Abstract
The coagulation and complement pathways simultaneously promote homeostasis in response to injury but cause tissue damage when unregulated. Mechanisms by which they cooperate are poorly understood. To delineate their interactions, we studied the effects of thrombin and C5 convertase on C5 in purified and plasma-based systems, measuring release of the anaphylatoxin C5a, and generation of C5b, the initial component of the lytic membrane attack complex. Thrombin cleaved C5 poorly at R751, yielding minimal C5a and C5b. However, thrombin efficiently cleaved C5 at a newly identified, highly conserved R947 site, generating previously undescribed intermediates C5T and C5bT. Tissue factor-induced clotting of plasma led to proteolysis of C5 at a thrombin-sensitive site corresponding to R947 and not R751. Combined treatment of C5 with thrombin and C5 convertase yielded C5a and C5bT, the latter forming a C5bT-9 membrane attack complex with significantly more lytic activity than with C5b-9. Our findings provide a new paradigm for complement activation, in which thrombin and C5 convertase are invariant partners, enhancing the terminal pathway via the generation of newly uncovered C5 intermediates. Delineating the molecular links between coagulation and complement will provide new therapeutic targets for diseases associated with excess fibrin deposition and complement activation.
Introduction
Throughout evolution, organisms have developed means to simultaneously contain wounds by limiting bleeding and fighting pathogens, thereby enabling rapid healing. This is accomplished partly by simultaneous activation of 2 biochemical systems in the blood: coagulation and complement.1
Coagulation is the fundamental process by which organisms, in response to injury, are able to limit bleeding, restore vascular integrity, and promote healing.2 Subsequent to vessel damage, tissue factor is exposed to the circulation, setting off a cascade of proteolytic reactions that leads to the generation of the central coagulation enzyme thrombin, which converts fibrinogen into a fibrin clot.
Via an analogous cascade, complement activation protects the organism by disposing of invading pathogens and damaged host cells.3,4 The complement system comprises more than 30 soluble and membrane-bound proteins, activation of which is achieved via 3 main pathways, all converging with proteolytic activation of the central complement component C3. This is followed by formation of C5 convertases, which cleave C5 at arginine 751 (R751) to liberate a chemotactic and anaphylatoxic C5a fragment and generate C5b. C5b is the initiating factor for assembly of the C5b-dependent lytic membrane attack complex (MAC; C5b-9), responsible for destroying damaged cells and pathogens.
Although coagulation and complement are generally viewed separately, they are coordinately activated in an overlapping spatiotemporal manner in response to common pathophysiologic stimuli to maintain homeostasis.5 Disease emerges when there is unchecked activation of the innate immune and coagulation responses, a situation that is common and evident in, for example, atherosclerosis,6 stroke,7,8 coronary heart disease,9 diabetes,10 ischemia-reperfusion injury, trauma,11 paroxysmal nocturnal hemoglobinuria,12 age-related macular degeneration,13 and atypical hemolytic-uremic syndrome.14 Recent successful introduction of complement inhibitors that simultaneously treat the inflammatory and thrombotic disturbances associated with some of these disorders15 underline the critical importance of delineating the interplay between coagulation and complement.
Several molecular links between complement and coagulation have been identified.16–20 Most notably in what was described as a new complement activation pathway, thrombin was found to be capable of directly promoting activation of complement by cleaving C5, presumably at R751, thereby releasing C5a.18 These studies did not compare thrombin with the bona fide C5 convertase, and only limited biochemical analyses were performed. Thus, the physiologic relevance of the pathway was not evaluable.19,21
We further explored the relative effects of thrombin and C5 convertase on C5 cleavage and subsequent formation of the MAC. Thrombin-mediated cleavage of C5 at R751 was indeed inefficient, generating barely detectable amounts of C5a. Strikingly, however, low concentrations of thrombin rapidly cleaved C5 and C5b at a previously unidentified, yet highly conserved thrombin cleavage site, R947, generating new C5 products, which we refer to as C5T and C5bT, respectively. In purified and serum systems, C5bT efficiently initiated generation of a MAC that exhibited more lytic activity than one comprising C5b.
Our findings provide a new paradigm for complement activation in which thrombin is an invariant and critical partner with C5 convertase in initiating formation of a more active MAC via formation of previously unidentified C5 products that are generated via cooperative proteolysis by the 2 enzymes. These discoveries are a departure from years of dogma, providing new insights into the regulation of innate immunity in the context of coagulation activation that occurs in many diseases.
Methods
Reagents
Healthy single-donor citrated fresh frozen plasma was obtained from the Canadian Blood Services NetCAD laboratory with approval from the Human Ethics Committee of the University of British Columbia. Normal human serum and gelatin veronal-buffered saline (GVB) containing 5mM barbital, 145mM NaCl, pH 7.4, and 0.1% gelatin was purchased from Complement Technologies. GVB-M7EGTA10 was GVB containing 7mM MgCl2 and 10mM EGTA, and GVB-EDTA10 was GVB containing 10mM EDTA. HEPES-buffered saline (HBS) contained 20mM HEPES, pH 7.4, and 150mM NaCl. HBS-M7 was HBS containing 7mM MgCl2. Maleimide-PEG2-Biotin (#21901) and avidin agarose (#20219) were from Thermo Scientific. L-α-phosphatidylcholine and L-α-phosphatidyl-L-serine were from Sigma-Aldrich. Unilamellar vesicles with 25% L-α-phosphatidyl-L-serine and 75% L-α-phosphatidylcholine were prepared as described.22 Chicken erythrocytes were isolated from chicken blood purchased from Colorado Serum Company and were diluted in GVB-EDTA10.
Antibodies and purified proteins
IRDye 800CW Streptavidin and goat anti–rabbit IgG (H + L) IRDye 680 antibodies were from LI-COR Biosciences. Human C5a (A144) and polyclonal rabbit antibodies against human rC5a (A221) were from Complement Technologies. Recombinant tissue factor was from Dade Innovin. Plasma-derived thrombin was from Enzyme Research Laboratories. Wild-type and S195A thrombin were gifts from Dr Isis Carter (Center for Blood Research, Vancouver, BC) and characterized previously.23 Trypsin was from Promega and hirudin from Sigma-Aldrich or Merck KGaA. Human complement proteins, including the preformed C5b,6 complex, were from Complement Technologies. Cobra venom factor (CVF) was from Cedarlane Laboratories Ltd.
Tissue factor-dependent coagulation assay
Thrombin generation was initiated in 100 μL citrated plasma by the sequential addition of 17.7mM MgCl2, 40.7μM 25% L-α-phosphatidyl-L-serine/75% L-α-phosphatidylcholine vesicles, 18.4nM biotinylated C5 (C5-biotin), 0.45pM tissue factor, and 17.7mM CaCl2 in HBS. Reactions were incubated for 1 hour at 37°C and centrifuged for 5 minutes at 14 000g to pellet the clot. To isolate biotinylated C5 and C5-containing fragments, the supernatant was diluted with buffer (300 μL PBS, 0.1% SDS) and subjected to immunoprecipitation with avidin agarose for 1.5 hours at 22°C. C5-biotin bound beads were pelleted, washed 6 times with immunoprecipitation buffer, resuspended in 30 μL 2× urea loading sample buffer (500mM Tris, 4% SDS [weight/volume], 24% urea [weight/volume], pH 6.8) containing 71.5mM β-mercaptoethanol, boiled and subjected to electrophoresis on a 4%-20% Tris-HCl gradient gel. Proteins were transferred to Immobilon-FL membrane and incubated with a polyclonal rabbit antibody against human C5a and secondary goat anti–rabbit IgG (H + L) IRDye 680. Biotinylated C5 fragments were detected by IRDye 800CW Streptavidin.
