Abstract
The Spanish Myeloma Group conducted a trial to compare bortezomib/thalidomide/dexamethasone (VTD) versus thalidomide/dexamethasone (TD) versus vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, doxorubicin, dexamethasone/bortezomib (VBMCP/VBAD/B) in patients aged 65 years or younger with multiple myeloma. The primary endpoint was complete response (CR) rate postinduction and post–autologous stem cell transplantation (ASCT). Three hundred eighty-six patients were allocated to VTD (130), TD (127), or VBMCP/VBAD/B (129). The CR rate was significantly higher with VTD than with TD (35% vs 14%, P = .001) or with VBMCP/VBAD/B (35% vs 21%, P = .01). The median progression-free survival (PFS) was significantly longer with VTD (56.2 vs 28.2 vs 35.5 months, P = .01). In an intention-to-treat analysis, the post-ASCT CR rate was higher with VTD than with TD (46% vs 24%, P = .004) or with VBMCP/VBAD/B (46% vs 38%, P = .1). Patients with high-risk cytogenetics had a shorter PFS and overall survival in the overall series and in all treatment groups. In conclusion, VTD resulted in a higher pre- and posttransplantation CR rate and in a significantly longer PFS although it was not able to overcome the poor prognosis of high-risk cytogenetics. Our results support the use of VTD as a highly effective induction regimen prior to ASCT. The study was registered with http://www.clinicaltrials.gov (NCT00461747) and Eudra CT (no. 2005-001110-41).
Introduction
Autologous stem cell transplantation (ASCT) is an essential part of the treatment of young patients with multiple myeloma (MM).1–4 Indeed, MM is the most frequent indication for ASCT in both Europe and the United States.4 The level of tumor burden reduction after ASCT, particularly the achievement of complete response (CR) or at least very good partial response (VGPR), has emerged as the most important factor associated with a prolonged progression-free survival (PFS) and overall survival (OS).5–8 Furthermore, the sensitivity to the initial chemotherapy, measured by the M-protein reduction at the time of transplantation, is the most important predictor of residual disease after ASCT.7,9,10 Regarding induction therapy, both a meta-analysis on trials using induction with conventional chemotherapy11 and a recent study using bortezomib12 have shown a strong association between maximal response at the time of transplantation and long-term outcome. As a consequence, the choice of the induction therapy is crucial for survival after transplantation. In this regard, the availability of novel antimyeloma drugs, particularly thalidomide and bortezomib, has provided the frame for improving the results of the pretransplantation induction therapy. Thus, the combination of thalidomide and dexamethasone (TD) was superior to vicristine, adriamycin, dexamethasone (VAD)13,14 and its use was approved by the US Food and Drug Administration (FDA) as a pretransplantation induction regimen. The association of bortezomib and dexamethasone (VD) has also been superior to VAD15 and has become a frequently used induction regimen before ASCT.1–4 However, the improvement achieved with TD or with VD over VAD or other chemotherapy regimens in terms of response rate and PFS has been modest. In more recent trials, thalidomide and bortezomib are used in the so-called triple combinations, the most common being thalidomide/adriamycin/dexamethasone (TAD),16 cyclophosphamide/thalidomide/dexamethasone (CTD),17 bortezomib/adriamycin/dexamethasone (PAD),18,19 and bortezomib/thalidomide/dexamethasone (VTD).20 Importantly, it must also be considered that the greatest benefit should be obtained at a reasonable toxicity cost.
In this evolving area of pretransplantation induction regimens for MM, the Spanish Myeloma Group activated a 3-arm randomized trial comparing the efficacy of triple combination VTD versus the “standard” TD versus the alternating combination chemotherapy vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, doxorubicin, dexamethasone (VBMCP/VBAD) used in the previous Spanish transplantation trials21 followed by 2 courses of bortezomib. The primary endpoint was aimed at determining whether VTD could result in higher postinduction and posttransplantation CR rates.
Methods
Patients
Patients with newly diagnosed and untreated symptomatic MM who were 65 years of age or younger with measurable serum and/or urine M protein were eligible for entering the study. The main inclusion criteria required performance status < 3, hemoglobin level ≥ 8 g/dL, neutrophil count ≥ 1 × 109/L, platelet count ≥ 50 × 109/L, liver enzymes < 100 IU/L, serum bilirubin < 1.5 mg/dL, serum calcium < 14 mg/dL, and serum creatinine ≤ 2 mg/dL. The main exclusion criteria were peripheral neuropathy grade ≤ 2, systemic amyloidosis, and a positive serology for HIV or hepatitis B or C. From April 6, 2006 to August 5, 2009, the 390 planned patients entered the study. Four patients failed the eligibility criteria: 3 had monoclonal gammopathy of undetermined significance and 1 had primary systemic amyloidosis. Thus, 386 patients from 66 institutions from Spain were analyzed. The study was approved by the Spanish National Health Service and by all the local institutional ethics committees and was conducted in accordance with the principles of the Declaration of Helsinki. All patients gave written informed consent.
