Abstract
Adult T-cell leukemia-lymphoma (ATL) is an intractable mature T-cell neoplasm. We performed a nationwide retrospective study of allogeneic hematopoietic stem cell transplantation (HSCT) for ATL in Japan, with special emphasis on the effects of the preconditioning regimen. This is the largest study of ATL patients receiving HSCT. Median overall survival (OS) and 3-year OS of bone marrow or peripheral blood transplantation recipients (n = 586) was 9.9 months (95% confidence interval, 7.4-13.2 months) and 36% (32%-41%), respectively. These values for recipients of myeloablative conditioning (MAC; n = 280) and reduced intensity conditioning (RIC; n = 306) were 9.5 months (6.7-18.0 months) and 39% (33%-45%) and 10.0 months (7.2-14.0 months) and 34% (29%-40%), respectively. Multivariate analysis demonstrated 5 significant variables contributing to poorer OS, namely, older age, male sex, not in complete remission, poor performance status, and transplantation from unrelated donors. Although no significant difference in OS between MAC and RIC was observed, there was a trend indicating that RIC contributed to better OS in older patients. Regarding mortality, RIC was significantly associated with ATL-related mortality compared with MAC. In conclusion, allogeneic HSCT not only with MAC but also with RIC is an effective treatment resulting in long-term survival in selected patients with ATL.
Introduction
Adult T-cell leukemia-lymphoma (ATL) is an aggressive peripheral T-cell neoplasm caused by human T-cell lymphotropic/leukemia virus type-1. It has a very poor prognosis.1–4 A recent phase 3 trial for previously untreated patients with aggressive ATL (acute, lymphoma, or unfavorable chronic type) aged 33 to 69 years demonstrated that the dose-intensified multidrug regimen VCAP-AMP-VECP resulted in a median overall survival (OS) and OS at 3 years of 12.7 months and 24%, respectively. The OS plot for this treatment did not reach a plateau.5 Alternatively, based on a meta-analysis, Bazarbachi et al proposed that zidovudine (AZT) and interferon (IFN)–α should be considered the standard for first-line therapy in patients with acute, chronic, or smoldering types of ATL. They reported median OS and 5-year OS for acute-type ATL treated with AZT/IFN-α to be 9 months and 28%, respectively, whereas these values were 7% and 0%, respectively, for lymphoma-type ATL.6 These results indicate that conventional chemotherapeutic agents alone, even including AZT/IFN-α, yield few or no long-term remissions or potential cures in ATL patients.
Although early experience in myeloablative chemoradiotherapy together with autologous hematopoietic stem cell rescue for ATL was associated with a high incidence of relapse and fatal toxicities,7 allogeneic hematopoietic stem cell transplantation (HSCT) has been explored as a promising alternative treatment that can provide long-term remission in a proportion of patients with ATL.8–10 Therefore, we previously performed a nationwide retrospective study of ATL patients who received allogeneic HSCT in Japan before December 31, 2005, with special emphasis on the effect of the graft source: 296 patients received bone marrow (BM) and/or peripheral blood stem cells (PBSCs) and 90 received cord blood.11 We concluded that allogeneic HSCT using currently available sources is an effective treatment in selected patients with ATL, although greater effort is warranted to reduce treatment-related mortality (TRM). In addition, the use of unrelated cord blood as a stem cell source was associated with lower survival, with a median OS and unadjusted 3-year probability of OS of 2.6 months and 17% (95% confidence interval [CI], 9%-25%), respectively. Because the results suggested that allogeneic BM and PBSCs could be considered to be the more standard donor forms, rather than unrelated cord blood, for transplantation in ATL, as a next step, here we report results of a nationwide retrospective study of Japanese ATL patients receiving allogeneic HSCT, especially focusing on bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT), with special emphasis on the effects of the preconditioning regimen. Our current analysis included the previous cohort11 (January 1996–December 2005) with updated clinical information as well as data on one patient who received allogeneic HSCT in February 1992 and patients who received allogeneic HSCT after December 2005. It is thought that allogeneic HSCT with reduced intensity conditioning (RIC) depends more on donor cellular immune effects after transplantation and less on the cytotoxic effects of the conditioning regimen to eradicate residual tumor cells than conventional myeloablative conditioning (MAC). In this context, RIC might be suitable for ATL because several reports have suggested the existence of graft-versus-T-cell lymphotropic/leukemia virus type-1 or graft-versus-ATL effects.12–18 In addition, RIC might be associated with reduced TRM, which has represented a significant obstacle to successful allogeneic HSCT for ATL patients.11 Furthermore, ATL has a long latency and occurs in older individuals at a median age of nearly 60 years.19,20 There is the possibility that HSCT with RIC can provide clinical benefits for those older patients who hardly benefit from allogeneic HSCT with MAC. Here, we performed multivariate analyses of OS and treatment-related or ATL-related mortality after allogeneic BMT and PBSCT and have identified factors influencing transplantation outcomes in ATL patients.