Formation of C5 convertase
CVF-dependent C5 convertase CVF,Bb was used as it has a longer half-life (∼ 7 hours) than the human alternative pathway C5 convertase C3bBbC3b (1.5-3 minutes). The 2 enzyme complexes have similar kinetic properties with essentially indistinguishable Km and kcat for cleavage of C5 at R75120,24 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CVF,Bb was generated as reported.20,24 Briefly, excess CVF (500nM) was incubated with limiting factor B (250nM) and excess factor D (500nM) in a total volume of 500 μL HBS containing 0.5mM MgCl2. After 20 minutes at 37°C, 125 μL of HBS containing 15mM EDTA was added. Conditions were established to produce 200nM CVF,Bb.
In vitro cleavage assays
Reactions were carried out at 37°C in HBS-M7 with a reaction volume of 60 μL that contained substrate (C5 or C5-biotin or C5b,6) and one or both of thrombin and CVF,Bb. Reactions were initiated with enzyme(s) and terminated by transfer of an aliquot (15 μL) into 10 μL Laemmli loading buffer containing 5% β-mercaptoethanol. Samples (25 μL) were boiled and centrifuged, and 23 μL was subjected to SDS-PAGE. The 11% gels were used for C5 fragments involving α-chain and α′-chain; 4%-15% or 4%-20% Tris-HCl gradient gels (Bio-Rad) were used for thrombin-derived C5 fragments. Where shown, hirudin and thrombin were premixed for 5 minutes at 37°C before incubation with substrate.
Generation and quantification of C5a anaphylatoxin
Cleavage reactions were performed as described in “In vitro cleavage assays.” Reactions (30 μL) without CVF,Bb were stopped with reducing Tris-tricine urea buffer (20 μL; 295mM Tris, 50mM Tricine, 4% SDS [weight/volume], 24% urea [weight/volume], 71.5mM β-mercaptoethanol, pH 8) and an aliquot (20 μL) was electrophoresed on a 15% Tris-tricine SDS-PAGE gel. Reactions with CVF,Bb were diluted 1:10 before an aliquot (20 μL) was subjected to SDS-PAGE. An in-gel concentration standard curve was generated using purified C5a (15 μL of 2.5 to 100nM), detected after SDS-PAGE by Western immunoblot using polyclonal rabbit anti-rC5a antibody. C5a bands were quantified with a LI-COR Version 3.0.21 imaging system and software.
Scanning densitometry of SDS gels and calculation of the rate of C5 consumption
The rate of activation of plasma-derived C5 was measured as α-chain consumption expressed as mol C5 consumed/min per (mol enzyme) using either 50nM thrombin or 50nM C5 convertase with 700nM C5. Reactions were linear with respect to α-chain consumption for the first 15 minutes (thrombin; r2 = 0.97) and 30 minutes (C5 convertase; r2 = 0.98). Stained gels were scanned and bands corresponding to the 115-kDa α-chain were analyzed. Linearity of the relationship between the amount of protein loaded and the intensity measured by densitometry was confirmed by loading known quantities of C5. In-gel standards were used to quantify residual α-chain during a reaction time course.
Scanning densitometry of SDS gels and calculation of the rate of C5b generation
Formation of C5b from C5 (400nM) was measured as α′-chain generation expressed as a percentage produced maximally after 90 minutes by C5 convertase (100nM CVF,Bb). Background corrected total pixel intensities of the 104-kDa α′-chain were determined in reactions containing C5 convertase, thrombin, or both enzymes. All experiments were performed 3-5 times.
Hemolytic assays
Chicken erythrocyte lysis was used to measure C5b,6 function by established methods with modifications.25 C5 or C5b,6 cleavage assays were performed as described in “In vitro cleavage assays,” except that C5 cleavage assays also contained an excess of C6 (600nM) and reactions were diluted 2000-fold to 160 000-fold at the indicated time with GVB-EDTA10. These dilutions stopped reactions from proceeding further in the time required to conduct the assays. Components to a final volume of 300 μL were added at 37°C in the following order: chicken erythrocytes (3.3 × 107/mL), C5/C6 cleavage assay aliquot, or C5b,6 cleavage assay aliquot or C5b,6 (0.5-50pM) as control followed by 5 minutes incubation, C7 (15nM) followed by 15 minutes incubation, and a premix of C8 (10nM) and C9 (25nM) followed by 30 minutes incubation. Hemolytic assays were also conducted using pooled normal human serum (18 μL or 0.03x) as a source of C7-C9. Unlysed cells were removed by centrifugation. Lysis was calculated by measuring the absorbance at 405 nm of supernatant compared with the control of 100% lysis with H2O. Background signal was established with control assays containing C5 and C6, but no C5 convertase. No detectable C5b,6 was formed from the C5 and C6 in serum when it was used as a source of C7-C9.
Statistics
Data analyses were performed with GraphPad Prism Version 5 software. For differences in erythrocyte lysis, a nonparametric ANOVA was applied (Kruskal-Wallis test) followed by a nonparametric Mann-Whitney t test. Data are mean ± SD. Statistical significance refers to P < .05.
Results
Contribution of thrombin to C5 convertase-mediated generation of C5a in a purified system
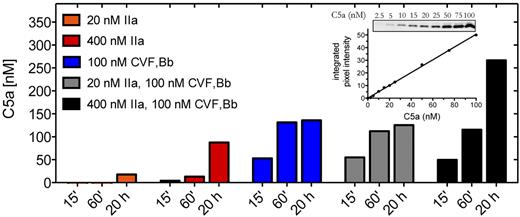
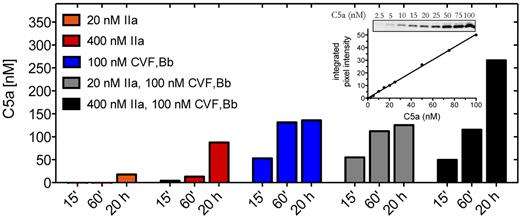
We first examined the contribution of thrombin to C5a generation compared with that of C5 convertase. C5 is a 2-chain disulfide-linked molecule that comprises a 75-kDa β-chain and a 115-kDa α-chain. Cleavage of C5 at the peptide bond between R751-L752 of the α-chain releases the 11-kDa C5a activation fragment, leaving the 2-chain disulfide-linked C5b composing the 75-kDa β-chain and a 104-kDa α′-chain. Using purified components, we compared the effects of thrombin and C5 convertase on the generation of C5a. The CVF26 dependent C5 convertase CVF,Bb was used throughout this study as it is more stable than the alternative pathway C5 convertase C3bBbC3b (half-life of ∼ 7 hours vs 1.5-3 minutes) yet otherwise has almost identical enzymatic properties.20,24 Where noted, limited confirmatory studies were performed with zymosan bound alternative pathway C5 convertase (ZymC3bnBb). The amount of C5a detected by anti-C5a antibodies was quantified by densitometry of Western blots, using an in-gel standard curve (Figure 1 inset).
C5a generation by thrombin and C5 convertase. Reactions containing C5 (400nM) were incubated with thrombin (IIa) (20nM or 400nM) or C5 convertase (CVF,Bb; 100nM) alone or in combination in HBS-M7, pH 7.4, for 15 minutes, 60 minutes, or 20 hours at 37°C. Reactions were subjected to 15% Tris-tricine SDS-PAGE under reducing conditions. C5a was detected by Western blotting using a polyclonal rabbit antibody against C5a. Results are representative of 3 independent experiments, each with similar results. Inset: C5a in-gel standards from Western blot and corresponding standard curve.