Study design and endpoints
Patients were centrally randomly assigned to receive VBMCP/VBAD/B versus TD versus VTD. Combination chemotherapy with VBMCP/VBAD chemotherapy plus bortezomib consisted of a total of 4 cycles of alternating VBMCP and VBAD at doses and schedules as previously described21 followed by 2 cycles of bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11 at 3-week intervals). TD consisted of thalidomide 200 mg daily (escalating doses in the first cycle: 50 mg on days 1 to 14, and 100 mg on days 15 to 28), and dexamethasone 40 mg orally on days 1-4 and 9-12 at 4-week intervals for 6 cycles. The VTD arm was identical to TD plus bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle. The duration of the induction therapy was 24 weeks in all arms. Either low-molecular-weight heparin or aspirin thromboprophylaxis was recommended for patients receiving induction therapy with thalidomide. All patients were planned to undergo ASCT with high-dose melphalan at 200 mg/m2 in a single dose or in 2 divided doses of 100 mg/m2 on days −3 and −2 followed by stem cell support. Peripheral blood progenitor cells were collected after mobilization with granulocyte colony-stimulating factor (G-CSF) at a dose of 10 mcg/kg subcutaneously every 12 hours for 5 days. In case of inadequate collection, subsequent mobilizations with cyclophosphamide plus G-CSF, with chemotherapy or with perixaflor according to the local practice was allowed. Patients failing to achieve sufficient stem cell collection to support the ASCT procedure were removed from the study. Three months after ASCT, patients were randomized to receive maintenance therapy with interferon alfa-2b (3 MU subcutaneously 3 times per week) versus thalidomide 100 mg per day orally versus thalidomide 100 mg per day orally plus one cycle of bortezomib on days 1, 4, 8, and 11 every 3 months. Maintenance therapy was planned for 3 years. Treatment was discontinued at any time in case of disease progression, undue toxicity/adverse events, or consent withdrawal. Concomitant medication consisted of zoledronic acid 4 mg IV every 4 weeks for up to 2 years. Antibiotic prophylaxis and/or post-ASCT G-CSF were administered accordingly with local clinical practice. The main endpoints were the CR rate after induction and after ASCT. Secondary endpoints were PFS, OS, and safety.
Safety
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (Version 3.0). Bortezomib or combination chemotherapy was withheld in cases of grade 4 hematologic toxicity or grade ≥ 3 nonhematologic toxicity until toxicity returned to at least grade ≤ 2. Concerning bortezomib, if the toxicity resolved, it was restarted at a dose reduced by 25% (1.3-1 mg/m2 or 1.0-0.7 mg/m2). Bortezomib-induced peripheral neuropathy or neuropathic pain was managed as previously described.22,23 In the case of grade 2 thalidomide-related peripheral neuropathy, the dose of thalidomide was reduced by 50%; in the case of grade 3, thalidomide was discontinued until it became grade ≤ 1 and then restarted with a dose reduction of 50%. A subsequent 50% dose reduction was permitted when necessary. For any grade 3 or 4 adverse event related to dexamethasone, the drug was withheld until toxicity returned to grade 2 or less. Dexamethasone was restarted at a dose reduced by 50%, and if toxicity recurred, a second 50% reduction was allowed.
Response assessment
Response and progression were assessed according to the criteria of the European Group for Blood and Marrow Transplantation (EBMT).24 In a post-hoc analysis, the VGPR category, as defined in the Uniform Response Criteria for MM, was also assessed.25 In short, CR required the disappearance of the original myeloma protein in serum and urine immunofixation and less than 5% bone marrow plasma cells (BMPCs). Partial response (PR) required a serum M protein decrease of 50% or more and a urine M protein decrease ≥ 90% or more, and/or to < 200 mg/24 hours as well as a reduction in 50% or more in the cross-sectional areas of extraosseous plasmacytomas. VGPR required a decrease of the serum M protein ≥ 90% and a 24-hour urine M-protein excretion lower than 100 mg. Responses were evaluated on an intention-to-treat basis. Responses reported by the investigators were centrally reassessed. Transient responses with subsequent progression before or at the completion of the 6 cycles of the induction therapy were considered as treatment failures.
FISH studies
BMPCs were isolated with anti-CD138–coated magnetic beads using the AutoMACs automated separation system (Miltenyi Biotec). Interphase fluorescence in situ hybridization (FISH) analysis was performed with specific probes (Abbott Molecular/Vysis) for 13q and 17p deletions, and immunoglobulin heavy chain (IGH) translocations including t(11;14), t(4;14), and t(14;16), as previously described.26 All cytogenetic studies were centrally performed at the laboratories of Hospital Clínico of Salamanca and at the Hospital Doce de Octubre in Madrid.
