Abstract
Although peripheral blood stem cells (PBSCs) have replaced bone marrow (BM) as the most common unrelated donor progenitor cell product collected, a direct comparison of concurrent PBSC versus BM donation experiences has not been performed. We report a prospective study of 2726 BM and 6768 PBSC donors who underwent collection from 2004 to 2009. Pain and toxicities were assessed at baseline, during G-CSF administration, on the day of collection, within 48 hours of donation, and weekly until full recovery. Peak levels of pain and toxicities did not differ between the 2 donation processes for most donors. Among obese donors, PBSC donors were at increased risk of grade 2 to 4 pain as well as grade 2 to 4 toxicities during the pericollection period. In contrast, BM donors were more likely to experience grade 2 to 4 toxicities at 1 week and pain at 1 week and 1 month after the procedure. BM donors experienced slower recovery, with 3% still not fully recovered at 24 weeks, whereas 100% of PBSC donors had recovered. Other factors associated with toxicity included obesity, increasing age, and female sex. In summary, this study provides extensive detail regarding individualized risk patterns of PBSC versus BM donation toxicity, suggesting donor profiles that can be targeted with interventions to minimize toxicity.
Key Points
BM and PBSC donors experience similar levels of mild/moderate discomfort; timing of onset and recovery from discomfort varies by procedure.
Variations in intensity and time course of donation-associated discomfort occur in donors who are obese, older, and female.
Introduction
Over the past 2 decades, several organizations, including the National Marrow Donor Program (NMDP), the Center for International Blood and Marrow Transplant Research (CIBMTR), the World Marrow Donor Association, and a host of international hematopoietic cell transplant registries, have worked to ensure that the process of hematopoietic progenitor cell (HPC) donation is performed safely and ethically.1-3 Although unrelated bone marrow (BM) or peripheral blood stem cell (PBSC) donors may possibly benefit if health issues are discovered during the medical evaluation they receive before donation, the procedures of BM or PBSC collection themselves result in no direct medical benefit to the donor and have the potential for harm. Hence, physicians and donor center personnel have an obligation to understand the risks associated with both procedures and to inform prospective donors as they decide whether and how to donate.
In the late 1990s, the NMDP developed comprehensive data tools that allow a more detailed understanding of the donor's experience. These tools include assessments modeled on the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) of 14 key toxicities that donors experience regularly. In addition, the tools include a comprehensive assessment of pain by location and intensity, specific Food and Drug Administration–based criteria for serious adverse events, and careful long-term follow-up. Using these tools, a description of the experience of NMDP PBSC donors from 1999 to 2004 was published previously.4 In 2004, the data tools developed for the NMDP unrelated donor PBSC study were adapted and put into place for BM donors, allowing a direct comparison of the 2 donation experiences. The procedures themselves vary in regard to the timing of adverse events in relation to donation; however, most of the toxicities donors experience (eg, pain, fatigue, low blood counts) occur as a result of both methods of HPC procurement and can thus be compared. This publication provides the first direct comparison of concurrently enrolled unrelated BM and PBSC donors using these detailed tools, permitting new insight into the donation experience not previously understood or reported.
Methods
Study population
The study population comprised unrelated donors from the United States whose nonstimulated bone marrow or filgrastim (recombinant human G-CSF)–mobilized PBSC donation was facilitated by the NMDP between January 2004 and July 2009. Only first-time HPC donors for whom data are available from baseline to 2 days after donation on the NMDP 700-series forms (standardized with overlapping elements for BM and PBSC donors) were included. Donors enrolled on the BMT CTN protocol 0201 (a randomized BM vs PBSC donor trial, reported separately5 ) and rare donors who donated bone marrow after filgrastim administration were excluded. All donors included in the study provided written informed consent for participation in CIBMTR research studies approved by the NMDP institutional review board. This study was conducted in accordance with the Declaration of Helsinki. The final number of donors eligible for inclusion in this study included 2726 BM and 6768 PBSC donors. All donors were evaluated for medical suitability, transplantation-transmissible infectious diseases, and contraindications (eg, pregnancy, autoimmune disease, history of thromboembolic disease) for BM collection or PBSC donation.