C5a generation by thrombin and C5 convertase. Reactions containing C5 (400nM) were incubated with thrombin (IIa) (20nM or 400nM) or C5 convertase (CVF,Bb; 100nM) alone or in combination in HBS-M7, pH 7.4, for 15 minutes, 60 minutes, or 20 hours at 37°C. Reactions were subjected to 15% Tris-tricine SDS-PAGE under reducing conditions. C5a was detected by Western blotting using a polyclonal rabbit antibody against C5a. Results are representative of 3 independent experiments, each with similar results. Inset: C5a in-gel standards from Western blot and corresponding standard curve.
Incubation of C5 (400nM) with C5 convertase (CVF,Bb) generated ∼ 50nM of C5a within 15 minutes and more than 125nM by 20 hours (Figure 1). Using a concentration of thrombin (20nM) that exceeds that necessary to induce a clot,27,28 no detectable C5a (< 2.5nM) was generated after a 15-minute incubation with C5. Even at 20 hours, only 18nM C5a was detected. It is estimated that a peak concentration of 300-500nM of free thrombin may accumulate within 20 minutes of tissue factor-induced clot formation.27,28 We therefore tested the effects of thrombin (400nM) on C5 and showed that low levels of C5a were indeed detectable after 15 minutes, but the amount was less than 10% of that generated with C5 convertase alone after the same reaction time (n = 3 experiments). Only after 20 hours with the higher concentration of thrombin was C5a generated in amounts approaching that seen with C5 convertase. The findings suggest that thrombin may contribute to C5a generation only when thrombin generation is chronically elevated, a situation that might occur, for example, during disseminated intravascular coagulation. In that setting, thrombin may contribute to C5 convertase-mediated C5a production in an additive manner (Figure 1 black bars).
Lack of C5a generation during tissue factor-initiated plasma clotting
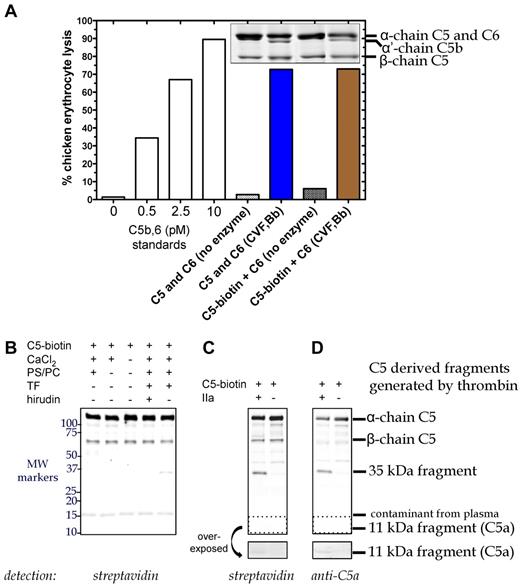
We next examined thrombin-mediated C5 cleavage in an ex vivo plasma clotting system. Initiation of fibrin clot formation was achieved by the addition of tissue factor, CaCl2 and phospholipid to normal human citrated plasma, conditions that yield thrombin concentrations of ∼ 400nM29 and generate a fibrin clot in 8-10 minutes. Despite a detection sensitivity of ∼ 400 pg C5a by Western blot, no C5a was detected in the clot supernatant 1 hour after tissue factor addition (data not shown).
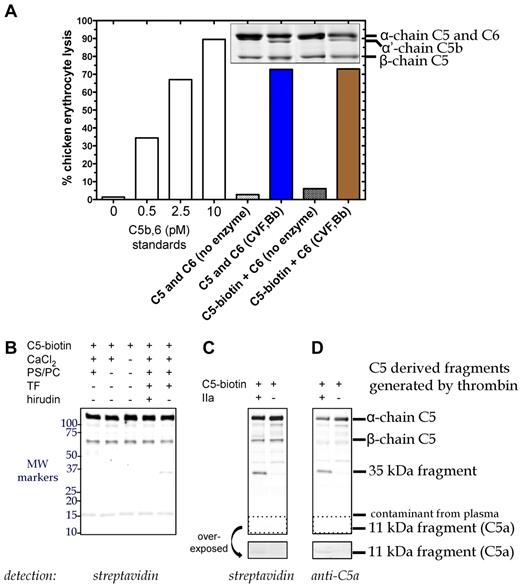
To enhance the sensitivity, we labeled C5 with biotin (C5-biotin) using a reagent that binds to C5a and the β-chain of C5, and used C5-biotin (20nM) as a tracer during tissue factor-initiated plasma clotting. C5-biotin retained full activity compared with unmodified C5, as (1) similar amounts of C5b were generated by C5 convertase (assessed by α′-chain on SDS-PAGE), and (2) C5b-biotin exhibited normal lytic activity in a terminal pathway hemolytic assay (Figure 2A). One hour after clot initiation, the fluid phase was immunoprecipitated for Western blotting (Figure 2B). Detection with labeled streptavidin revealed a band between 50 and 75 kDa, confirmed to be biotinylated β-chain of C5-biotin. An ∼ 15-kDa band was identified as a streptavidin cross-reacting species found in plasma and not derived from C5-biotin (data not shown). Notably, no C5a-biotin was detectable with streptavidin (Figure 2B), although in a purified system, after 90 minutes of incubation, 20nM thrombin could generate minute amounts (Figure 2C-D overexposed box).
C5 cleavage during tissue factor-induced clot formation. (A) Hemolytic activity of biotinylated C5 was confirmed. Reactions containing C6 (600nM) and either C5 (400nM) or C5-biotin (400nM) were incubated with C5 convertase (CVF,Bb; 100nM) for 90 minutes at 37°C. Aliquots of the reaction were subjected to SDS-PAGE followed by Coomassie staining to assess activation cleavage or diluted 160 000-fold for the terminal pathway (TP) assay to measure erythrocyte lytic activity. Lack of enzyme with C6 and either C5 or C5-biotin resulted in minimal background lysis. A standard curve (left side) was generated by inducing chicken erythrocyte lysis with purified C5b,6 and the addition of C7, C8, and C9. (B) C5 cleavage in plasma during tissue factor (TF)–dependent clot initiation. Thrombin (IIa) generation was initiated in citrated plasma by the addition of TF, phospholipid vesicles, and CaCl2 in the presence of C5-biotin, and reactions proceeded for 1 hour at 37°C. Coimmunoprecipitation was performed on the clot fluid phase using avidin agarose to isolate all biotinylated C5-containing fragments. Reactions were analyzed on a 4%-20% Tris-HCl gel under reducing conditions and detected by Western blotting using IRDye 800CW Streptavidin. No C5a was detected, but a 35-kDa fragment was generated that was absent when thrombin was inhibited with hirudin. (C-D) Cleavage of C5-biotin (400nM) was assessed in vitro by incubating it with or without 20nM thrombin for 1.5 hours at 37°C. Reaction products were detected by Western blotting using IRDye 800CW Streptavidin (C) or a polyclonal rabbit antibody against C5a and secondary goat anti–rabbit IgG (H + L) IRDye 680 (D), both of which revealed a novel C5-derived 35-kDa fragment. An area of the blot (dotted box) corresponding to the location of C5a was overexposed and depicted below to show the presence of tiny amounts of C5a formed by thrombin in the in vitro cleavage reaction.