Sample size and statistical analysis
A sample size of 390 patients (130 per arm), considering a loss of 10%, was calculated to achieve 80% statistical power to detect a 15% difference among groups in postinduction and posttransplantation CR rates with a type I error of 0.05. The χ2 test was used to assess the statistical significance of multiple comparisons. The postinduction and post-ASCT CR rates were calculated on an intention-to-treat basis including all randomized patients. PFS was calculated from the date of randomization to the date of relapse, progression, or death from any cause. Patients who were removed from the study during the induction period because of toxicity and received alternative therapy before progression were censored for PFS at the time when the alternative treatment was initiated. OS was calculated from randomization to the date of death or last visit. Survival curves were plotted according to the method of Kaplan and Meier27 and statistically compared by the log-rank test.28
Study monitoring
The study was monitored by an external contract research organization (CRO). Both response to therapy and toxicity was centrally reassessed by the principal investigators. Although the recruitment of patients was finished in August 2009 the study is still in a continuous updating process. The cutoff for this report was August 13, 2011.
Results
Randomization and comparability of treatment groups
Four of the 390 randomized patients did not fulfill the diagnostic criteria of MM (3 patients had monoclonal gammopathy of undetermined significance and one had primary systemic amyloidosis) and were excluded from the analysis for violation of inclusion criteria. The 386 eligible patients (median age: 56 years; male: 207, female: 179) were allocated to: VBMCP/VBAD/B (129 patients), TD (127 patients), or VTD (130 patients). The M-protein type was: IgG in 233 patients, IgA in 85, light chain: 57, IgD: 9, and IgM: 2). The stage according to the International Staging System (ISS) was I in 147 patients, II in 160, III in 75, and unknown in 4. Sixty-six patients (17%) had extramedullary soft-tissue plasmacytomas defined as soft-tissue masses resulting either from metastatic spread or arising from lytic bone lesions. FISH cytogenetics were performed in 330 of the patients included in the trial and 70 of them (21%) had high-risk cytogenetics: t(4;14), t(14;16), and/or 17p deletion. The pretreatment characteristics of patients according to treatment arm are shown in Table 1. Prognostic factors, including ISS stage and cytogenetic status, were well balanced among the 3 treatment groups.
Response to the induction therapy
The response to induction therapy according to treatment arm is shown in Table 2. The immunofixation electrophoresis (IFE)–negative CR rate was significantly higher with VTD (35%) compared with TD (14%) and VBMCP/VBAD/B (21%; P = .0001 and P = .01, respectively). Of interest, in the VBMCP/VBAD/B arm, the CR rate increased from 8% after the 4 cycles of VBMCP/VBAD to 21% after the completion of the 2 bortezomib courses. The progressive disease (PD) rate during induction was significantly lower with VTD than with TD (7% vs 23%, P = .0004). In patients with extramedullary soft-tissue plasmacytomas, the CR rate was significantly higher with VTD compared with TD (42% vs 14%, P = .02) and the PD rate was significantly lower with VTD (12% vs 40%, P = .02; Table 3). In all these subanalyses, the VBMCP/VBAD/B arm showed an intermediate efficacy between VTD and TD. In the overall series, the PD rate was significantly higher in patients with extramedullary plasmacytoma (EMP) compared with patients without (24% vs 11%, P = .01). This higher PD rate in patients with EMP was observed in the 3 treatment arms (12% vs 6% with VTD, 24% vs 9% with VBMCP/VBAD/B, and 40% vs 19% with TD). In patients with high-risk cytogenetics, the CR rate was significantly higher with VTD compared with TD (35% vs 0%, P = .002) and with VBMCP/VBAD/B (35% vs 22%, P = .02; Table 4). Among the 45 patients with t(4;14), the CR rate was significantly higher with VTD than with VBMCP/VBAD/B (38% vs 25%, P = .05) or with TD (38% vs 0%, P = .01). Similarly, among the 22 patients with 17p deletion, the CR rate with VTD was 58% while none of the patients with this cytogenetic abnormality entered CR with VBMCP/VBAD/B or with TD (P = .03 and P = .02, respectively).
Toxicity, adverse events, and discontinuations within the induction period
Grade 3 and 4 toxicities as well as grade 3 and 4 adverse events according to each treatment arm are shown in Table 5. The number of patients who developed shingles during induction was 10, 5, and 5 in the VBMCP/VBAD/B, TD, and VTD arms, respectively. Peripheral neuropathy grade ≥ 3 with VTD (14%) was significantly higher than with TD (5%; P = .01) and differences with VBMCP/VBAD/B (9%) were not significant. An additional 46% of patients in the VTD arm developed grade 2 peripheral neuropathy compared with 8% and 15% in the TD and VBMCP/VBAD/B arms, respectively (P < .001). In the VTD arm, bortezomib dose reduction because of peripheral neuropathy was required in 33 (25%) of the 130 patients, the dose reductions being required in 19 (57%) of the 33 in cycles 5 and 6. The number of discontinuations because of peripheral neuropathy in the VTD arm was 3 (2%); 2 of these discontinuations were required during cycles 5 and 6. Grade 3 and 4 neutropenia was significantly higher with VBMCP/VBAD/B than with the remaining 2 arms (22% vs 14% with TD, P = .05 and 22% vs 10% with VTD, P = .007). No significant difference in grade ≥ 3 adverse events was recorded among the 3 treatment groups.