Bone marrow donation
Before the bone marrow collection, to minimize the likelihood of needing allogeneic transfusion, donor centers were advised to collect 1 to 2 autologous blood units from the donor in relation to the marrow volume they were to donate. Marrow was collected from the donor's posterior iliac crests in an operating room under either general or regional (spinal or epidural) anesthesia. The NMDP guidelines suggest removing no more than 20 mL of marrow per kilogram of donor weight, and recommend that duration of anesthesia is less than 150 minutes and duration of the collection procedure itself is less than 120 minutes. Seventy-one percent of BM donors received 1 to 3 units of previously collected autologous packed red blood cells during or immediately after their collection procedures (43% received 1 unit, 27% received 2 units, and 1% received 3 units).
PBSC donation
All PBSC collections were performed according to the NMDP-sponsored and institutional review board–approved research protocol for manufacturing PBSC products, operated under an Investigational New Drug application with the Food and Drug Administration. Filgrastim-mobilized PBSC collection involved subcutaneous administration of filgrastim for 4 (for recipients weighing less than 35 kg) or 5 consecutive days at a daily dose of approximately 10 μg/kg. Administered filgrastim doses were rounded to some combination of 300-μg and 480-μg vials based on the donor's total body weight so that protocol defined targets were 13.3 μg/kg or less per day. The protocol included provisions for filgrastim dose reductions in the presence of high-grade symptoms or low platelet counts. On day 5 alone, or on days 5 and 6, the donor's PBSCs were collected by leukapheresis. Donors with preapheresis platelet counts less than 120 × 109/L on the first day of collection or 80 × 109/L on the second day of collection were required to discuss whether collection could be performed safely with an NMDP medical officer, as the goal was to avoid platelet counts less than 50 × 109/L after collection. The volume of whole blood processed was targeted to be between 12 and 24 L per collection, depending on recipient weight and/or immediate preprocedure donor blood CD34+ cell count. The protocol allowed a maximum total blood volume processed to be 24 L, whether collected over 1 or 2 days. If the PBSC product could not be collected using peripheral veins, a central venous catheter (CVC) was used. The insertion of any central catheter took place in a hospital. The subsequent apheresis procedure(s) was then also performed in a hospital, and donors with CVCs remained in the hospital between apheresis procedures.
Data collection
The bone marrow donation processes were facilitated by 81 donor centers and 83 collection centers; the PBSC donation processes were facilitated by 76 donor centers and 98 apheresis centers. Donor centers manage donor medical evaluations, infectious disease marker testing, and filgrastim administration. They are also responsible for coordinating the donation process, monitoring the donor's recovery, and data reporting. Collection centers perform marrow collections, assist donor centers with data reporting, and are responsible for submitting Marrow Product Analysis Forms. Apheresis centers perform PBSC collections, coordinate donor filgrastim administration in conjunction with local donor centers as needed, assist donor centers with data reporting, and they are responsible for submitting PBSC Product Analysis Forms.
Data collection began at the time of the donor's medical evaluation to determine suitability to donate HPCs. For bone marrow donations, the data collection continued on the day of marrow collection. For PBSC donations, the data collection continued through each day of filgrastim and on the day of each apheresis procedure. Both bone marrow and PBSC donors were contacted by the donor center after donation at 2 days after donation, and then at 1 week after donation, and weekly thereafter until complete recovery. “Complete recovery” was judged by the donor center coordinator or medical director based on reports of return to baseline and no ongoing symptoms associated with the collection procedure as ascertained by the weekly follow up call with the donor. Further contact with the donor occurred at 1 month, 6 months, and annually to assess for the presence of any new or residual symptoms. Detailed questions using the toxicity criteria modeled on CTCAE were used to assess specific symptoms, to measure the donors overall health, and to capture any toxicity the donor may have occurred as a result of the HPC donation process. Symptoms assessed included fever, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, syncope, pain, and infections. In addition, a complete blood count and white cell differential were performed at the initial medical evaluation, on the first day of filgrastim, the day(s) of collection, and at annual follow-ups.