C5 cleavage during tissue factor-induced clot formation. (A) Hemolytic activity of biotinylated C5 was confirmed. Reactions containing C6 (600nM) and either C5 (400nM) or C5-biotin (400nM) were incubated with C5 convertase (CVF,Bb; 100nM) for 90 minutes at 37°C. Aliquots of the reaction were subjected to SDS-PAGE followed by Coomassie staining to assess activation cleavage or diluted 160 000-fold for the terminal pathway (TP) assay to measure erythrocyte lytic activity. Lack of enzyme with C6 and either C5 or C5-biotin resulted in minimal background lysis. A standard curve (left side) was generated by inducing chicken erythrocyte lysis with purified C5b,6 and the addition of C7, C8, and C9. (B) C5 cleavage in plasma during tissue factor (TF)–dependent clot initiation. Thrombin (IIa) generation was initiated in citrated plasma by the addition of TF, phospholipid vesicles, and CaCl2 in the presence of C5-biotin, and reactions proceeded for 1 hour at 37°C. Coimmunoprecipitation was performed on the clot fluid phase using avidin agarose to isolate all biotinylated C5-containing fragments. Reactions were analyzed on a 4%-20% Tris-HCl gel under reducing conditions and detected by Western blotting using IRDye 800CW Streptavidin. No C5a was detected, but a 35-kDa fragment was generated that was absent when thrombin was inhibited with hirudin. (C-D) Cleavage of C5-biotin (400nM) was assessed in vitro by incubating it with or without 20nM thrombin for 1.5 hours at 37°C. Reaction products were detected by Western blotting using IRDye 800CW Streptavidin (C) or a polyclonal rabbit antibody against C5a and secondary goat anti–rabbit IgG (H + L) IRDye 680 (D), both of which revealed a novel C5-derived 35-kDa fragment. An area of the blot (dotted box) corresponding to the location of C5a was overexposed and depicted below to show the presence of tiny amounts of C5a formed by thrombin in the in vitro cleavage reaction.
Identification of a new thrombin-dependent C5 activation pathway in plasma
Although almost no C5a was generated by thrombin, tissue factor-initiated clot formation in plasma did yield a previously undescribed C5-biotin-derived ∼ 35-kDa fragment (Figure 2B) that was not observed in the presence of hirudin. The same band was observed in a purified system in which C5-biotin was incubated with thrombin 20nM (Figure 2C-D). Its immunodetection with an anti-C5a antibody suggested that this new thrombin-generated fragment of C5-biotin encompasses C5a.
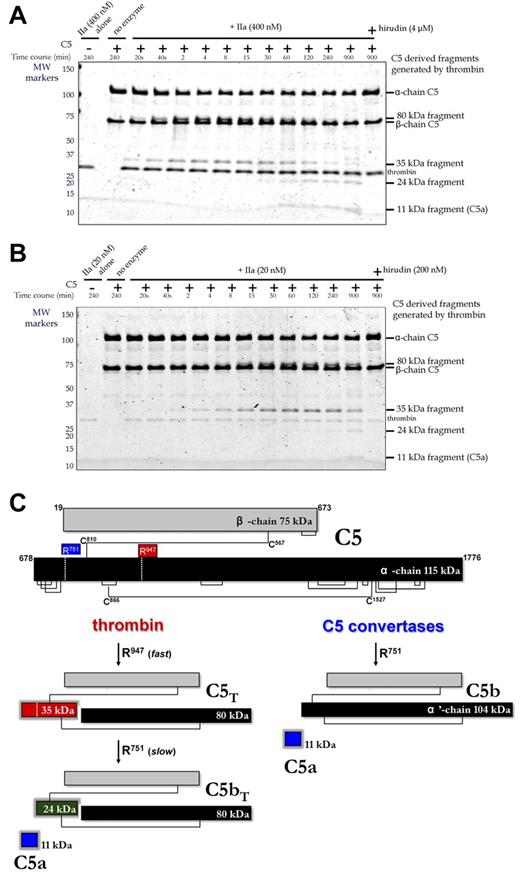
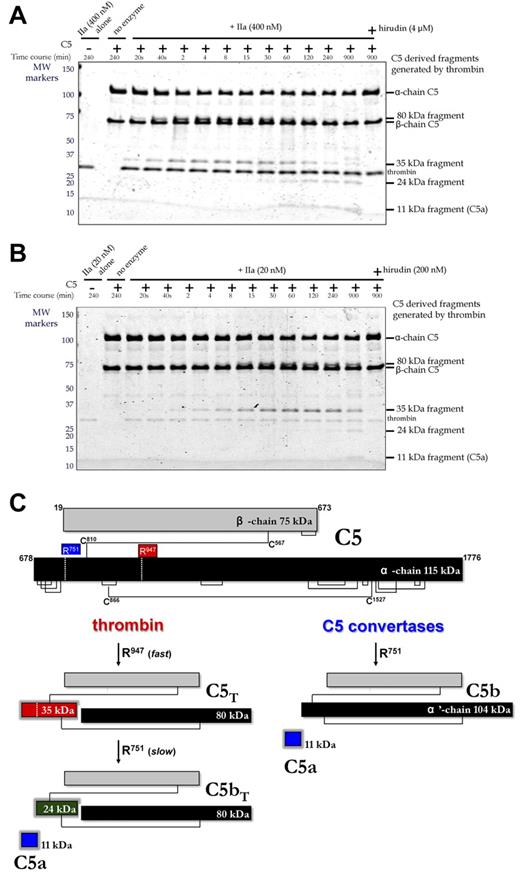
The origin of this fragment was first studied by treating purified C5 with 2 different concentrations of thrombin in vitro (Figure 3A with 400nM thrombin; Figure 3B with 20nM thrombin), monitoring the reaction on Coomassie-stained reducing gels. The 75-kDa β-chain of C5 was not proteolysed even after overnight incubation. However, the 115-kDa α-chain was cleaved and coincided with generation of 35-kDa and 80-kDa fragments that were visible within 20 seconds, becoming more prominent over the ensuing 30 minutes. Longer incubations resulted in the disappearance of the 35-kDa fragment and appearance of 11-kDa and 24-kDa fragments. Hirudin blocked all cleavages (Figure 3A-B, last lane). Identification of the 11-, 24-, 35-, and 80-kDa fragments was accomplished by in-gel trypsin digestion and mass spectrometry. All peptides analyzed from the bands in question (Table 1) were derived from the α-chain (T678-C1676) of C5 (NCBI accession no. P01031.4). The 11-kDa fragment corresponded to T678-R751 representing C5a; the 35-kDa fragment corresponded to T678-R947, which encompasses C5a; the 24-kDa fragment corresponded to L752-R947, which is immediately C-terminal to C5a; the 80-kDa fragment corresponded to G948-C1676 at the most C-terminus of the α-chain.
Temporal analysis of thrombin-mediated cleavage of plasma-derived C5. (A-B) C5 (400nM) was incubated with plasma-derived thrombin (IIa; 400nM, A; 20nM, B) in the presence and absence of hirudin (4μM, A; 200nM, B) in HBS-M7 at 37°C. Aliquots from each reaction were removed at selected time intervals for analysis. For comparison, thrombin and C5 alone are shown in lanes 1 and 2, respectively. Molecular weight (MW) markers on the left are in kDa. (C) Schematic of C5 proteolysis by thrombin and C5 convertase is shown with the number and position of relevant amino acid residues. Thrombin-derived cleavage products were determined by mass spectrometry after in-gel trypsin digestion. C5 convertases cleave only at R751 generating C5a and C5b, the latter comprising the β-chain and a disulfide linked 104-kDa α′-chain.