In the VBMCP/VBAD/B arm, 4 patients died and the other 4 were removed because of toxicity within the induction period. In the TD arm, 2 patients withdrew their consent, 3 died, and 4 developed severe toxicity precluding the continuation of treatment. Concerning the VTD group, one patient withdrew her consent, 3 died, and 9 were removed because of toxicity. A total of 4 patients died within the first 2 months from the initiation of therapy (early deaths): one in the VBMCP/VBAD/B arm, one in the TD group, and 2 in the VTD arm (Table 2). The number of patients discontinued within the induction therapy period because of disease progression was 15, 29, and 9 for the VBMCP/VBAD/B, TD, and VTD arms, respectively.
Stem cell mobilization and response after ASCT
In the overall series, the median time between day 1 cycle 6 and stem cell infusion was 12.1 weeks (95% confidence interval [CI]: 11.6-12.5) with no significant differences among the 3 arms: 11.3 vs 12.6 vs 12.3 weeks for the VBMCP/VBAD/B, TD, and VTD, respectively. No significant differences in stem cell collection among the 3 treatment groups were observed. The median number of CD34-positive cells was 3.1 106/kg in the VBMCP/VBAD/B arm, 4.5 in the TD arm, and 3.8 in the VTD group. The number of patients failing G-CSF mobilizations and requiring cyclophosphamide plus G-CSF was 15 in the VBMCP/VBAD/B arm, 9 in the TD group, and 4 in the VTD arm. One patient in the VBMCP/VBAD/B arm failed cyclophosphamide plus G-CSF and received plerixafor on a compassionate use. There were 2 mobilization failures in the VBMCP/VBAD/B arm.
Apart from patients who died or were discontinued because of toxicity, consent withdrawal, or progression during the active induction period, the other 17 patients did not undergo the ASCT (progression between the end of induction and the planned ASCT: 10; mobilization failure: 2; investigator decision: 2; patient refusal:1; adenocarcinoma of the colon: 1; or death: 1). A total of 283 patients underwent ASCT (101 in the VBMCP/VBAD/B arm, 79 in the TD arm, and 103 in the VTD arm). The median duration of neutropenia (< 0.5 × 109/L) after stem cell infusion was 11, 12, and 12 days for the VBMCP/VBAD/B, TD, and VTD arms, respectively. The median duration of thrombocytopenia (< 50 × 109/L) was 17, 14, and 17 days, respectively. There were 2 transplantation-related deaths (1 in the VBMCP/VBAD/B and 1 in the VTD arm). On an intention-to-treat basis, including all randomized patients, the post-ASCT CR rate was significantly higher in the VTD arm compared with TD (46% vs 24%, P = .004) and there was a trend toward a higher CR rate compared with VBMCP/VBAD/B (46% vs 38%, P = .2). The upgrade to CR with ASCT was from 21% to 38% in the VBMCP/VBAD/B arm, from 14% to 24% with TD, and from 35% to 46% with VTD. When considering only the patients who actually underwent the ASCT, the CR rate was 48%, 40%, and 57% for the VBMCP/VBAD/B, TD, and VTD arms, respectively. The increase in CR rate was from 21% to 48% with the VBMCP/VBAD/B, from 14% to 40% with TD, and from 35% to 57% in the VTD group.
