonal B-cell activation triggered by nonspecific stimuli.
Of note, all 4 eluates that did not show clear epitopes (PTs 2, 5, 7, and 37) were collected > 6 years after the diagnosis; in contrast, all 5 eluates collected within 1 year after diagnosis were categorized as group B or C (PTs 1, 6, 34, 36, and 45; Table 1). These results suggested that restricted epitopes tended to be more common in the early stages of ITP and that epitopes may spread out in the later, chronic phase of the disease, although in some cases, such as PTs 17 and 23, highly specific epitopes were identified in patients with a long ITP history. This time dependence was also found in other autoimmune diseases.34,35 In this context, it is intriguing that PA Abs which were categorized as group C were only found in patients with a diagnosis made < 1 year ago in this study. In fact, the PA Abs from PT 12, which showed highly impaired reactivity with the substituted epitopes in the KO mutation in our previous study (PT 6 [patient no. 6] in the previous study15 ), appeared to react with extended or changed epitopes in the W1:2-3, W2:3-4, and W3:3-4 loops in the present study, with eluates collected > 10 years later. These results suggest that W3:4-1 loop may be the target epitope of the early phase of ITP. Clearly, concrete evidence of epitope spreading will require long-term studies of patients with ITP.
This study had several limitations. First, we used human-mouse αIIb chimeras for epitope mapping; thus, we could not evaluate whether identical residues between human and mouse were significant. Second, there was an inevitable bias for patient and sample selection, because patients with severe thrombocytopenia could not provide sufficient platelet eluates for the study. Finally, we analyzed only PA anti-αIIbβ3 Abs; thus, we could not rule out the possibility that PA Abs that recognized other GPs (such as GPIb/IX/V) might play a role in the pathogenesis of ITP in our patients.
In summary, we have shown that PA anti-αIIbβ3 Abs tend to recognize highly restricted regions in the N-terminal half of the β-propeller domain of αIIb with clonality. These results may contribute to a better understanding of the pathogenesis of chronic ITP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Junichi Takagi (Institute for Protein Research, Osaka University) for assisting with the structural analysis of αIIbβ3.
This work was supported in part by a Grant in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan; by the Ministry of Health, Labor, and Welfare in Japan; and by a grant from the SENSHIN Medical Research Foundation.
Authorship
Contribution: K.K., H.K., and Y.T. designed the study; K.K. performed most of the experiments and wrote the paper; H.K. and Y.T. edited the paper; T.N. and S.T. performed the transfection studies; and S.H. and Y.K. supervised several aspects of the projects and helped with manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hirokazu Kashiwagi, Department of Hematology and Oncology, Osaka University Graduate School of Medicine, 2-2 Yamada-oka Suita, Osaka565-0871, Japan, e-mail: kashi@hp-blood.med.osaka-u.ac.jp.