Temporal analysis of thrombin-mediated cleavage of plasma-derived C5. (A-B) C5 (400nM) was incubated with plasma-derived thrombin (IIa; 400nM, A; 20nM, B) in the presence and absence of hirudin (4μM, A; 200nM, B) in HBS-M7 at 37°C. Aliquots from each reaction were removed at selected time intervals for analysis. For comparison, thrombin and C5 alone are shown in lanes 1 and 2, respectively. Molecular weight (MW) markers on the left are in kDa. (C) Schematic of C5 proteolysis by thrombin and C5 convertase is shown with the number and position of relevant amino acid residues. Thrombin-derived cleavage products were determined by mass spectrometry after in-gel trypsin digestion. C5 convertases cleave only at R751 generating C5a and C5b, the latter comprising the β-chain and a disulfide linked 104-kDa α′-chain.
Thrombin cleavage sites in C5 yield novel activation products C5T and C5bT
Thrombin cleavage sites thus reside at R751 and R947 of the C5 α-chain (Figure 3C). The R947 site was cleaved efficiently, yielding a 3-chained disulfide-linked molecule resembling C5, herein referred to as C5T. At high thrombin concentrations (400nM), the C5T species, coinciding with generation of the 80/35-kDa band pair, was observed at the earliest reaction time point of 20 seconds (Figure 3A). At low thrombin concentrations (20nM), C5T was generated 4-8 minutes into the reaction (Figure 3B). The R751 site was cleaved more slowly by thrombin (ie, apparently after the R947 cleavage), a reaction recognized by the appearance of the 11-kDa C5a fragment (overnight with 20nM thrombin; at 1 hour with 400nM thrombin) and generation of a 3-chained disulfide-linked molecule that resembles C5b, herein referred to as C5bT.
Comparative analyses of C5 cleavage by thrombin and a C5 convertase
With the discovery of a novel thrombin-mediated C5 activation pathway, we compared the proteolytic efficiency of thrombin to C5 convertase. Because the α-chain of C5 is cleaved by thrombin and by C5 convertase, we compared initial α-chain consumption, quantified by densitometry of bands detected by Coomassie staining of reduced gels during a C5 reaction (not shown). The rate of initial α-chain consumption determined for C5 convertase (CVF,Bb) was 0.088 + 0.01 mol/min per (mol of enzyme; n = 3), which agrees with reported values.24 The rate of cleavage of the α-chain of C5 by thrombin, 0.195 + 0.04 mol/min per (mol of enzyme; n = 3) was similar or higher compared with that of the C5 convertase. Thus, thrombin is at least as efficient as C5 convertase in activating C5. However, C5 convertases cleave C5 only at R751 to form C5a and C5b. Thrombin preferentially cleaves at R947 to generate C5T but can also cleave the R751 site, albeit inefficiently, to yield C5bT.
Simultaneous activation of C5 by thrombin and C5 convertase
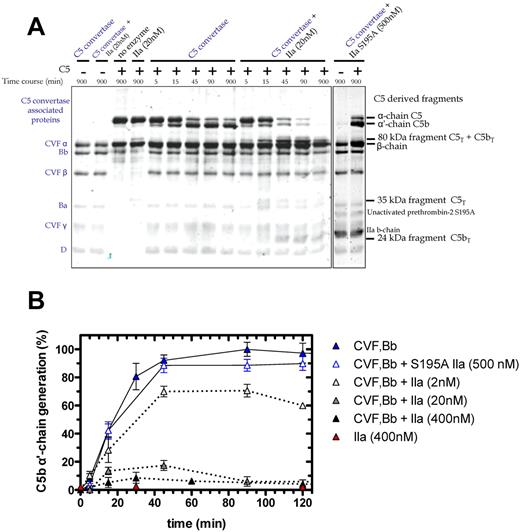
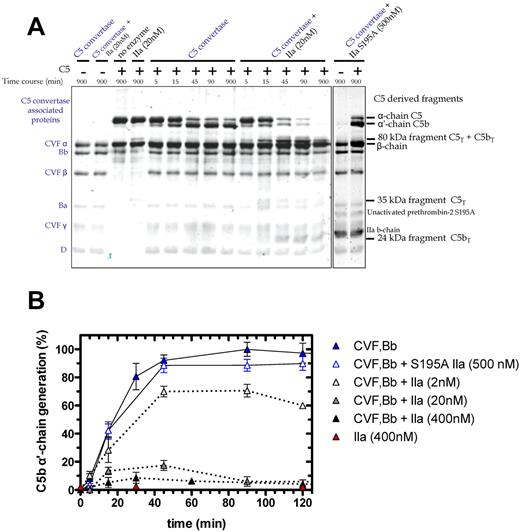
We examined C5 cleavage by the combination of thrombin and C5 convertase (Figure 4A). The C5 convertase (CVF,Bb; left lane of gel) is represented in the Coomassie-stained gel by the 3 chains of CVF, and single bands for Bb, the activation fragment Ba, and factor D. Overnight coincubation of thrombin (20nM) and CVF,Bb (100nM) did not affect the integrity of either enzyme (Figure 4A, second lane). As before, C5 activation by thrombin alone (20nM) generated the 35-kDa fragment (Figure 4A fourth lane). CVF,Bb activation of C5 yielded the expected time-dependent loss of the α-chain and appearance of the α′-chain (reflecting C5b), peaking at 45-90 minutes (C5a is not shown on this gel). Notably, addition of thrombin with a fixed concentration of CVF,Bb had a profound effect on C5 activation compared with CVF,Bb alone. Dual enzyme catalysis of C5 resulted in (1) rapid and complete proteolysis of C5 (monitored by the α-chain) and (2) complete cleavage of the C5 convertase product, C5b (monitored by the α′-chain). Where CVF,Bb alone consistently generated a maximum amount of C5b at 45-90 minutes, the additional presence of thrombin drastically reduced C5b formation (Figure 4A). This effect was dependent on the thrombin concentration (Figure 4B). A low concentration (2nM) resulted in a 30% reduction in C5b, whereas 20nM and 400nM resulted in a further 80% and > 90% reduction, respectively. At the higher thrombin concentrations, C5b was only transiently detected, disappearing with longer incubation (> 60-90 minutes; Figure 4B).
Time course of C5 cleavage by thrombin and C5 convertase. (A) SDS-PAGE analysis of plasma-derived C5 (400nM) cleavage with C5 convertase (CVF,Bb; 100nM) alone or combined with thrombin (20nM) or with S195A thrombin (500nM). At selected time intervals, aliquots of the reactions were subjected to SDS-PAGE under reducing conditions. Proteins were visualized by Coomassie staining. Additional lanes on right are shown to match bands to their corresponding protein. (B) Generation of the C5b α′-chain is shown over time during C5 cleavage with C5 convertase alone (CVF,Bb; 100nM; blue) or thrombin alone (IIa: 400nM; red) or with both C5 convertase (CVF,Bb; 100nM) and thrombin (IIa: 2nM; light gray, 20nM; dark gray or 400nM; black) or with inactive S195A thrombin (S195A IIa: 500nM; clear with blue border). C5b α′-chain–containing bands were quantified by scanning densitometry. C5b α′-chain generated by C5 convertase after 90 minutes was considered 100%. Data are mean ± SD of 2-5 independent experiments.
Time course of C5 cleavage by thrombin and C5 convertase. (A) SDS-PAGE analysis of plasma-derived C5 (400nM) cleavage with C5 convertase (CVF,Bb; 100nM) alone or combined with thrombin (20nM) or with S195A thrombin (500nM). At selected time intervals, aliquots of the reactions were subjected to SDS-PAGE under reducing conditions. Proteins were visualized by Coomassie staining. Additional lanes on right are shown to match bands to their corresponding protein. (B) Generation of the C5b α′-chain is shown over time during C5 cleavage with C5 convertase alone (CVF,Bb; 100nM; blue) or thrombin alone (IIa: 400nM; red) or with both C5 convertase (CVF,Bb; 100nM) and thrombin (IIa: 2nM; light gray, 20nM; dark gray or 400nM; black) or with inactive S195A thrombin (S195A IIa: 500nM; clear with blue border). C5b α′-chain–containing bands were quantified by scanning densitometry. C5b α′-chain generated by C5 convertase after 90 minutes was considered 100%. Data are mean ± SD of 2-5 independent experiments.