PFS, OS, and survival after progression
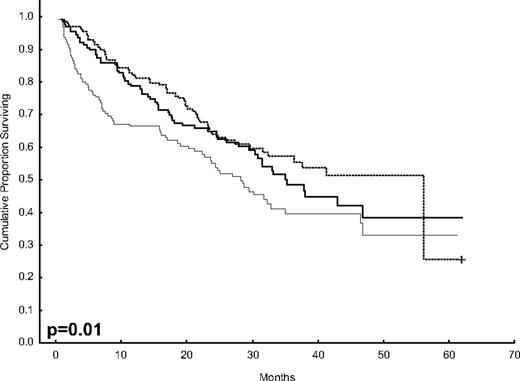
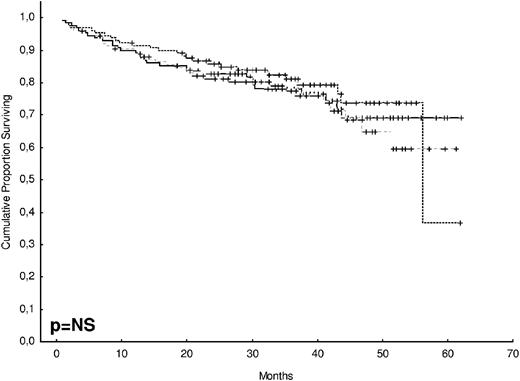
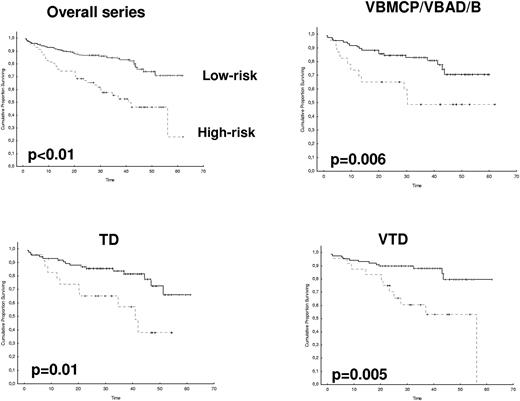
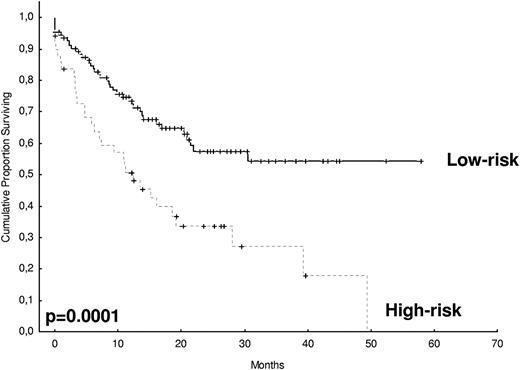
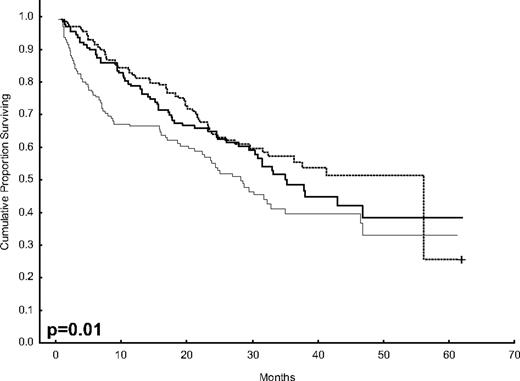
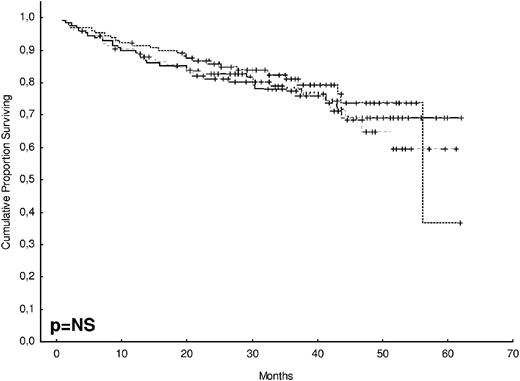
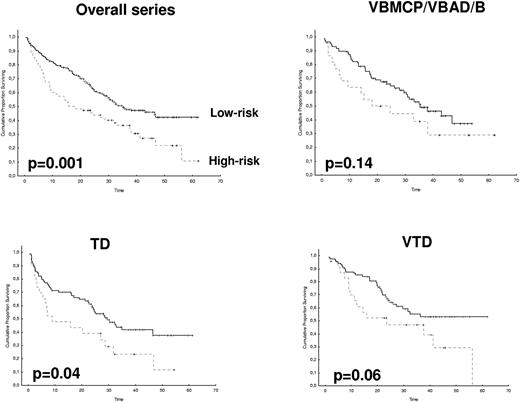
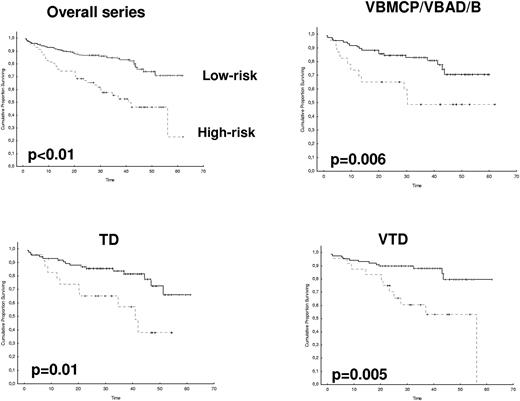
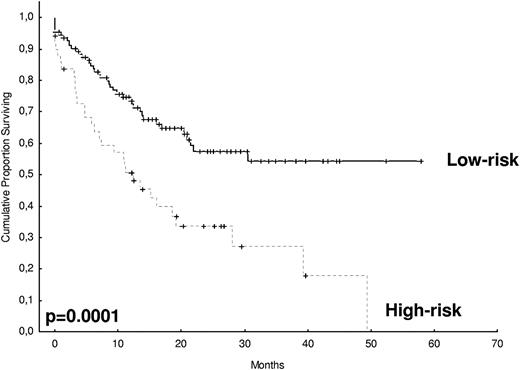
After 2 years from the inclusion of the last patient and a median follow-up of 35.2 months, the median PFS for the overall series was 33.1 months, being significantly higher with VTD than with VBMCP/VBAD/B or with TD (56.2 vs 35.3 vs 28.2 months, respectively; P = .01, Figure 1). The estimated overall survival at 4 years from randomization was 74% for VTD, 70% for VBMCP/VBAD/B, and 65% for TD (P = NS, Figure 2). There were no significant differences in PFS among patients with and without EMPs (median median 33 months vs 27.8 months, P = .25). However, the OS was significantly shorter in patients with extramedullary disease (median not reached vs 46.7 months, P = .03). In the overall series, patients with high-risk cytogenetics had a significantly shorter PFS compared with the good-risk group (median 18.1 vs 35 months; P = .0017, Figure 3). In addition, patients with poor-risk cytogenetics had a significantly shorter PFS when treated with TD (median 8.9 vs 29.4 months, P = .04) and there was also a trend toward a shorter PFS after induction with VTD (median 23.5 months vs not reached, P = .06) and with VBMCP/VBAD/B (median 18 vs 35.3 months, P = .14; Figure 3). In the overall series, patients with high-risk cytogenetics had a significantly shorter OS (median 41 months vs not reached; P = .0001, Figure 4), irrespective of the treatment arm: VBMCP/VBAD/B (median 31 months vs not reached, P = .006), TD (median 41.5 months vs not reached, P = .01), and VTD (median 56.6 months vs not reached, P = .005). Finally, survival from progression (median 28.1 months) was significantly shorter in patients with high-risk cytogenetics compared with that of those with standard-risk cytogenetics (median 12.3 months vs not reached; P = .00015, Figure 5). This significantly shorter survival after progression for patients with high-risk cytogenetics was observed in the 3 treatment arms (Figure 5). In the overall series, the survival after relapse was not significantly different among the 3 induction arms (median 28 months vs 30.6 months vs 20.3 months, P = .4). After a median follow-up of 24 months from the initiation of maintenance, the PFS was significantly longer with thalidomide/bortezomib compared with thalidomide alone and with alfa2-IFN (78% vs 63% vs 49% at 2 years, P = .01). However, the OS was not significantly different among the 3 maintenance arms.
OS according to treatment arm. The estimated OS at 4 years was 74% for VTD, 70% for VBMCP/VBAD/B, and 65% for TD (P = NS).