End points
The following end points were analyzed in multivariate models: incidence of grade 2 to 4 or 3 to 4 skeletal pain, fatigue, and highest toxicity level across selected body symptoms frequently associated with collection (fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope). Skeletal pain was defined as pain in at least 1 of the following sites: back, bone, headache, hip, limb, joint, or neck. The severity of skeletal pain was defined as the maximum grade among these pain sites. End points were analyzed at the following time points: the day with the highest level of toxicity (day + 5 from start of G-CSF for PBSCs and first assessment after BM collection, 1 to 2 days after collection); and at 1 week and at 1 month after donation, if sufficient frequencies of events were present. Time to recovery from donation was defined as the time in days from the BM collection or first PBSC collection to report of complete recovery as defined herein. Hematologic parameters (white blood cell [WBC], platelet, hemoglobin, absolute neutrophil, and mononuclear cell counts) were measured before collection; immediately after collection; and at 1 year, 2 years, and 3 years after collection.
Statistical methods
The analysis quantified a variety of donor and collection characteristics by product type. Variables were compared between BM and PBSCs using the Pearson χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. If a second donation occurred, all outcomes were censored at the time of second donation; for subsequent bone marrow donations, at time of second collection; and for subsequent peripheral blood donations, at day 1 of filgrastim administration.
Logistic regression was used to compare BM and PBSC donors for the incidence of bone pain, highest toxicity, and fatigue, after adjusting for donor characteristics and baseline measurements as listed in Table 1. The effects were estimated via odds ratios (ORs). Univariate probabilities of complete recovery from donation were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons between BM and PBSC donors; the χ2 test was used for pointwise comparisons.6 A multivariable model using Cox proportional hazards regression was used to compare the time to complete recovery between BM and PBSC donors after adjusting for donor characteristics and baseline measurements.7 The estimated effects of each significant risk factor were given as relative risks (RRs). The proportional hazards assumption was assessed using graphical approaches and time-dependent covariates. Mean changes in donor WBC, platelet, hemoglobin, neutrophil, and mononuclear cell recovery from baseline to each postdonation time point were compared between BM and PBSC donors using a 2-sample t test. Models were built separately for each time point because of complex interactions with time identified in an initial analysis using mixed models for repeated measures data. In all multivariate models, donation type was forced into the model and stepwise model selection was used to determine additional donor characteristics to be included. Interactions between donation type and each donor characteristic were tested for in all multivariate models.
Results
Characteristics of NMDP BM and PBSC donors
Table 1 shows clinical characteristics of BM and PBSC donors enrolled in the study, and Table 2 shows collection characteristics. Although donors were more often male (61%), this ratio was similar in BM versus PBSC donors. There were no notable differences between BM and PBSC donors with regard to age and body mass index (BMI). Only a small portion of BM donors underwent procedures using spinal or epidural anesthesia (4%), and a similar percentage had more than 20 cc/kg donor body weight collected. The majority of the single-day donations were large volume (77% > 18 L), whereas 96% (day 5) and 99% (day 6) of donors undergoing 2 days of apheresis had intermediate- or small-volume collections (Table 2).