Methods
Collection of data
Data on patients with ATL who had received their first allogeneic BMT, PBSCT, or BMT + PBSCT between February 1992 and December 2009 were collected from nationwide survey data of the Japan Society for Hematopoietic Cell Transplantation (JSHCT). Cases with missing preconditioning or survival data were excluded, with the result that 586 patients were included in the analysis. Data collected for analysis included the patients' clinical characteristics such as age at transplantation, sex, disease status at transplantation, date of transplantation, time from ATL diagnosis to transplantation, performance status (PS) according to the Eastern Cooperative Oncology Group criteria at transplantation, source of stem cells, relationship between recipient and donor, ATL clinical subtype,1 preconditioning regimens, date alive at last follow up, date and cause of death, and incidence and severity of acute graft-versus-host disease (GVHD). When serologic or molecular typing for HLA-A, HLA-B, and HLA-DR were identical between the recipient and the related donor, we determined the relationship as HLA-matched related. As a control, data on patients with ATL who had received their first unrelated cord blood transplantation (CBT) between March 2001 and December 2009 were collected from the nationwide survey data of the JSHCT. Cases with missing survival data were excluded, resulting in the inclusion of 174 patients in the present study. The study was approved by the data management committees of the JSHCT, as well as by the institutional ethics committee of Nagoya City University Graduate School of Medical Sciences.
Definitions
OS was defined as the time from transplantation until death, and patients who remained alive at the time of the last follow-up were censored. For analysis, patients were divided into 2 age groups, either > or ≤ 55 years, because the Japanese Clinical Oncology Group is currently conducting a phase 2 study of strategies including allogeneic HSCT other than CBT with MAC for ATL patients aged 20 to 55 years (UMIN000004147). Reported causes of death were reviewed and categorized into ATL-related or TRM. ATL-related mortality was defined as death caused by relapse or progression of ATL in patients who survived for at least 1.0 month after transplantation based on the judgment of each institution. TRM was defined as any death other than ATL-related mortality. Acute GVHD was diagnosed and graded using traditional criteria21 by the physicians who performed transplantations at each institution. Patients undergoing allogeneic BMT or PBSCT were divided into 2 groups based on the preconditioning regimens, with 1 group being MAC and the other group RIC. MAC or RIC was defined according to the proposals by Giralt et al22 and Bacigalupo et al,23 with a slight modification. In the present study, MAC was defined as any regimen that includes (1) ≥ 5 Gy of total body irradiation (TBI) as a single fraction or ≥ 8 Gy fractionated, (2) busulfan (BU) > 8 mg/kg orally or the intravenous equivalent, or (3) melphalan (Mel) > 140 mg/m2. All other regimens were classified as RIC. MAC was further subdivided into 4 groups as follows: TBI (n = 208), BU (n = 46), Mel (n = 21), and other types (n = 3). RIC also was subdivided into 3 groups: fludarabine (Flu) + BU (n = 165), Flu + Mel (n = 86), and other types (n = 49).