OS according to treatment arm. The estimated OS at 4 years was 74% for VTD, 70% for VBMCP/VBAD/B, and 65% for TD (P = NS).
Discussion
The achievement of the lowest possible tumor burden with the induction therapy is crucial because it is associated with the enhancement in the quality of response reached after ASCT, which in turn is the best surrogate for a prolonged PFS and OS. In the present trial, we have compared the “standard” combination of TD with VBMCP/VBAD chemotherapy plus 2 courses of bortezomib and with the triple combination VTD. The rationale for using VBMCP/VBAD plus bortezomib was the high response rate after 4 to 6 cycles of VBMCP/VBAD observed in our previous PETHEMA studies5,21 which could be theoretically further enhanced before ASCT by bortezomib once the tumor burden has been already reduced with the chemotherapy component. In our study, VTD resulted in 35% CR and 60% at least VGPR rates, which were significantly superior to those achieved with TD and with VBMCP/VBAD/B. In fact, this 35% CR rate is the highest reported so far with any induction pretransplantation regimen. This efficacy is likely because of the dose intensity administered in our trial (ie, 6 full-dose cycles). Thus, in the Italian study, the CR rate with 3 cycles of VTD was 19%29 while in the Intergroupe Francophone du Myelome (IFM) trial, using 4 cycles of VTD with reduced doses of bortezomib and thalidomide, the CR rate was 13%.30 In addition, in the seminal study by the MD Anderson group administering “no more than 3” cycles of VTD, the CR rate was 16%.20 The impact of dose intensity in triple induction regimens containing bortezomib is further illustrated by the results reported with PAD (24% CR rate with 4 full-dose cycles vs only 11% with the bortezomib-reduced dose).31 In patients with high-risk cytogenetics [t(4;14) and/or 17p deletion], the CR rate obtained with VTD was as high as in the overall series and also significantly higher than with the other 2 arms.
In contrast with other reports, a PD rate of approximately 13% during the induction period was observed in the present series, being the highest PD rate for patients in the TD arm and the lowest in the VTD group. It is likely that our longer induction period allowed the emergence and recognition of early progressions in patients with dexamethasone-associated transient responses not becoming apparent when shorter induction treatments were administered. It is of interest that a significantly increased rate of PD was observed in patients with soft-tissue plasmacytomas. Thus, VTD resulted in the highest CR and VGPR rates as well as in the lowest PD rate, both in the overall series and in patients with high-risk cytogenetics, while TD was suboptimal, particularly in patients with high-risk cytogenetics as well and in those with extramedullary involvement.
