In this issue of Blood, García-Ojeda et al demonstrate a key mechanism that drives T and B lymphocytes to divergent fates from early in their development.1
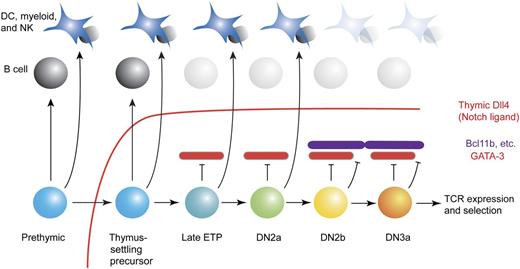
T-cell early developmental stages and alternative options: GATA-3 is depicted as an intrathymically induced, specific inhibitor of access to the B-lineage pathway. Recognized stages in early intrathymic T-cell development are shown, along with the degrees of access to the B-cell program and other alternative programs that the cells can demonstrate at these stages if the extrinsic barrier of Notch/Dll4 signaling (red line) is removed. Red bricks represent GATA-3 when expressed at critical levels, and purple bricks represent other T-lineage commitment factors (eg, Bcl11b6 ) that are activated later. Not shown are the positive roles of GATA-3 that permit the efficient generation of DN2 cells. Full commitment is complete by the DN2b stage.6
T-cell early developmental stages and alternative options: GATA-3 is depicted as an intrathymically induced, specific inhibitor of access to the B-lineage pathway. Recognized stages in early intrathymic T-cell development are shown, along with the degrees of access to the B-cell program and other alternative programs that the cells can demonstrate at these stages if the extrinsic barrier of Notch/Dll4 signaling (red line) is removed. Red bricks represent GATA-3 when expressed at critical levels, and purple bricks represent other T-lineage commitment factors (eg, Bcl11b6 ) that are activated later. Not shown are the positive roles of GATA-3 that permit the efficient generation of DN2 cells. Full commitment is complete by the DN2b stage.6
T cells and B cells share many developmental requirements, starting with the parallel checkpoints in both pathways that are dependent on the activation of Rag1-Rag2 DNA recombinase and Rag-mediated immune receptor gene rearrangements. A close developmental relationship has seemed self-evident: the first partially restricted hematopoietic progenitor cell type to be defined prospectively was the common lymphoid precursor,2 a stem cell descendant that retains both T- and B-lineage potential while other potentials are reduced or eliminated. Thus, it has remained perplexing that, within the thymus, T-cell precursors lose access to the B-cell developmental fate substantially earlier than they lose access to myeloid, dendritic, and NK cell fates.3,4 The environmental signaling imposed on these precursors through Notch1 Dll4 interaction is known to block the B-cell developmental program as long as the cells remain in situ.5 However, Notch signaling also limits access to other fate alternatives during exposure to Dll4. Yet even when early T-lineage precursors are removed from the Dll4-rich thymic environment, they prove to have become unable to generate B cells at stages when they can still generate myeloid or dendritic cells in these conditions3,4 (see figure). This implies that early T cells must have activated an endogenous, specific antagonist of the B-cell program. In this issue, García-Ojeda et al present compelling evidence that it is the activation of GATA-3 in the precursors that accounts for this profound barrier to B-cell fate.1
GATA-3 has long been a tempting candidate for this role. It is expressed in prethymic cells at a low level, and then strongly upregulated by Notch signals as one of the earliest events in T-cell development (reviewed in Rothenberg et al6 ). Once turned on, it is not highly dependent on continuing Notch signals. Thus, it is upregulated around the time that B-lineage exclusion is first imposed, and then it remains expressed (albeit at modulating levels) in all subsequent stages of T-cell development. It is one of the few transcription factors in T cells that do not have a family member shared between any phases of B- and T-cell lineage programs (unlike family members TCF-1/LEF-1 and Bcl11b/Bcl11a, as well as other factors that are explicitly shared). Furthermore, forced GATA-3 expression profoundly antagonizes B-cell development,7,8 whereas without this factor, Gata3−/− precursors can generate some B cells even when weak Notch signals are present.1 However, the dose effects of GATA-3 have complicated the arguments for a specific role in B-lineage exclusion. First, GATA-3 becomes so important for T-lineage precursor survival and development at an early stage that the Gata3 loss of function depletes most of the cells that need to be assayed.9 This has invited concern that any B cells alleged to have emerged from GATA-3–deficient T-cell precursors might in fact be products of contaminants. Second, GATA-3 overexpression not only inhibits B-cell development, but it also inhibits T-cell development itself.8,10
García-Ojeda et al have carefully structured their case to circumvent these problems.1 They have dissected the effects of Gata3 deletion by using highly defined precursor cell populations, efficient and quantitative precursor frequency assays, gene expression monitoring and T-cell receptor gene rearrangement analyses, and temporally controlled excision of Gata3 in cells that have already reached a definitive T-lineage developmental stage. Their results extend our understanding of both the roles of GATA-3 in T-cell developmental progression and the specific dependence of T-cell precursors on GATA-3 for B-lineage fate exclusion. The authors find that a loss of GATA-3 function at an early stage handicaps the generation of definitive DN2-stage, T-lineage progenitors from less-defined DN1 precursors. The inhibition is confirmed by the reduced expression of a suite of genes that normally turn on during the transition to T-lineage commitment (at DN2b stage) and just afterward, that is, Cd3e, Itk, Bcl11b, Rag1, and Ets1. However, some DN2 cells are made, and these can be confirmed to be of early T-cell lineage through their robust expression of Tcf7. Importantly, the GATA-3–deficient DN2 cells also show exaggerated, not reduced, expression of Notch-activated genes Dtx1 and Ptcra, proving that their Notch signaling is not inhibited. The appropriate cells exist, therefore, in which to ask whether GATA-3 is truly an intrinsic requirement for B-lineage exclusion.
When removed from Notch stimulation, Gata3−/− DN2 cells indeed display B-lineage potential along with reduced NK-lineage potential.1 B-cell precursor activity is detected at ∼10% frequency (comparable to wild-type NK precursor frequency), in marked contrast to the complete lack of detectable B-cell potential in normal DN2 cells. The converted cells switch to a B-lineage transcriptional profile, eliminating any trace of previous T-lineage gene expression. However, at least some of them retain TCRβ gene rearrangements attesting to a T-lineage past. The concern that they might represent the outgrowth of an aberrant minority within the mutant DN2 population is countered by using an acute deletion system to remove Gata3 only after the cells have reached the DN2 stage with their GATA-3 function intact. Such cells should have had the full opportunity to activate GATA-3 as well as many of the T-cell genes shown to be dependent on GATA-3. Nevertheless, when Gata3 is removed, they, too, reveal B-cell precursor competence, a ≥102-fold increase in frequency from GATA-3–replete DN2 cells that lack detectable B-cell potential.
These experiments may still be affected by GATA-3 contributions to proliferation and survival; however, García-Ojeda et al make a strong case that GATA-3 has a nonredundant role in B-lineage exclusion specifically, once cells have entered the T-cell pathway. GATA-3 is not the answer for all aspects of T-lineage commitment: loss of GATA-3 reduces, rather than increases, access to the NK pathway, and the conditions used would not optimally reveal the role of GATA-3 in access to myeloid- or dendritic-cell fates. The steps in the pathway of lineage conversion, especially for cells starting from a normal DN2 stage, also remain to be defined. Nevertheless, GATA-3 induction and the mechanisms that sustain its expression now appear to constitute the key with which common lymphoid precursors and any other uncommitted progenitors that enter the thymus are persuaded to lock the door to the B-lineage pathway.
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
National Institutes of Health