Key Points
Both immature and mature neutrophils differentiate into a previously unrecognized hybrid population when cultured with GM-CSF.
The resulting hybrids exhibit dual phenotype and functionality of both neutrophils and dendritic cells.
Abstract
Neutrophils have been reported to acquire surface expression of MHC class II and co-stimulatory molecules as well as T-cell stimulatory activities when cultured with selected cytokines. However, cellular identity of those unusual neutrophils showing antigen presenting cell (APC)-like features still remains elusive. Here we show that both immature and mature neutrophils purified from mouse bone marrow differentiate into a previously unrecognized “hybrid” population showing dual properties of both neutrophils and dendritic cells (DCs) when cultured with granulocyte macrophage-colony-stimulating factor but not with other tested growth factors. The resulting hybrid cells express markers of both neutrophils (Ly6G, CXCR2, and 7/4) and DCs (CD11c, MHC II, CD80, and CD86). They also exhibit several properties typically reserved for DCs, including dendritic morphology, probing motion, podosome formation, production of interleukin-12 and other cytokines, and presentation of various forms of foreign protein antigens to naïve CD4 T cells. Importantly, they retain intrinsic abilities of neutrophils to capture exogenous material, extrude neutrophil extracellular traps, and kill bacteria via cathelicidin production. Not only do our results reinforce the notion that neutrophils can acquire APC-like properties, they also unveil a unique differentiation pathway of neutrophils into neutrophil-DC hybrids that can participate in both innate and adaptive immune responses.
Introduction
Neutrophils are the most abundant leukocytes, whose turnover is extremely robust and fast. In humans, ∼109 neutrophils/kg of body weight are released every day from bone marrow (BM), and their half-life is shorter than 12 h in the steady state. Circulating neutrophils are rapidly recruited to the sites of tissue damage or microbial invasion, where they execute a number of preassigned defense tasks, including extrusion of neutrophil extracellular traps (NETs), engulfment and killing of microorganisms, recruitment of monocytes, and remodeling of damaged tissues.1 Thus, neutrophils are generally regarded as professional phagocytes playing important roles in the resolution of tissue injury and infection.
Recent studies have suggested functional contributions of neutrophils to adaptive immune responses.2 Activated human neutrophils trigger dendritic cell (DC) maturation and promote T-cell activation and migration.3,4 When cultured with granulocyte macrophage-colony-stimulating factor (GM-CSF) and interferon-γ (IFNγ), human neutrophils begin to express MHC II molecules and to function as accessory cells by promoting superantigen-induced T-cell activation.5 After culturing with GM-CSF, interleukin (IL)-4, and tumor necrosis factor (TNF)α, human neutrophils exhibit surface expression of MHC II, CD1, and co-stimulatory molecules and gain a potent ability to activate allogeneic T cells.6 Depending upon the cytokine composition, they acquire additional markers of antigen presenting cells (APCs), such as CD14, CD64, CD83, CCR6 (CD196), macrophage colony-stimulating factor (M-CSF) receptor (CD115), and macrophage mannose receptor (CD206).7-9 However, the cellular identities or functional properties of those unusual neutrophils showing APC-like properties have remained relatively unclear. Here we demonstrate that murine neutrophils can differentiate into a previously unrecognized “hybrid” leukocyte population exhibiting dual phenotype and functionality of neutrophils and DCs.
Methods
Mice
Animals were purchased from Jackson Laboratories, except the cathelicidin-related antimicrobial peptide (CRAMP) knockout mice.10 All experiments were performed in accordance with the NIH guidelines after approval by the Institutional Animal Care and Use Committee of the University of Toledo.