Statistical analysis
Descriptive statistics were used for summarizing variables related to patient demographics and transplant characteristics. Comparisons among the groups were performed by Fisher exact test as appropriate for categorical variables. The probability of OS was estimated according to the Kaplan-Meier method. The Cox proportional hazard model was used for multivariate analyses for OS using all independent variables in the model and then using a stepwise selection method by minimizing the Akaike Information Criterion (AIC). The AIC penalizes overparametrization, and variables are retained only when the model improves enough to balance the number of parameters. The lower the AIC, the better the predictive model fits the data.24 Our inspection of plots of OS estimates versus follow-up time indicated that the assumption of proportional hazards for all variables used seemed to be valid. In the Cox proportional hazard model, incidence and severity of acute GVHD was treated as a time-varying covariate25 as described previously.12 Fine and Gray proportional hazard modeling was used to estimate the effect of the same variables used in multivariate analysis of OS on the cumulative incidence of TRM and ATL-related mortality, respectively.26,27 All analyses including competing risk analysis28,29 were performed using the cmprsk package of R Version 2.9.0 for Windows statistics software. Statistical significance was set at P < .05.
Results
Patients' characteristics
Among 586 ATL patients who received allogeneic BMT or PBSCT (mean age, 52 years; median, 53 years; range, 15-72 years), 280 received MAC (mean age, 48 years, median, 49 years; range, 15-69 years) and the remaining 306 received RIC (mean age, 56 years; median, 57 years; range, 28-72 years). Characteristics of these ATL patients are shown in Table 1. In comparison with MAC recipients, significantly more RIC recipients belonged to the older age group (56-72 years), more often received PBSCs as the stem cell source and more frequently had a related donor transplantation. There was no significant difference between MAC and RIC recipients regarding PS distribution from 0 to 4, but unknown PS was observed in significantly more MAC recipients than RIC recipients. There were no significant differences between MAC and RIC recipients regarding sex, disease status at transplantation (in complete remission [CR], not in CR, or unknown), and ATL clinical subtypes (chronic/smoldering, acute, lymphoma, or unknown). There were also no significant differences between MAC and RIC recipients regarding the date of transplantation and time from diagnosis to transplantation, both of which were equally distributed in quartiles among the 586 cases.
The 174 ATL patients who received unrelated CBT were aged 54 years, on average, with a median of 55 years and range of 27 to 79 years. There were 69 females and 105 males, with an ATL status at transplantation of CR (n = 50), not in CR (n = 115), and unknown (n = 9).
As for infectious complications, 145 of the 280 MAC recipients had bacterial infection, and 94 did not. Information on bacterial infection was missing for the remaining 41 MAC recipients. As for fungal infection, 23 and 219, respectively, did and did not have fungal infection; no such information was available on 38 patients. As to viral infection, 65 and 177, respectively, did and did not experience a viral infection, with such data missing on the remaining 38 patients. When we examined data on infectious complications in the RIC recipients, we found that of the 306 RIC recipients 134 had bacterial infection and 121 did not, with data unavailable for the remaining 51 patients. Twenty-three RIC recipients had fungal infection and 232 did not; no such information was available for 51 patients. As to viral infection, 57 and 199 patients, respectively, had and did not have viral infection; no information was available on the remaining 50 patients.
OS of patients receiving allogeneic HSCT
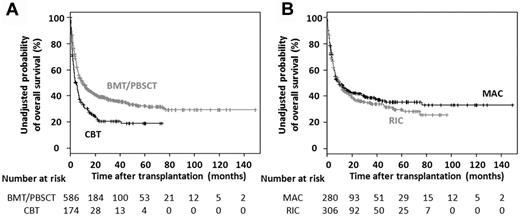
The unadjusted 3-year probability of OS was 36% (95% CI, 32%-41%) in the 586 ATL patients receiving allogeneic BMT or PBSCT and 21% (95% CI, 15%-29%) in the 174 patients receiving unrelated CBT. The median OS of the former was 9.9 months (95% CI, 7.4-13.2 months) and of the latter, 4.3 months (95% CI, 3.2-6.5 months; Figure 1A).