Hematologic toxicities and recovery of BM versus PBSC donors
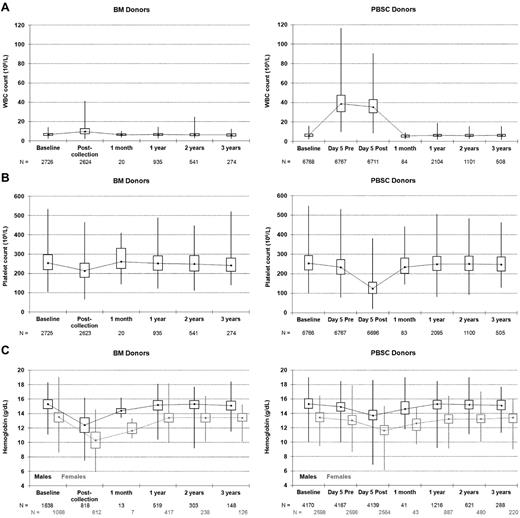
As expected, total peripheral WBC counts were much higher for PBSC versus BM donors in the pericollection period because of cytokine stimulation (Figure 1A). The WBCs of BM donors increased on average by 3.7 × 109/L after collection, with 84% of BM donors experiencing an elevated WBC count and the highest postcollection WBCs reaching just over 40 × 109/L. Only a small fraction of BM donors had documented marrow site infections after collection (all grade 2, n = 41 [1.5%]; in comparison 37 [0.55%] PBSC donors had grade 2 local IV site infections after PBSC collection); thus, the elevated WBCs likely reflected a stress reaction to the procedure in the large majority of BM donors. The mean WBCs noted for PBSC donors before the apheresis procedure on day 5 was just above 40 × 109/L. Twenty percent of PBSC donors had WBC counts exceeding 50 × 109/L, but only 1 donor had a WBC count exceeding 100 × 109/L. Platelet counts decreased to below 100 × 109/L in 8 BM donors (0.3%) after collection but never went below 50 × 109/L. However, after day 5 PBSC collections, platelet counts were below 100 × 109/L in 26% of donors, below 50 × 109/L in 30 donors (< 1%), but never below 20 × 109/L (Figure 1B). After the second collection on day 6 in those PBSC donors who underwent 2 aphereses, the platelet counts were below 100 × 109/L in 50% of donors, below 50 × 109/L in 22 donors (1%), but they were never below 20 × 109/L. No platelet transfusions were reported. Hemoglobin levels fell after both procedures but fell on average 1.34 g/dL more after BM collection (P < .001); 0.2% of men and 5.7% of women had hemoglobin fall below 8 g/dL after a BM collection compared with 0.1% of men and 0.2% of women after a PBSC collection. Thus, women were more likely to experience significant anemia (< 8 g/dL) compared with men after BM collection (P < .001).
Donor blood counts at baseline, on the day of collection, and at postdonation follow-ups. (A) Donor white blood cell (WBC) counts. (B) Donor platelet counts. (C) Donor hemoglobin levels. Minimum, lower quartile, median, upper quartile, maximum; day 5 is the first day of apheresis; Pre and Post refer to the apheresis procedure.
Donor blood counts at baseline, on the day of collection, and at postdonation follow-ups. (A) Donor white blood cell (WBC) counts. (B) Donor platelet counts. (C) Donor hemoglobin levels. Minimum, lower quartile, median, upper quartile, maximum; day 5 is the first day of apheresis; Pre and Post refer to the apheresis procedure.
A small number of BM donors (13 of 2726, 0.48%) received allogeneic (homologous) packed red blood cell transfusions. Although allogeneic transfusions were very rare, women were 5 times more likely to receive them compared with men (10 of 1088 women vs 3 of 1638 men; P = .009, Fisher exact test).
Figure 1 and supplemental Table A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) show the pattern of hematologic recovery after BM collection and PBSC collection procedures. By 1 month, mean WBC, platelets, and hemoglobin recovered to near-normal levels after both procedures. Of note, there is a small but statistically significant decrease in mean WBCs of PBSC donors from baseline that resolved by year 3 (supplemental Table A). Mean platelet counts were slightly decreased from baseline after both BM and PBSC donation through year 3 and were lower after PBSCs compared with BM through the first year. Hemoglobin levels were also slightly lower than baseline values for up to 3 years after both procedures, but the magnitude of the change in values did not differ by type of collection procedure.