BM cell culture
Crude BM cells were cultured in 6-well plates (6 × 106 cells/well) in complete RPMI 1640 with 10 ng/mL GM-CSF (R&D Systems, Minneapolis, MN) or 200 ng/mL Flt3L (PeproTech, Rocky Hill, NJ). Culture medium was replaced every 2 d by returning floating cells resuspended in fresh medium back to the plates containing adherent cells. Both floating and adherent cells were harvested on d 6 unless otherwise mentioned. In some experiments, BM cells were cultured according to Inaba’s11 standard protocol by removing nonadherent cells every 2 d. Monocyte-derived DCs (Mo-DCs) were generated by culturing CD11b+/Ly6G−/CD11c− BM cells with GM-CSF for 6 d. To generate macrophages, BM cells were cultured for 7 d in the presence of 10 ng/mL M-CSF (R&D Systems); floating cells were removed every 2 d, and firmly adherent cells were harvested as macrophages. Methods for culturing human band cells are described in supplemental Methods.
Band cell culture with BM feeders
Band cells purified from C57BL/6 mice (CD45.2) and crude BM feeder cells isolated from B6 SJL mice (CD45.1) were co-cultured in a 1:4 ratio in the presence of 10 ng/mL GM-CSF. In some experiments, co-cultures were supplemented with 20 ng/mL granulocyte colony-stimulating factor (G-CSF) (PeproTech), 100 ng/mL M-CSF, or 200 ng/mL Flt3L. CD45.2+/CD45.1− cells purified from these co-cultures were analyzed for morphological and functional properties.
Cell purification
Gr-1high/CD48− band cells were sorted from BM cells with >99.5% purity with FACSAria (BD Biosciences San Jose, CA). All other populations were sorted with >95% purity, and the sorting procedure was repeated if necessary to achieve the above purity. The staining procedures, list of monoclonal antibodies (mAbs), and methods for morphological analyses are described in supplemental Methods.
Microarray analysis and quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted with Trizol (Invitrogen, Grand Island, NY) and purified with an RNeasy Mini kit (Qiagen, Valencia, CA). After hybridization with Affymetrix Mouse Genome 2.0 Array (Genome Explorations, Memphis, TN), data were analyzed by GeneSifter (Geospiza, Seattle, WA) and TreeView software (Michael Eisen Laboratory). The above data as well as the datasets extracted from the Gene Expression Omnibus were normalized with MAS5.0 algorithm using GeneChip Command Console Software. Quantitative reverse transcription-polymerase chain reaction was performed with a LightCycler System (Roche, Indianapolis, IN) using the primer pairs listed in supplemental Table 1. The whole-gene datasets have been deposited in Gene Expression Omnibus with accession number GSE28408.
Western blotting and cytokine analyses
Samples were analyzed in western blotting with rabbit anti-mouse CRAMP antibodies.12 Samples were cultured for 24 h in complete RPMI with GM-CSF and culture supernatants examined for matrix metalloproteinase 9 (MMP9) by enzyme-linked immunosorbent assay. After 24-h incubation with Pam3CSK4 (1 μg/mL), LPS (10 μg/mL), R848 (10 μg/mL), or CpG oligonucleotide (5 μM) (InvivoGen, San Diego, CA), supernatants were analyzed with the Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences).
Assays to measure phagocyte functionality
After incubation with fluorescein isothiocyanate (FITC)-dextran (DX), green fluorescent protein (GFP)-E.coli, or fluorescent latex beads, samples were examined for the uptake of fluorescently labeled probes by FACSCalibur. Bacterial killing activities were examined as described.10 NET formation was assessed under confocal microscopy after staining for DNA and histone13 and by measuring DNA release into the supernatants.14 These methods are described in detail in supplemental Methods.
APC assays
Fluorescence-activated cell sorter (FACS)-purified samples were pulsed for 60 min with ovalbumin (OVA)257–264 or OVA323–339 peptide (10 μg/mL), soluble OVA protein (1 mg/mL), OVA-coated latex beads (10 μg OVA protein/mL), or pnir15.OVA-E. coli (multiplicity of infection = 20).15 After extensive washing, samples were co-cultured with CD8 or CD4 T cells (0.5 or 1 × 105 cells/well) purified from OT-I or OT-II TG mice, respectively. T-cell proliferation was measured by 3H-thymidine uptake on d 3 or 4. In some experiments, OT-II CD4 T cells (0.2 × 105 cells/well) were stimulated for 3 d with the test samples (105 cells/well) and OVA323-339 peptide. Supernatants were examined for IFNγ, IL-4, and IL-17 by enzyme-linked immunosorbent assay, and the cells were stained with mAbs against CD4 and FoxP3 (eBioscience, San Diego, CA).