OS of ATL patients receiving allogeneic HSCT. (A) Kaplan-Meier curves of estimated OS in ATL patients receiving allogeneic BMT, PBSCT, or unrelated CBT. (B) Kaplan-Meier curves of estimated OS in ATL patients receiving allogeneic BMT or PBSCT with MAC or RIC.
OS of ATL patients receiving allogeneic HSCT. (A) Kaplan-Meier curves of estimated OS in ATL patients receiving allogeneic BMT, PBSCT, or unrelated CBT. (B) Kaplan-Meier curves of estimated OS in ATL patients receiving allogeneic BMT or PBSCT with MAC or RIC.
The unadjusted 3-year probability of OS was 39% (95% CI, 33%-45%) in the 280 ATL patients receiving MAC and 34% (95% CI, 29%-40%) in the 306 patients receiving RIC. The median OS of the former was 9.5 months (95% CI, 6.7-18.0 months), and of the latter 10.0 months (95% CI, 7.2-14.0 months; Figure 1B).
Multivariate analysis of factors influencing OS in ATL patients receiving allogeneic BMT or PBSCT
Of the 586 ATL patients receiving allogeneic HSCT other than unrelated CBT, 4 were excluded because of lack of data on the time from diagnosis to transplantation, 2 were excluded because of receiving BMT and PBSCT together, and 2 were excluded because of lack of data on HLA. Multivariate analysis of OS was therefore conducted on a total of 578 patients (Table 2). The following 10 variables were analyzed: age (15-55 or 56-72 years), sex, disease status (CR, not CR, or unknown), date of transplantation (1992.2-2004.12, 2004.12-2006.10, 2006.10-2008.4, or 2008.4-2009.12), time from diagnosis to transplantation (0.5-4.9, 4.9-6.9, 6.9-10.1, or 10.1-143.2 months), PS (0, 1, 2-4, or unknown), source of stem cells (BM or PBSCs), relationship between recipient and donor (HLA-matched related, HLA-mismatched related, or unrelated), ATL clinical subtype (chronic/smoldering, acute, lymphoma, or unknown), and preconditioning regimen (MAC or RIC). Five variables, age, sex, disease status, PS, and relationship between recipient and donor, were retained by stepwise Cox regression analysis by minimizing the AIC, as was the preconditioning regimen, which received special emphasis in this study. Of these 6 variables, the following 5 significantly affected OS: older age (56-72 years compared with 15-55 years; hazard ratio [HR], 1.334; 95% CI, 1.035-1.719), male sex (HR, 1.376; 95% CI, 1.113-1.702), not being in CR compared with CR (HR, 1.940; 95% CI, 1.511-2.490), worse PS (1 compared with 0; HR, 1.498; 95% CI, 1.171-1.916, 2-4 compared with 0; HR, 4.057; 95% CI, 2.957-5.565), and transplantation from an unrelated donor compared with HLA-matched related donor (HR 1.276; 95% CI, 1.009-1.613).
Multivariate analysis of factors influencing OS including acute GVHD in ATL patients receiving allogeneic BMT or PBSCT
Of the 586 ATL patients receiving allogeneic HSCT other than unrelated CBT, 2 were excluded because of lack of data on HLA and 57 were excluded because of missing any data on the time from transplantation to onset of acute GVHD or the severity of acute GVHD. Thus, multivariate analysis on 527 ATL patients was performed using the following 7 variables: age, sex, disease status, PS, relationship of the donor to the recipient, preconditioning regimen, and incidence and severity of acute GVHD. Of these, 5 variables significantly affected OS; they were male sex (HR, 1.472; 95% CI, 1.168-1.855), not in CR (HR, 1.943; 95% CI, 1.491-2.532), worse PS (1 compared with 0; HR, 1.534; 95% CI, 1.182-1.991, 2-4 compared with 0; HR, 3.223; 95% CI, 2.256-4.605), transplantation from an unrelated donor compared with that from an HLA-matched related donor (HR, 1.449; 95% CI, 1.115-1.882), and acute GVHD. HRs for death of recipients having grades 1 or 2 and 3 or 4 acute GVHD compared with recipients having no acute GVHD were 0.753 (95% CI, 0.576-0.984), and 1.538 (95% CI, 1.123-2.107), respectively (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This result suggesting that an appropriate level of acute GVHD contributed to better OS but that severe GVHD contributed to inferior OS was consistent with our previous report.12 In contrast, the inclusion of a posttransplant time-varying covariate, acute GVHD, into the present study resulted in a decrease in the number of evaluable patients. In addition, the inclusion of patients who died so early after transplantation that onset of acute GVHD would not yet have occurred provided unacceptable bias leading to the finding that recipients without acute GVHD had worse OS compared with recipients with acute GVHD. Thus, we conducted the present subsequent analyses that aimed to clarify the significance of the preconditioning regimen MAC versus RIC in ATL patients by only including time-fixed covariates that were present pretransplantation.