The C5 cleavage patterns were independent of the thrombin source (ie, plasma-derived human thrombin vs human wild-type recombinant thrombin; data not shown). An active site mutant S195A thrombin23 had no effect on C5 activation alone or in combination with C5 convertase (Figure 4A-B). Similarly, hirudin prevented the catalytic activity of thrombin on C5 (not shown). Finally, isolated components of the CVF,Bb enzyme mixture alone (CVF or complement factors B, Ba, Bb or D) or in combination with thrombin did not recapitulate the dual enzyme activity observed. Thus, both thrombin and the C5 convertase must be intact to achieve the observed C5 proteolysis.
Activity of C5T and C5bT in complement-mediated cell lysis
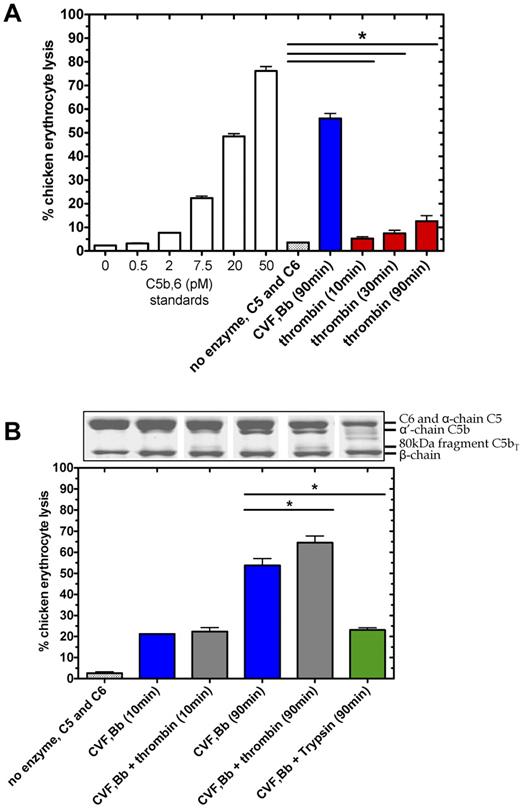
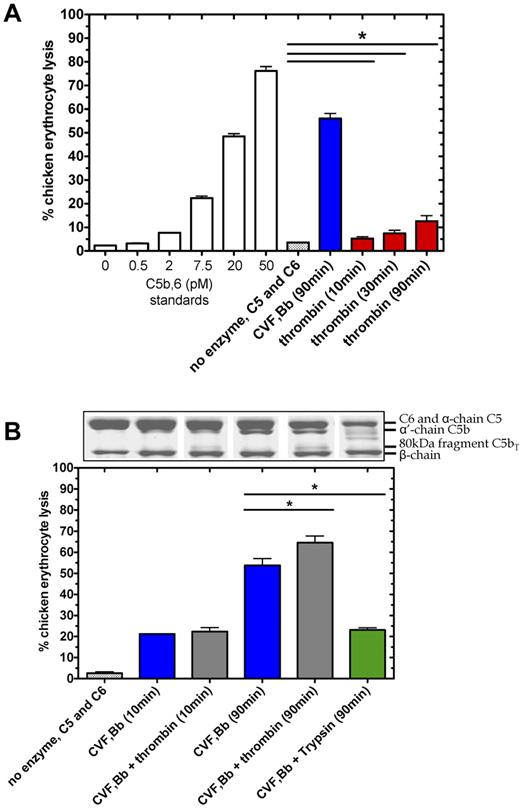
To test the functional consequence of thrombin-mediated proteolysis at R947 in C5 (C5T generation) and C5b (C5bT generation), we assessed the lytic properties of these molecules when exposed to chicken erythrocytes with excess purified terminal components (C6, C7, C8, and C9). As expected, C5 convertase generated a robust lytic response on C5 (400nM) activation in the presence of excess C6 after 90 minutes, corresponding to ∼ 55% lysis (Figure 5A) or equivalent to ∼ 300nM C5b,6 in the original activation reaction (after correction for dilution). Thrombin (400nM) cleavage of C5 in the presence of C6, without C5 convertase, also caused erythrocyte lysis to a significantly greater extent after 90 minutes (P < .05, n = 3) than that of C5 and C6 background lysis without thrombin. Although the thrombin-mediated lysis (equivalent to ∼ 4nM C5b,6 after 90 minutes) was considerably less than that attributed to the C5 convertase, the results suggest that thrombin proteolysis of C5 in the presence of C6, forms a lytic component. Similar data were obtained when the purified terminal components C7-C9 were replaced with serum, which contains natural inhibitors and competitive substrates of both enzymes (not shown).
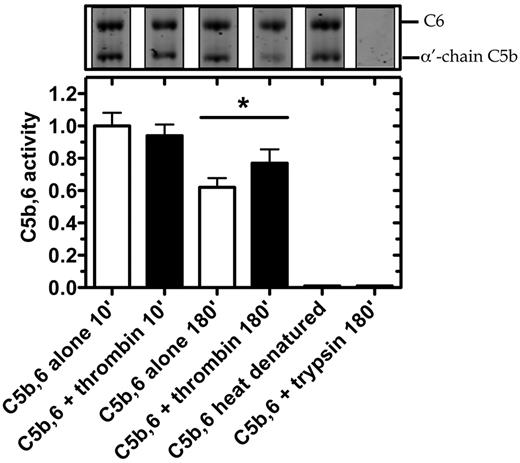
Thrombin-mediated hemolytic activity as modulated by C5 convertase. (A) Thrombin-mediated hemolytic activity was compared with C5 convertase hemolytic activity. In the terminal pathway assay, a standard curve was generated by measuring lysis of chicken erythrocytes after addition of a range of concentrations of purified C5b,6 and excess C7, C8, and C9. Percent lysis is expressed relative to 100% lysis in H2O. To measure enzyme-induced hemolysis, reactions containing C5 (400nM) and C6 (600nM) incubated alone, with C5 convertase (CVF,Bb; 100nM) or with thrombin (400nM), were diluted 2000-fold, 8000-fold, and 2000-fold, respectively, to measure hemolytic activity in the terminal pathway assay. (B) Thrombin-mediated hemolytic activity in the presence of C5 convertase was measured. The ability of thrombin to augment C5 convertase hemolytic activity was assessed in reactions as noted above but with a lower concentration of thrombin (20nM) and in the presence or absence of C5 convertase (CVF,Bb; 100nM) or trypsin (20nM). Reactions were either subjected to SDS-PAGE or diluted 160 000-fold for the terminal pathway hemolytic assay. Data are mean ± SD; n = 3. *P < .05.
Thrombin-mediated hemolytic activity as modulated by C5 convertase. (A) Thrombin-mediated hemolytic activity was compared with C5 convertase hemolytic activity. In the terminal pathway assay, a standard curve was generated by measuring lysis of chicken erythrocytes after addition of a range of concentrations of purified C5b,6 and excess C7, C8, and C9. Percent lysis is expressed relative to 100% lysis in H2O. To measure enzyme-induced hemolysis, reactions containing C5 (400nM) and C6 (600nM) incubated alone, with C5 convertase (CVF,Bb; 100nM) or with thrombin (400nM), were diluted 2000-fold, 8000-fold, and 2000-fold, respectively, to measure hemolytic activity in the terminal pathway assay. (B) Thrombin-mediated hemolytic activity in the presence of C5 convertase was measured. The ability of thrombin to augment C5 convertase hemolytic activity was assessed in reactions as noted above but with a lower concentration of thrombin (20nM) and in the presence or absence of C5 convertase (CVF,Bb; 100nM) or trypsin (20nM). Reactions were either subjected to SDS-PAGE or diluted 160 000-fold for the terminal pathway hemolytic assay. Data are mean ± SD; n = 3. *P < .05.