No relevant differences in adverse events, other than peripheral neuropathy, were observed among the 3 treatment groups. Concerning grade ≥ 3 peripheral neuropathy, it was significantly higher with VTD than with TD (14% vs 5%) and it was observed in 9% of patients receiving VBMCP/VBAD plus 2 cycles of bortezomib. Unfortunately, the 14% grade 3 and 4 peripheral neuropathy with 6 courses of VTD was higher than the 10% with 3 cycles reported in the Italian study29 and the only 3% in the IFM trial with the reduced dosing of bortezomib and thalidomide.30 In addition, the 46% of grade 2 peripheral neuropathy in our trial was significantly higher than that observed in the Italian and in the IFM trials, using a less-intensive VTD induction.29,30 However, it is also of note that grade ≥ 3 peripheral neuropathy after 4 cycles of bortezomib and dexamethasone in 2 consecutive IFM trials was as high as 9% and 11%, respectively.15,30 Thus, peripheral neuropathy remains the main issue of concern in all bortezomib-based combinations15,29,30 and the benefit in response rate of an intensive dose induction, as the one in the present study administering 6 cycles of VTD, should be weighed against the higher rate of peripheral neuropathy. In any event, careful prevention, as well as an appropriate management with dose and schedule adjustments, is crucial.22,23 The subcutaneous administration of bortezomib, which apparently results in a significantly lower peripheral neuropathy rate while maintaining the efficacy,32 may reduce current rates of bortezomib-induced peripheral neuropathy. Other possible future alternatives could be the use of the irreversible proteasome inhibitor carfilzomib, which has a significantly lower risk of peripheral neuropathy and a high antimyeloma activity,33 or oral protesome inhibitors.
No significant differences in either stem cell collection or engraftment were observed among the 3 treatment groups. It is worth mentioning that only 73% of our patients underwent the planned ASCT as per protocol. A similar failure rate was reported in the most recent Medical Research Council (MRC) trials.17 However, the proportion of patients receiving the ASCT in our trial was lower than the 89% in the GIMEMA study and the 90% in the IFM trial using VTD.29,30 The reason why 27% of our patients did not receive the planned ASCT was early study discontinuation mainly because of disease progression or toxicity as previously discussed. Despite this fact, in an intention-to-treat analysis on all randomized patients, the posttransplantation CR rate was significantly higher with VTD compared with TD (46% vs 24%) and there was also a trend toward a higher CR rate than with VBMCP/VBAD/B (46% vs 38%). When considering only patients who actually underwent the ASCT, the CR rates were increased from 35% to 57% in the VTD arm, from 21% to 48% in the VBMCP/VBAD/B arm, and from 14% to 40% in the TD group. This strongly supports the use of upfront ASCT for MM even in the era of novel agents. The CR rate of 46% after ASCT in the VTD arm is the highest reported so far in phase 3 trials, and it compares favorably with the 38% achieved in the Italian study with 3 courses of VTD and the first ASCT and approaches the 49% reached after tandem ASCT and VTD consolidation in the same trial.29 Further enforcing the impact of dose-intensity induction, the post-ASCT CR rate in the IFM study using VTD at reduced doses of bortezomib and thalidomide was only 29%.30 On the other hand, the posttransplantation CR rate achieved with TD is similar to that observed in large contemporary trials using VAD15,16,18 or cyclophosphamide, vincristine, adriamycin, dexamethasone (C-VAD)17 and, as a consequence, all of these regimens should be considered suboptimal, particularly in patients with high-risk cytogenetics or EMP. In contrast, the 38% rate obtained with VBMCP/VBAD/B plus 2 courses of bortezomib compares favorably to that recently reported with triple thalidomide combinations such as TAD16 or CTD,17 or with bortezomib plus dexamethasone,15,30 and therefore, this combination could be considered as a reasonable pre-ASCT induction option, although it is inferior to VTD.