Interactions of the preconditioning regimen with age, disease status, and PS for OS
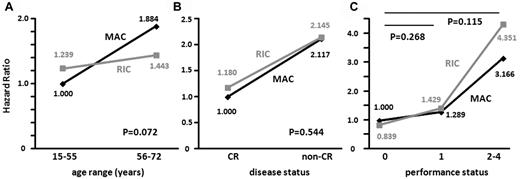
Statistical interactions between the preconditioning regimens and age, disease status, or PS at transplantation for OS were tested by adding an interaction term into the multivariate analysis that included the following 6 variables: age, sex, disease status, PS, relationship of the donor to the recipient, and preconditioning regimen. Among the 578 patients for whom multivariate analysis for OS was conducted (Table 2), when the HR for death of MAC recipients of a younger age (15-55 years) was determined as 1.000, the HRs of MAC recipients in the older age group (56-72 years) and RIC recipients in the younger and older age groups were 1.884, 1.239, and 1.443, respectively (Pinteraction = 0.072; Figure 2A). When the HR for death of MAC recipients with CR at transplantation was determined as 1.000, HRs of MAC recipients with non-CR and RIC recipients with CR and non-CR were 2.117, 1.180, and 2.145, respectively (Pinteraction = 0.544; Figure 2B). When the HR for death of MAC recipients with PS 0 at transplantation was determined as 1.000, HRs of MAC recipients with PS 1 and RIC recipients with PS 0 and 1 were 1.289, 0.839, and 1.429, respectively (Pinteraction = 0.268), and HRs of MAC and RIC recipients with PS 2 to 4 were 3.166 and 4.351, respectively (Pinteraction = 0.115; Figure 2C).
Interactions of the preconditioning regimen with age, disease status, and performance status for OS. Statistical interactions between the preconditioning regimens (MAC or RIC) and age range (15-55 vs 56-72 years; A), disease status (CR vs non-CR; B), and performance status (0 vs 1 or 2-4; C) were analyzed.
Interactions of the preconditioning regimen with age, disease status, and performance status for OS. Statistical interactions between the preconditioning regimens (MAC or RIC) and age range (15-55 vs 56-72 years; A), disease status (CR vs non-CR; B), and performance status (0 vs 1 or 2-4; C) were analyzed.
Multivariate analysis of factors influencing OS in the subgroup of ATL patients who had transplantation after MAC
Of the 280 ATL patients who received MAC, 1 patient was excluded because of missing data on the time from diagnosis to transplantation and one was excluded because of lack of data on HLA. Multivariate analysis was therefore conducted on 278 patients and included the variables of age, sex, disease status, PS, and relationship of the donor to recipient, which were found to have significantly affected OS in the entire subject population (Table 2). Also included was a sixth variable, the type of MAC (TBI, BU, Mel-based, or others). Of these 6 variables, 4 significantly affected OS, namely, older age (HR, 1.667; 95% CI, 1.051-2.643), male sex (HR, 1.458; 95% CI, 1.053-2.019), not in CR (HR, 2.071; 95% CI, 1.409-3.043), and worse PS (2-4 compared with 0; HR, 3.073; 95% CI, 1.920-4.919; Table 3).