Statistical analyses
All experiments were carried out at least 2 times to assess reproducibility. All data were processed by GraphPad Prism 5.0 program (GraphPad Software, San Diego, CA) together with SigmaPlot 10 (Systat Software Chicago, IL). Quantitative data are presented as the means ± SD. The statistical significance was assessed based on an unpaired Student t test with 2-tailed distributions or 1-way analysis of variance with Newman-Keuls multiple comparison test. P values < 0.05 were considered to be significant.
Results
Both immature and mature neutrophils acquire DC-like properties in GM-CSF–supplemented cultures
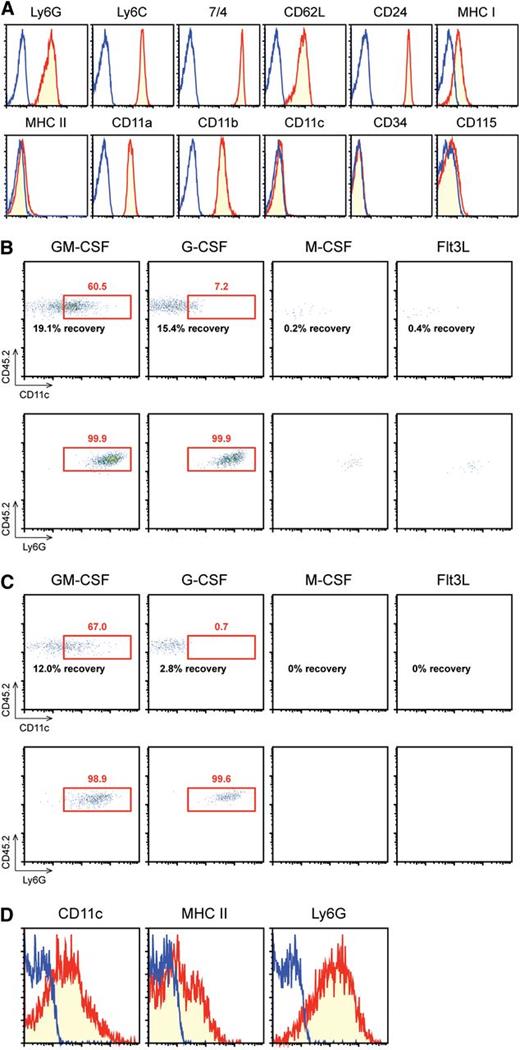
Gr-1high/CD48− band cells were FACS purified from the BM of C57BL/6 mice. The resulting preparations were always >99.5% pure (n = 31) as validated by post-sort analyses (supplemental Figure 1). Moreover, they were extremely homogeneous in terms of small cell size and inclusion of ring-shaped nuclei (supplemental Figure 2). By contrast, the Gr-1+/CD48+ fraction purified in parallel was mostly composed of monocytes (supplemental Figure 3), illustrating an advantage of our sorting strategy. Importantly, the Gr-1high/CD48− population uniformly expressed several neutrophil markers (eg, Ly6G, Ly6C, 7/4, CD62L, CD24, and CD11b) but not markers of DCs (eg, CD11c and MHC II), macrophages (eg, CD115), or granulocyte-macrophage progenitors (eg, CD34) (Figure 1A).
Purified band cells gain surface expression of CD11c and MHC II in GM-CSF–supplemented culture. (A) Gr-1high/CD48− band cells purified from BM of C57BL/6 mice (CD45.2) were examined for surface expression of the indicated markers. Blue lines indicate staining profiles with isotype-matched control IgG. (B-D) Band cells purified from C57BL/6 mice were cultured for 4 d (B) or 6 d (C) with BM feeder cells from B6 SJL mice (CD45.1) in the presence of GM-CSF, G-CSF, M-CSF, or Flt3L. The data show the expression profiles of CD11c (top panels) and Ly6G (bottom panels) within the CD45.2+ gated population and the recovery rates compared with the originally plated band cell number. (D) The data show the expression profiles of CD11c, MHC II, and Ly6G within the CD45.2+/CD45.1− gated population harvested on d 6 from GM-CSF–supplemented co-cultures. Data are representative of at least 3 independent experiments.