The median PFS with VTD was significantly longer than with the other 2 arms. However, as in all other studies15–18,29 and with the current follow-up, this has not resulted in a significant OS prolongation. The capacity of novel agents to overcome the poor outcome of patients with high-risk cytogenetics remains an issue of controversy.34 In our study, patients with high-risk cytogenetics had a significantly shorter PFS than those with “standard” cytogenetic risk. Importantly, the shorter PFS in patients with high-risk cytogenetics was observed in all 3 treatment arms. Concerning OS, it was significantly shorter in high-risk patients irrespective of the treatment group. Thus, in our series, and in contrast with the results of the Italian study,29 the VTD regimen was not able to overcome the poor prognostic impact of high-risk cytogenetics. In fact, the median PFS of 18 months in our patients is comparable with the median PFS of 18 and 20 months recently reported in the MRC IX trial using C-VAD and CTD, respectively.17 The short PFS in patients with high-risk cytogenetics was also similar to that reported in the HOVON-65/GMMG-HD4 trial comparing VAD versus PAD [17.6 and 21.7 months for patients with deletion 17p and t(4;14), respectively].18 The Arkansas group also reported that TP53 deletion was not an adverse prognostic feature in patients with Total Therapy 3 including VTD in induction, consolidation, and maintenance.35 Contrasting with the results of the previous paragraph, the IFM group reported in a large number of patients that induction with bortezomib plus dexamethasone versus VAD improved the outcome of patients with t(4;14) while no improvement for patients with deletion 17p was observed.36 However, in the latter study, the 2 cytogenetic abnormalities had a negative impact on both PFS and OS, despite the use of the induction bortezomib-based regimen. In a recent PETHEMA/GEM trial in elderly patients, induction with melphalan, prednisone, bortezomib (MPV) or with bortezomib, thalidomide, prednisone (VTP) followed by bortezomib maintenance was not able to overcome the adverse prognosis of high-risk cytogenetics.37 When looking at the results of the reported trials, it seems that, although some improvement can be achieved in patients with high-risk cytogenetics with the use of bortezomib-based regimens, this improvement is in general quite modest and not enough to overcome the poor prognosis of high-risk cytogenetics. In any event, pretransplantation induction therapy should be based on highly active triple combinations. In our trial, the PFS was significantly longer with thalidomide/bortezomib maintenance compared with thalidomide alone, with no significant impact on OS. It is unlikely that the maintenance component of this study has an impact on the PFS observed between the different induction arms because the maintenance groups were well balanced in terms of induction group and response status at the time of maintenance randomization after ASCT.38 One important finding of our study is that the survival from progression in patients with high-risk cytogenetics was only 12 months, significantly shorter than that of patients with standard cytogenetic risk. This significantly shorter survival after relapse was noted in the 3 treatment arms and was likely because of the limited efficacy of salvage therapies in this setting.
In summary, our study shows that induction with VTD results in a higher CR rate in both the overall series and in patients with high-risk cytogenetics. The post-ASCT CR rate is also higher with VTD. The VTD regimen was associated with a longer PFS although this regimen was not able to overcome the poor outcome of patients with high-risk cytogenetics. Our findings extend the results of the other 2 trials comparing VTD with TD29 or with bortezomib plus dexamethasone,30 and support the use of VTD as the best induction regimen reported so far for patients with MM who are eligible for ASCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arturo Touchard for his valuable support in data collection and analysis.
This work was supported by the Cooperative Research Thematic Network (RTICs; RD06/0020/0005, RD06/0020/0006, RD06/0020/0031, RD06/0020/0101, RD06/0020/1056, and G03/136) and FIS 08/0147. The present trial was sponsored by the Spanish PETHEMA Foundation. Janssen-Cilag and Pharmion supported the study costs through 2 grants to PETHEMA. Janssen-Cilag provided bortezomib free of charge. Pharmion (and later Celgene) also provided thalidomide free of charge.
Authorship
Contribution: L.R., M.V.M., J.J.L., J.S.M., and J.B. conceived the idea and designed the study protocol; N.C.G. and M.L.M.-R. analyzed the FISH data; L.R., A.O., A.I.T., D.H., J.L.J., J.d.l.R., M.G., J.B., L.P., Y.G., M.A.E., J.D.-M., M.T.H., F.d.A., M.T.C., M.V.M., J.M., and A.A. contributed with provision of study material or patients; L.R., J.J.L., J.S.M., and J.B. analyzed and interpreted data; L.R. performed statistical analysis; L.R. and J.B. wrote the manuscript; A.O., J.d.l.R., N.C.G., M.V.M., J.J.L., and J.S.M. made suggestions and modifications; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: L.R. has received honoraria and has served on the advisory board for Janssen-Cilag and Celgene. J.B. has received honoraria and has served on the advisory board for Janssen-Cilag and Celgene, and has also received grant support from Celgene and Janssen-Cilag. J.S.M. has served on the speaker's bureau and on the advisory board for, and has received honoraria from, Millenium, Janssen-Cilag, Onyx, Novartis, and Celgene. M.V.M. has served on the speaker's bureau for Milleniun, Celgene, and Janssen-Cilag. M.T.C., A.O., F.d.A., J.J.L., J.d.l.R., and A.A. have received honoraria from Celgene and Janssen-Cilag. The remaining authors declare no competing financial interests.
A complete list of the members of the PETHEMA/GEM participants appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Joan Bladé, MD, PhD, Hospital Clínic de Barcelona, Hematology Department, Villarroel 170, Barcelona 08036, Spain; e-mail: jblade@clinic.ub.es.