Pain and toxicity experiences of BM versus PBSC donors
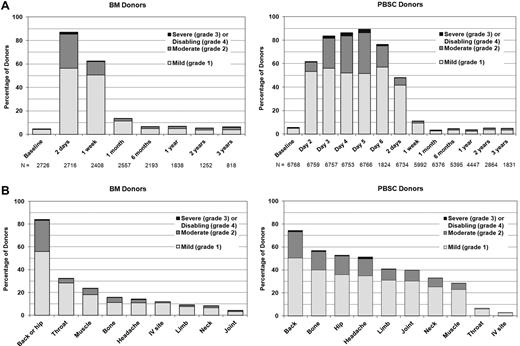
Figure 2 shows the time course, location, and extent of pain experienced by BM versus PBSC donors. PBSC donors were assessed daily before filgrastim administration and within 48 hours of completing the apheresis procedure. Onset of pain occurred within 24 hours of initiation of G-CSF administration for the majority of donors. Donors undergoing BM collection were assessed within 48 hours after completing the collection when pain and toxicities were expected to be at the highest levels. Both groups were then assessed at 1 week and weekly until full recovery, at 1 and 6 months, and then yearly. Pain in BM donors was generally localized to the site of collection, whereas pain associated with PBSC involved musculoskeletal locations throughout the body and higher rates of headache. Figure 3A shows the time course and degree of toxicities experienced by BM versus PBSC donors. Sixty to 70% of donors experienced at least 1 of these toxicities in the pericollection period. By 1 week after collection, 43% of BM donors and 15% of PBSC donors report at least 1 toxicity, but by 1 month after collection, the percentage of donors reporting toxicities had returned to levels near baseline. Figure 3B compares specific toxicities experienced by BM donors assessed within 48 hours of collection with PBSC donors on day + 5 of G-CSF (day 5 is the peak day for pain and toxicities, Figures 2B and 3B). Fatigue and insomnia were the most common complaints of both BM and PBSC donors, with more fatigue noted in BM donors and more insomnia in PBSC donors. Figure 3C shows the recovery pattern from these toxicities.
Pain. (A) Skeletal pain experienced by bone marrow (BM) and peripheral blood stem cell (PBSC) donors at baseline, during the pericollection period, and after donation. (Skeletal pain represents pain in at least 1 of the following sites: back, bone, headache, hip, limb, joint, and neck.) The severity of skeletal pain is defined as the maximum grade among these pain sites. Day 2 is the second day of filgrastim; day 5 is the first day of apheresis. (B) Sites of pain among BM and PBSC donors on the day noted to have the highest severity of pain (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC).
Pain. (A) Skeletal pain experienced by bone marrow (BM) and peripheral blood stem cell (PBSC) donors at baseline, during the pericollection period, and after donation. (Skeletal pain represents pain in at least 1 of the following sites: back, bone, headache, hip, limb, joint, and neck.) The severity of skeletal pain is defined as the maximum grade among these pain sites. Day 2 is the second day of filgrastim; day 5 is the first day of apheresis. (B) Sites of pain among BM and PBSC donors on the day noted to have the highest severity of pain (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC).
Toxicities. (A) Highest toxicity level of key symptoms (fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope) experienced by BM and PBSC donors at baseline, during pericollection period, and after donation. (Day 2 is the second day of filgrastim; day 5 is the first day of apheresis.) (B) Toxicities among BM and PBSC donors on the day noted to have the highest level of toxicity (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC). (C) Common toxicities among BM and PBSC donors on the day noted to have the highest level of toxicity (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC), and at selected time points after donation.
Toxicities. (A) Highest toxicity level of key symptoms (fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, insomnia, dizziness, and syncope) experienced by BM and PBSC donors at baseline, during pericollection period, and after donation. (Day 2 is the second day of filgrastim; day 5 is the first day of apheresis.) (B) Toxicities among BM and PBSC donors on the day noted to have the highest level of toxicity (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC). (C) Common toxicities among BM and PBSC donors on the day noted to have the highest level of toxicity (1-2 days post-BM collection and day + 5 from start of G-CSF for PBSC), and at selected time points after donation.