We next compared the hemolytic activity of products formed from C5 in the presence of excess C6 when subjected to proteolysis by the C5 convertase alone versus a low concentration of thrombin and the C5 convertase. C5 convertase alone (Figure 5B blue bars) caused a lytic response proportional to the amount of α′-chain (C5b) generated over the reaction time (Figure 5B aligned Coomassie-stained gel), corresponding to 22% lysis (C5b,6 ∼ 80nM) after 10 minutes and 55% lysis (C5b,6 ∼ 400nM) after 90 minutes. With both thrombin and C5 convertase, the amount of C5b was reduced compared with C5 convertase, replaced by C5bT. Nonetheless, the amount of lytic activity increased after 90 minutes (Figure 5B gray bars) to a significantly greater extent than seen with C5 convertase alone (P < .05). The specific nature of thrombin's activity was demonstrated using a similarly low concentration of trypsin to cleave C5, the latter which resulted in reduced hemolytic activity (Figure 5B green bar). Similar results of these functional experiments were found when the zymosan bound alternative pathway C5 convertase was used instead of the CVF-based C5 convertase (supplemental Figures 1 and 2).
Overall, thrombin-mediated cleavage of C5/C5b at R947 results in formation of C5bT,6 (and possibly C5T,6) that generates an intact MAC that exhibits more hemolytic activity than from C5b,6. Similar results were obtained when experiments were performed with serum in place of the terminal components C7-C9 (not shown).
Thrombin cleaves C5b,6 to generate C5bT,6
The essentially irreversibly bound complex, C5b,6 is considered the exclusive initiator of assembly of the MAC C5b-930 and was used as the substrate in the following experiments. We assessed whether thrombin alone or in combination with C5 convertase could alter C5b or C6 when complexed as C5b,6 (supplemental Figure 3). At thrombin concentrations of 20-500nM, C6 within C5b,6 remained resistant to proteolysis. However, the R947 site in C5b within C5b,6 was cleaved by thrombin to generate C5bT, defined by the 80- and 24-kDa fragments (supplemental Figure 3). Thus, even low concentrations of thrombin can convert C5b,6 to C5bT,6, and the addition of C5 convertase does not alter the activity of thrombin in this reaction (supplemental Figure 3 right lanes).
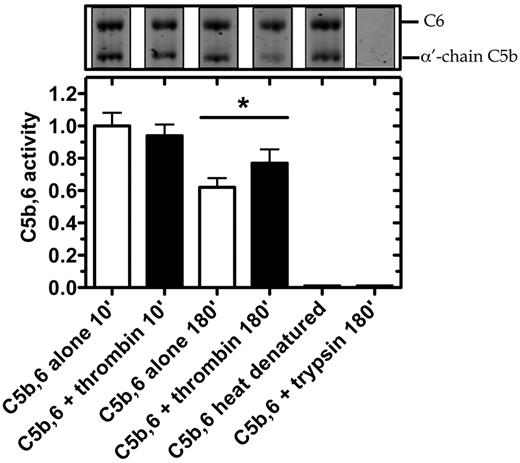
We next tested in serum whether thrombin could enhance the lytic activity of C5b within the preformed C5b,6 complex (Figure 6). After 10-minute incubation with C5b,6, thrombin had no effect on erythrocyte lysis. However, after 180 minutes, erythrocyte lysis was moderately but significantly increased by the addition of thrombin. Disrupting the structure of C5b,6 by heat denaturation or trypsin proteolysis abolished C5b,6 lytic activity (Figure 6), underlining thrombin's specificity. The findings indicate that the conversion of C5b,6 to C5bT,6 by low concentrations of thrombin results in effective assembly of a C5bT-9 MAC complex and that this MAC has greater lytic activity than that of the C5b-9 MAC.
Thrombin enhances hemolytic activity of C5b,6 in serum-based assay. C5b,6 (200nM) was incubated alone or with either thrombin (500nM) or trypsin (500nM; as control) at 37°C for the indicated times. At the indicated time, an aliquot was prepared for SDS-PAGE analysis or diluted (2000-fold) to quantify residual C5b,6 activity using the serum-based hemolytic assay. C5b,6 alone (after 10 minutes of incubation) resulted in 23.8% ± 2.7% lysis and was arbitrarily given an activity of 1.0. Heat-denatured C5b,6 (10 minutes at 95°C) is shown as an additional control. Data are mean ± SD of 3 independent experiments each performed in quadruplicate. *P < .05.
Thrombin enhances hemolytic activity of C5b,6 in serum-based assay. C5b,6 (200nM) was incubated alone or with either thrombin (500nM) or trypsin (500nM; as control) at 37°C for the indicated times. At the indicated time, an aliquot was prepared for SDS-PAGE analysis or diluted (2000-fold) to quantify residual C5b,6 activity using the serum-based hemolytic assay. C5b,6 alone (after 10 minutes of incubation) resulted in 23.8% ± 2.7% lysis and was arbitrarily given an activity of 1.0. Heat-denatured C5b,6 (10 minutes at 95°C) is shown as an additional control. Data are mean ± SD of 3 independent experiments each performed in quadruplicate. *P < .05.
Discussion
Complement activation proceeds through several multiprotein complexes called convertases. These are capable of proteolytically activating, in sequence, C3 and in turn, by enzyme amplification, C5.31 More recently, investigators have reported that several enzymes, including thrombin, may activate C5 in a C3-independent manner by bypassing the bona fide convertases.16–19 Regardless of the enzyme, C5 has always been thought to be singularly activated at R751, liberating 2 functional products: the anaphylatoxin C5a and the initiator of MAC assembly C5b. By identifying an additional and functionally relevant thrombin-sensitive cleavage site in C5 at R947, our findings challenge that dogma. The discovery of previously undescribed, biologically active C5 products presents a new paradigm for initiation and assembly of a more active MAC and demonstrates at a molecular level how the coagulation and complement pathways are fully integrated through activation of C5 by both thrombin and C5 convertase.