Multivariate analysis of factors influencing OS in the subgroup of patients receiving transplantations after RIC
Of the 306 ATL patients receiving RIC, 3 were excluded because of lack of data on the time from diagnosis to transplantation, 2 were excluded because of receiving BMT and PBSCT together, and 1 was excluded because of lack of data on HLA. Thus, multivariate analysis on 300 ATL patients was performed using the following 6 variables: age, sex, disease status, PS, relationship of the donor to the recipient, and type of RIC (Flu + BU, Flu + Mel-based, or others). Of these, 4 significantly affected OS, namely, male sex (HR, 1.475; 95% CI, 1.100-1.978), not in CR (HR, 1.743; 95% CI, 1.249-2.432), worse PS (1 compared with 0; HR, 1.803; 95% CI, 1.293-2.516, 2-4 compared with 0; HR, 6.175; 95% CI, 3.908-9.756), and type of RIC (Flu + Mel compared with Flu + BU based; HR, 0.645; 95% CI, 0.453-0.918; Table 4).
Multivariate analysis of TRM and ATL-related mortality
Among the 586 ATL patients receiving allogeneic BMT or PBSCT, 14 could not be assigned to either the TRM or ATL-related mortality category because detailed information regarding cause of death was missing. The Fine and Gray proportional hazards model was applied to the remaining 572 patients to identify variables affecting TRM and ATL-related mortality, respectively. The variables included age, sex, disease status, PS, and relationship between recipient and donor, which was shown to significant affect OS in the entire patient population (Table 2), and the preconditioning regimen, namely, MAC or RIC. Among these variables, sex and PS were significantly associated with TRM. The HR for TRM of male patients was 1.383 (95% CI, 1.026-1.863). HRs for TRM of recipients with PS 1 and PS 2 to 4 compared with PS 0 were 1.509 (95% CI, 1.075-2.118) and 3.004 (95% CI, 1.915-4.714), respectively. Conversely, disease status, PS, and the preconditioning regimen were significantly associated with ATL-related mortality. HR for ATL-related mortality of recipients not in CR was 2.203 (1.469-3.302). The HR for ATL-related mortality of recipients with PS 2 to 4 compared with PS 0 was 1.679 (95% CI, 1.035-2.723), and the HR of patients receiving RIC compared with MAC was 1.579 (95% CI, 1.080-2.308; Table 5).
Cumulative incidence of TRM and ATL-related mortality
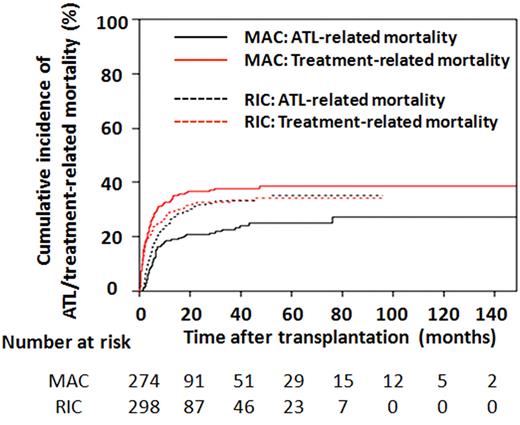
Among the 572 ATL patients receiving allogeneic BMT or PBSCT, the cumulative incidence of TRM one year after transplantation was 32.7% (95% CI, 27.1-38.4) in MAC recipients and 29.2% (95% CI, 24.0-34.5) in RIC recipients. These figures at 3 years were 37.7% (95% CI, 31.8-43.6) and 33.3% (95% CI, 27.7-38.9), respectively (Figure 3). The cumulative incidence of ATL-related mortality 1 year after transplantation was 18.5% (95% CI, 14.1-23.4) for MAC and 25.0% (95% CI, 20.1-30.1) for RIC recipients and was 22.5% (95% CI, 17.5-27.9) and 33.2% (95% CI, 27.6-38.9), respectively, at 3 years (Figure 3).