Multivariate analysis of risks associated with experiencing significant levels of toxicities and pain showed that risk of toxicity varied between donors of PBSC versus BM and that the relative risks of these toxicities changed over time (Table 3). PBSC donors were monitored more frequently compared with BM donors in the pericollection period. To avoid ascertainment bias we compared a single pericollection time point when pain and toxicities were noted to be highest (day + 5 of G-CSF administration for PBSC donors and within 48 hours of collection for the BM donors). At the pericollection time point, pain and toxicities were generally comparable; however, key differences were noted when donors were analyzed by BMI groups. All PBSC donors were more likely to experience grade 3 to 4 pain compared with all BM donors, but among overweight and obese donors, PBSC donors were also more likely to complain of grade 2 pain. Grade 2 to 4 fatigue was more common among BM donors except obese donors, where rates of fatigue were similar regardless of collection technique. Other grade 3 to 4 toxicities were comparable between BM and PBSC donors. At later evaluations, the rates of persistent pain and toxicities were low, but BM donors were more likely to report persistent pain at both 1 week and 1 month after donation. Other toxicities and fatigue were more common after BM donation when assessed at 1 week, but these differences disappeared by 1 month after donation.
Whether donors give BM or PBSCs, age and sex are associated with different risks of toxicities, fatigue, and pain. Women were more likely to experience pain, toxicities, and fatigue compared with men in both the pericollection time frame and postdonation recovery period. Older donors were at less risk of grade 2 to 4 pain in the pericollection period, but they were more likely to be among the small percentage of donors with persistent pain at 1 week after collection. In addition, older donors were also at higher risk for grade 2 to 4 toxicities and fatigue at 1 week after collection. One other factor that was independently associated with an increase in moderate fatigue and skeletal pain in the pericollection period was a baseline mononuclear cell count (combination of lymphocyte and monocyte counts) of more than 2.7 × 109/L (Table 4).
Probability of complete recovery
Donors were followed weekly until they reported complete recovery. More than half of PBSC donors were completely recovered by 1 week after collection, whereas only 18% of BM donors reported complete recovery. More than 90% and 100% of PBSC donors reported complete recovery at 1 and 6 months, respectively, whereas 67% and 97% of BM donors reported recovery at those same time points (P < .001; Figure 4).
Univariate probability of reported complete recovery from stem cell donation.
Multivariate analysis showed that undergoing BM versus PBSC collection put donors at twice the risk for delay or failure to achieve complete recovery (RR, 0.45 [95% CI, 0.43%-0.48%]; P < .001). Women were less likely than men to experience complete recovery, regardless of HPC source donated (RR, 0.92 [95% CI, 0.88%-0.96%]; P < .001).
Although BM donors are delayed in reporting full recovery compared with PBSC donors, the persistent symptoms are almost all grade 1 (mild) pain or toxicities. By 1 week after the collection procedure, less than 1% of BM and PBSC donors reported grade 3 pain or toxicities; 11% and 1% of BM and PBSC donors reported grade 2 (moderate) pain, and 6% and 2% reported at least one grade 2 toxicity. By 1 month, less than 1% reported toxicities and 2% and 1% of BM and PBSC donors reported grade 2 pain. PBSC donors are back to baseline for all toxicity and pain measures at 1 month; the real difference between BM and PBSC donors at 1 month is an 11% rate of grade 1 (mild) pain in BM donors and 3% in PBSC donors (baseline values 4%-5%).
Other adverse events associated with BM or PBSC donation
Approximately a third of both men and women were hospitalized overnight after BM collection; most cases were the routine practice of the collection center. However, approximately 1% of donors required 2 or more nights of hospitalization because of complications, and women were almost twice as likely as men to require extended hospitalization (P = .028). Fifteen BM donors required 2 nights of hospitalization, 6 donors required 3 to 7 nights of hospitalization, and 1 donor required 20 nights of hospitalization. As observed in an earlier NMDP analysis, women were more likely to require a CVC (21% vs 5%; P < .001) and to have apheresis-related adverse events (P < .001). Specific apheresis-related events not previously reported that occur more frequently in female donors include nausea/vomiting (2 vs < 1% [60 of 2598 female donors vs 17 of 4170 male donors]), transient numbness or tingling (45% vs 33% of male vs female donors), persistent moderate numbness or tingling (8% vs 2%), severe numbness or tingling (1% vs < 1% [23 vs 3 donors]), and tetany (1 vs < 1% [24 vs 6 donors]). Female donors were more likely to require prolonged hospitalization after PBSC collection. Seventy-eight female PBSC donors (3%) were hospitalized for 1 night compared with 24 male PBSC donors (1%); 7 female PBSC donors and 1 male PBSC donor required 2 to 3 nights in the hospital (P < .001).