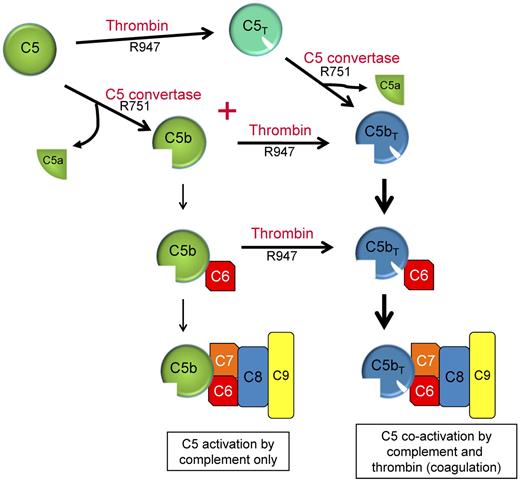
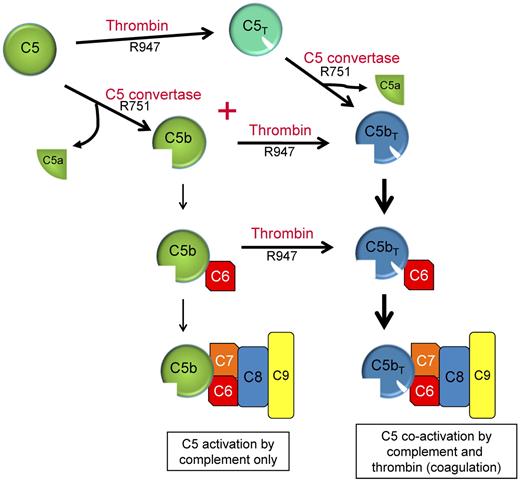
Our studies indicate that thrombin's role in C5 activation is predominantly linked to the coexistence of a C5 convertase. Efficient proteolysis of C5 at R751 and R947 requires the availability of both a C5 convertase and thrombin, a situation that probably occurs with all tissue injury. With both enzymes present, the catalysis pathway of C5 is dependent on the concentrations of C5 convertase and thrombin and proceeds via 2 activation intermediates (either C5b or C5T) before yielding the final activation products C5a and C5bT (Figure 7). During pathologies in which complement activation occurs in the setting of low thrombin concentrations, the predominant C5 activation intermediate is C5b, with minimal C5T generation. However as thrombin is efficient at cleaving R947 in C5b, even at low concentrations, the C5b has a transient existence before being converted to C5bT. During a potent coagulation response with thrombin concentrations of 200-500nM, the pathway shifts to the formation of both C5b and C5T intermediates generated at similar rates. The existence of C5b is however short-lived, as thrombin immediately converts the nascent-formed C5b intermediate to C5bT. C5T, the other intermediate formed when both complement and coagulation are active, can be cleaved by thrombin and presumably by the C5 convertase to generate C5a and C5bT.
Dual enzyme activation of C5. C5b, the product of C5 activation, has been regarded as the exclusive initiator of MAC complex assembly (left: C5 activation by complement only). C5 convertases and thrombin are often simultaneously formed in response to many pathologies in vivo to maintain homeostasis. We propose that activation of C5 proceeds via 2 pathways using either C5T or C5b as reaction intermediates and C5a and C5bT as final reaction products. C5b,6 that is formed is probably rapidly converted to C5bT,6.
Dual enzyme activation of C5. C5b, the product of C5 activation, has been regarded as the exclusive initiator of MAC complex assembly (left: C5 activation by complement only). C5 convertases and thrombin are often simultaneously formed in response to many pathologies in vivo to maintain homeostasis. We propose that activation of C5 proceeds via 2 pathways using either C5T or C5b as reaction intermediates and C5a and C5bT as final reaction products. C5b,6 that is formed is probably rapidly converted to C5bT,6.
Our results indicate that thrombin proteolysis of R947 has a faster rate of cleavage in the intermediate C5b compared with C5. This is apparent if C5b α′-chain consumption (Figure 4A; fast R947 cleavage) is compared with that of the C5 α-chain (Figure 3B; slow R947 cleavage). Conformational changes in the α-chain, including structures around R947, have been shown to accompany the C5 to C5b transition,32 which may explain the preference of thrombin for C5b as a substrate over C5.
Surprisingly, we observed that thrombin alone could, to a minor extent, promote hemolysis by cleaving C5 in the presence of C6. Formation of 3 possible complexes may provide an explanation: (1) C5bT,6: this would be the likeliest explanation, as tiny amounts of C5bT were generated at 10-90 minutes. The ability of thrombin to convert C5b,6 to C5bT6 also supports this hypothesis. (2) C5T,6: C5 and C6 do associate but do not exhibit lytic function.33,34 It is possible that R947 cleavage produces a C5T,6 complex that contrary to C5,6, initiates lysis. (3) C5b,6: although we never detected the C5b-specific α′-chain during thrombin-mediated C5 cleavage, it is possible that C5b (and thus C5b,6) was generated in amounts sufficient to lyse the highly sensitive chicken erythrocytes. If the thrombin cleavages in C5 are not order-dependent, thrombin conceivably could generate some C5b, allowing C5b,6-dependent lysis.
Our detection of a highly thrombin-sensitive cleavage site in C5 at R947 prompted comparative analyses with other thrombin substrates. The active site of thrombin prefers a large- to middle-sized hydrophobic residue at P4, usually a proline at P2, an arginine at P1, a small residue in P1′, a bulky hydrophobic residue at P2′, and a small- or middle-sized hydrophobic residue at P4′. Residues flanking R947 of C5 have all these features, overall being most similar to protein C (supplemental Figure 4A).35 The site in human C5 is well conserved in nonhuman primates, mice, rats, pigs, and possibly hagfish (supplemental Figure 4B), further supporting the likelihood of it being physiologically relevant. Although the R751 site has a P1 Arg residue, it otherwise contains little similarity to the cleavage sites in other classic thrombin substrates (P4-P4′; supplemental Figure 4A). In line with this, thrombin only cleaves R751 at a slow rate of ∼ 0.001 mol of C5a/min per (mol of thrombin; data not shown). In comparison, C5 convertase cleaves R751 ∼ 100-fold more efficiently (0.088 + 0.01 mol of C5a/min per (mol of CVF,Bb). Admittedly, we cannot exclude the possibility that circulating blood and/or endothelial cells enhance thrombin-dependent C5a generation in vivo. An obvious candidate is thrombomodulin to which thrombin binds and accelerates protein C activation.36 However, in preliminary studies, the soluble ectodomain of thrombomodulin did not accelerate thrombin-mediated C5 proteolysis at either R751 or R947 (not shown).
Taken together, we propose a new paradigm in which thrombin partners with the traditional C5 convertases during complement activation through the formation of C5bT and possibly C5T. C5bT that is generated either from C5b immediately after its generation and before C6 binding, or from within the C5b,6 complex, gives rise to the C5bT6 complex, which in turn leads to assembly of a functional MAC (Figure 7) with increased lytic activity. Although it has long been accepted that C5b is the initiator of MAC assembly, our data suggest that in pathologies in which complement is activated in the presence of thrombin, even small amounts, the predominant initiator of the terminal pathway leading to MAC assembly is C5bT. The mechanism(s) of enhancement of thrombin-mediated lytic activity via C5bT is currently unresolved. It is noteworthy that C6, C7, and C8 all have domains that bind to regions of the C5b α′-chain.33,37 It is therefore probable that R947 cleavage in the C5b α′-chain, with an accompanying conformational change, affects these interactions, as well as the capacity of regulators of complement activation to suppress the lytic activity of the C5bT-derived MAC. Further study is necessary to understand these pathways, which may impact on many diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jason C. Rogalski at the Center for Blood Research proteomics hub for assistance with mass spectrometric analyses.
E.M.C. is supported by the Canadian Institutes of Health Research and the Canada Foundations for Innovation. E.M.C. holds a CSL Behring Research Chair and a Canada Research Chair in Endothelial Cell Biology and is an adjunct Scientist with the Canadian Blood Services. E.L.G.P. is supported by the Heart and Stroke Foundation of British Columbia and Yukon. E.M.C. and E.L.G.P. are members of the University of British Columbia Life Sciences Institute.
Authorship
Contribution: E.M.C. supervised and designed all studies and wrote the manuscript; M.J.K. designed and performed experiments and wrote the manuscript; E.L.G.P. helped in the design of experiments; V.G. designed and performed experiments; and Z.L., S.C.M., and T.M. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward M. Conway, Centre for Blood Research, 4306-2350 Health Sciences Mall, University of British Columbia, Vancouver, BC, Canada V6T 1Z3; e-mail: emconway@exchange.ubc.ca.