Cumulative incidence of ATL-related and TRMs in patients receiving BMT or PBSCT. Probabilities of ATL-related and TRMs in recipients of MAC or RIC were estimated using cumulative incidence curves to accommodate competing events.
Cumulative incidence of ATL-related and TRMs in patients receiving BMT or PBSCT. Probabilities of ATL-related and TRMs in recipients of MAC or RIC were estimated using cumulative incidence curves to accommodate competing events.
Discussion
To the best of our knowledge, the present study is the largest retrospective study of ATL patients receiving allogeneic HSCT. Results showed that for allogeneic BMT or PBSCT for ATL, RIC was applied more frequently in older patients, as is reasonable and expected. RIC patients more often received PBSCT and had related donors. We surmise this was because RIC was initially proposed in the setting of PBSCT from HLA-matched sibling donors.30
The OS plot of ATL patients receiving allogeneic HSCT reached a plateau, leading to long-term survival of a subgroup of ATL patients. Recipients of CBT had a significantly worse prognosis than recipients of BMT or PBSCT, which was consistent with our previous report.11 Direct comparison of transplantation outcomes between unrelated CBT and the other types of allogeneic HSCT was not possible because the selection of the graft source is an individual process strongly influenced by donor availability and the patient's ATL status. However, even considering such potential biases, the outcome of unrelated CBT seems clearly unsatisfactory. Thus, novel strategies to further improve the outcomes of unrelated CBT are warranted.
Among ATL patients receiving allogeneic BMT or PBSCT, multivariate analysis revealed 5 significant independent variables affecting OS, namely, age, sex, disease status, PS, and relationship between the recipient and donor. Of these factors, younger age, good ATL disease status, and PS at transplantation contributing to better OS were to be expected. The contribution to a better OS of HSCT from HLA-A, -B, and -DR–matched related donors also would be expected. The reason why the female sex was an independent favorable factor is not fully understood but is consistent with results of our previous study.11 With respect to preconditioning, there was no significant difference in OS between MAC and RIC recipients. To further clarify the clinical significance of preconditioning in allogeneic BMT or PBSCT for ATL, we analyzed the interactions of preconditioning with age, disease status, and PS. There was a clear trend indicating that RIC contributed to better OS in older patients compared with MAC. In contrast, the associations between MAC and RIC to OS were almost similar even if ATL patients at transplantation were in CR or not. In general, when considering allogeneic HSCT for many other types of leukemia/lymphoma patients who are in non-CR, it seems more usual to apply MAC for those patients because MAC should have the more potent effect in eradicating residual leukemia/lymphoma cells than RIC. However, the present study does not support this strategy at least in HSCT for ATL. The associations between MAC and RIC to OS were almost similar even when the PS at transplantation was 0, 1, or 2 to 4. In general, considering allogeneic HSCT for patients who have a worse PS, it seems to be more usual to apply RIC because RIC should be less toxic for recipients than MAC. However, the present study also does not support this strategy, at least in HSCT for ATL.
In the subgroup analyses stratified by MAC or RIC, older age was an independent unfavorable prognostic factor in MAC recipients, but not in RIC recipients. Female sex, good ATL disease status, and PS significantly contributed to better OS in both groups. Among MAC recipients, there was no significant difference in OS according to the type of MAC, but among RIC recipients, a Flu + Mel–based regimen contributed to better OS compared with a Flu + BU–based regimen. Although RIC regimens that contain alemutuzumab have been widely used in various parts of the world,31 we had no data available as to whether any of the regimens used included alemtuzumab. Thus, we were not able to clarify the significance of the inclusion of alemtuzumab as a conditioning agent.