Discussion
In an earlier report describing toxicities experienced by NMDP PBSC donors collected between 1999 and 2004, we noted a higher rate of toxicities, pain, and the need for CVC placement in women compared with men.4 We further showed that obese donors experienced more pain and toxicities compared with donors of normal weight. Miller et al described levels of pain and serious toxicities reported by NMDP BM donors donating between 2001 and 2006,1 but because different data instruments were used to report BM and PBSC donor safety during this era, a direct and more detailed prospective comparison of BM and PBSC toxicities experienced by NMDP unrelated donors has not been possible until now.
Previous reports directly comparing BM and PBSC donation experiences have been secondary analyses of either randomized trials comparing the HPC sources in related donors or single center related donor experiences.8-13 These comparative studies are relatively small (total of 807 donors in 6 studies) and the methodology used to assess donors has varied widely, with no use of CTCAE elements, little use of standardized pain scales, and varied times when data were assessed before and after collection. The general observations noted in published related donor comparisons describe more site-directed pain, more days of restricted activity, and more overall adverse events in BM donors compared with PBSC donors. Not all donors from the parent trials were included in some of the randomized studies, and the ability to draw strong conclusions based on these studies is limited by low numbers and methodological weaknesses.14
This study is the first to prospectively compare the experiences of unrelated BM and PBSC donors using validated, high-quality instruments that give detailed understanding of what donors experience on a day-to-day basis through the donation process. The study includes nearly 9500 donors collected through a large network of donor/collection centers between 2004 and 2009 and therefore should be considered a standard for expected safety outcomes of unrelated donors. Because NMDP donor screening and collection procedures are standardized, these results may be better than one would expect compared with sibling donor outcomes, where older donors, very young pediatric donors, and donors with comorbidities who would have been excluded by the NMDP may be used. A large study of related donors using the standardized instruments included in this study is currently open at nearly 60 U.S. transplant centers; a direct comparison between related and concurrently enrolled NMDP unrelated donor outcomes is planned.
Our study shows that BM and PBSC donors experience roughly the same levels of pericollection pain and toxicity on their peak days of discomfort (day + 5 of G-CSF for PBSC donors, within 48 hours of collection for BM donors). However, donors who are overweight or obese tend to have more moderate-severe pain with PBSC donation. PBSC donors of any size are more likely to experience more severe pain, but the percentage of donors experiencing this level of pain is low. In addition, women were more likely than men to experience pain, toxicities, and fatigue during the pericollection and at 1 week and 1 month regardless of the type of collection they had undergone. Finally, this study shows that age at donation has a surprising impact; older donors have a lower likelihood of pain in the early collection period but a higher risk of persistent pain at 1 week after donation. Although older donors have a similar risk of experiencing toxicities in the pericollection period, they have a much higher risk of reporting persistent toxicities and fatigue at 1 week, reflecting a slower recovery period than younger donors for all aspects of the donation process.
The most striking differences between BM and PBSC donation are the timing of toxicities relative to the collection procedure and the timing of recovery after completion of the procedure. The main period of discomfort associated with PBSC donation starts early, several days before the first collection day (within 24-48 hours of administration of the first dose of filgrastim). The discomfort persists throughout the administration of G-CSF and the apheresis procedure(s), but it resolves fairly quickly after collection is complete, with just over 10% of donors reporting discomfort at 1 week after collection. The peak time of discomfort after BM collection is immediately after the collection and persists for several days, with more than 60% reporting continued pain at 1 week. Although this “week of discomfort” seems to be very similar for both BM and PBSC donors (PBSC before collection, BM after collection), a much larger percentage of BM donors report mild persistent symptoms weeks after the collection with only 37%, 67%, and 90% of BM donors reporting full recovery at 2, 4, and 8 weeks after collection compared with 84%, 94%, and 98% of PBSC donors.
Another key difference between the procedures is that a small percentage of BM donors report long-term, persistent discomfort. In this series, 3% of BM donors did not report full recovery at 6 months compared with 0% of PBSC donors; 6.6% and 3.9% of BM donors reported pain and toxicities at 6 months (baseline 4.4% and 3.6%) compared with 4.6% and 4.0% of PBSC donors. A more detailed study of rare severe and prolonged toxicities associated with these 2 procedures is underway and will provide further insight into the rare donors who experience prolonged symptoms or rare severe events after donation.
Although differences were noted in WBC, hemoglobin, and platelet levels after both BM and PBSC collection compared with baseline levels (all 3 were slightly lower at 1 month and beyond after apheresis/BM collection, but had normalized by year 3), and there were slight differences in the magnitude of decrease in blood counts depending on the type of collection procedure performed, it is important to stress that none of these differences were clinically meaningful and there were no significant differences in the magnitude of decrease in blood counts comparing the 2 groups of donors by 3 years after the collection procedure. Other investigators have noted early changes in blood counts and immunologic profiles after G-CSF stimulation, but the significance of these changes is unknown.1,15-18 We have yet to discover any long-term adverse effects on marrow function associated with either procedure. There is ongoing discussion about whether filgrastim or other cytokines used to mobilize PBSC donors are associated with long-term risks of malignancy or autoimmunity.4,19-23 Although this analysis does not specifically address this issue, studies to further assess this risk in our cohort and larger cohorts of NMDP donors are ongoing, complemented by similar efforts in other registries throughout the world.
In conclusion, although the procedures of BM and PBSC donation are generally considered to be safe, they are associated with different timing of discomfort and recovery profiles. These findings should alert donor centers to the fact that toxicity profiles associated with HPC source vary, and understanding this along with the age, sex, and weight of their donors should help them modify supportive care measures to decrease rates of toxicity, pain, or both in groups at higher risk. In addition, the findings of this study can be used to educate prospective donors of their risks and assist them with their choice to become a donor.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This project was supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration (HHSH234200637020C) to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program. The CIBMTR is supported by Public Health Service grant/cooperative agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; grant/cooperative agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration (Department of Health and Human Services); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos Inc; Amgen Inc; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; Fresenius-Biotech North America Inc; Gamida Cell Teva Joint Venture Ltd; Genentech Inc; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Optum Healthcare Solutions Inc; Otsuka America Pharmaceutical Inc; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix Inc; Swedish Orphan Biovitrum; THERAKOS Inc; and Wellpoint Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government. M.A.P.'s effort in this work is supported by the NHLBI (R01 HL085707).
National Institutes of Health
Authorship
Contribution: M.A.P. and D.L.C. designed the research, analyzed and interpreted the data, wrote the manuscript, and had responsibility for the entire manuscript as an accurate, verifiable report; P.C. designed the research, prepared the data file, performed the statistical analysis, analyzed and interpreted the data, and wrote the manuscript; B.R.L. designed the research, performed the statistical analysis, analyzed and interpreted the data, and wrote the manuscript; B.E.S., J.R.W., H.M.L., E.K.W., M.S., D.F.S., A.M.L., D.M., P.H., P.V.O., A.W.L., P.A., S.C.G., J.E.L., W.H.N., and J.P.M. analyzed and interpreted the data, and wrote the manuscript; and S.F.L. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, Division of Hematology/Blood and Marrow Transplant, University of Utah School of Medicine, 30 N 1900 E, Rm 5C402, Salt Lake City, UT 84132; e-mail: michael.pulsipher@hsc.utah.edu.