Multivariate analysis of variables contributing to mortality demonstrated that there was significantly more ATL-related mortality in RIC recipients. Although not statistically significant, a clear trend showed an association of increased TRM but not ATL-related mortality in older patients. Male sex was significantly associated with increased TRM, which might contribute to the better OS of female recipients. ATL patients not in CR had greater ATL-related mortality, but not TRM. A poor PS was significantly associated with both ATL-related mortality and TRM, but the association was closer with TRM. HSCT from unrelated donors was significantly associated with increased TRM but not with ATL-related mortality.
Cumulative incidence curves of TRM and ATL-related mortalities in MAC and RIC recipients showed characteristic features as illustrated in Figure 3. In comparison with the black lines indicating ATL-related mortality, the red lines showing TRM rise in the early phase after transplantation. Two solid lines for MAC had quite different trajectories, with TRM being greater than ATL-related mortality at any time after transplantation. In contrast, the 2 dotted lines for RIC nearly joined at 24 months after transplantation and were almost identical thereafter. Both lines for RIC were between those for MAC TRM and ATL-related mortality.
Currently, several promising new agents for ATL are being developed.32–35 These novel treatments should increase the number of ATL patients with a sufficient disease control status and who have maintained a good PS who could become suitable candidates for transplantation. This would require further improvement in allogeneic HSCT for ATL as well as better rescue strategies for patients relapsing after HSCT. Although treatment by AZT/IFN-α6 and/or alemutuzumab34,36 are applied for ATL patients in many countries, none of these agents are currently approved in Japan for the treatment of ATL under the national health insurance. Therefore, there are currently no data on their clinical impact on outcome after allogeneic HSCT for ATL. We do expect, however, that the application of AZT/IFN and alemutuzumab would contribute to improved outcomes of HSCT for ATL.
Although this study reports significant novel findings for allogeneic HSCT for ATL patients, it also has inherent limitations common among observational retrospective studies. Eligibility for transplantation as well as choice of transplantation protocol, including the selection of MAC or RIC, was determined by the physicians at each institution. Regarding mortality analysis, it is not easy to determine whether death of an ATL patient after allogeneic HSCT is TRM or ATL-related mortality. This is partially because relapsed ATL patients sometimes achieve partial or complete remission on decreasing or discontinuing immunosuppressive agents, donor lymphocyte infusions, or chemotherapy, which can result in long-term remission and survival.9,13,18
In conclusion, allogeneic BMT or PBSCT not only with conventional MAC but also RIC is an effective treatment that results in long-term survival of selected patients with ATL. Posttransplantation outcomes are influenced by the recipient's age, sex, PS, disease status at transplantation, and the relationship between recipient and donor. Although no significant difference in OS between MAC and RIC recipients was observed, there was a clear trend that RIC contributed to better OS in older patients. Regarding results of analysis of mortality, RIC was more significantly associated with ATL-related mortality in comparison with MAC. More definitive conclusions on the role of allogeneic HSCT in the therapeutic algorithm for ATL will need to be drawn from well-designed prospective clinical trials.
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all the physicians and data managers at the institutes who contributed valuable data on transplantation for ATL to the JSHCT. The authors thank all the members of the data management committees of JSHCT and Prof Sadao Suzuki (Department of Public Health, Nagoya City University Graduate School of Medical Sciences) for valuable advice on the statistical analyses.
This work was supported by a Grant-in-Aid for Scientific Research (H22-Clinical Cancer Research-general-028; T.I. and A.U.) from the Ministry of Health, Labor, and Welfare, Japan.
Authorship
Contribution: T.I., M.H., K.K., R.T., and A.U. designed the research, organized the project, and wrote the paper; T.I. and T.N. performed statistical analysis; H.S. and R.S. collected data from JSHCT; Y.M. collected data from JMDP; K.K. collected data from JCBBN; and all authors interpreted data, reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: Nagoya City University Graduate School of Medical Sciences has received research grant support from Kyowa Hakko Kirin for works provided by T.I. The remaining authors declare no competing financial interest.
Correspondence: Takashi Ishida, Department of Medical Oncology and Immunology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-chou, Mizuho-ku, Nagoya, Aichi, 467-8601, Japan; e-mail: itakashi@med.nagoya-cu.ac.jp.