Purified band cells gain surface expression of CD11c and MHC II in GM-CSF–supplemented culture. (A) Gr-1high/CD48− band cells purified from BM of C57BL/6 mice (CD45.2) were examined for surface expression of the indicated markers. Blue lines indicate staining profiles with isotype-matched control IgG. (B-D) Band cells purified from C57BL/6 mice were cultured for 4 d (B) or 6 d (C) with BM feeder cells from B6 SJL mice (CD45.1) in the presence of GM-CSF, G-CSF, M-CSF, or Flt3L. The data show the expression profiles of CD11c (top panels) and Ly6G (bottom panels) within the CD45.2+ gated population and the recovery rates compared with the originally plated band cell number. (D) The data show the expression profiles of CD11c, MHC II, and Ly6G within the CD45.2+/CD45.1− gated population harvested on d 6 from GM-CSF–supplemented co-cultures. Data are representative of at least 3 independent experiments.
The purified band cells started to die in 48 h when placed in culture even with added GM-CSF. Addition of crude BM feeder cells (from CD45.1 mice) significantly enhanced the recovery of band cells purified from CD45.2 mice (supplemental Figure 4A). Addition of CD45− nonhematopoietic BM cells was sufficient to sustain the survival of band cells (supplemental Figure 4B). Consistent with the report that GM-CSF and G-CSF both prevent human neutrophil apoptosis by inducing an apoptosis inhibitor, survivin,16 both growth factors sustained the survival of CD45.2+/CD45.1− cells (Figure 1B-C left two panels). CD11c expression became detectable when cultured in the presence of GM-CSF but not G-CSF. Neither M-CSF nor Flt3L promoted the survival or CD11c expression by band cells (Figure 1B-C right two panels). Importantly, CD45.2+/CD45.1− cells recovered from GM-CSF–supplemented co-cultures expressed CD11c and MHC II while maintaining the expression of a neutrophil maker, Ly6G (Figure 1D). Thus, band cells are capable of acquiring the APC-like phenotype when cultured in the presence of GM-CSF but not other tested growth factors. Our culture protocol differs from the previously reported methods to generate APC-like neutrophils in that we employed BM feeder cells to sustain the survival of band cells and we eliminated IFNγ, which is known to induce MHC II expression by even nonhematopoietic cells.17 We failed to detect IFNγ in the co-culture supernatants.
To follow morphological changes, we co-cultured Gr-1high/CD48− band cells (CD45.2) with crude BM feeder cells (CD45.1) and GM-CSF and purified the CD45.2+/CD45.1− populations at various time points. The starting preparations uniformly showed ring-shaped nuclei and small cell size (Figure 2A, d 0). When examined 2 d after culture, many CD45.2+/CD45.1− cells exhibited segmented nuclei, thus resembling polymorphonuclear neutrophils. At later time points, CD45.2+/CD45.1− cells gradually acquired a DC-like morphology characterized by oval-shaped nuclei, enlarged cell size, and dendritic processes (Figure 2A, d 4-8). Oval-shaped nuclei, which were not detectable in the starting band cells (d 0), were observed in ∼40% to 60% of the CD45.2+/CD45.1− cells recovered on d 6-8 (Figure 2B). It should be stated that only 15% to 30% of the originally plated band cells were recovered as viable cells on d 6-8 (Figure 2B numbers on right). In summary, band cells acquired the expression of 2 DC markers, CD11c and MHC II, while retaining a neutrophil marker, Ly6G, and began to display DC-like morphology. The resulting population has, therefore, been termed the “neutrophil-DC hybrid.”
Differentiation of band cells into neutrophil-DC hybrids in culture. (A-B) Gr-1high/CD48− band cells purified from C57BL/6 mice were cultured with GM-CSF in the presence of crude BM feeder cells from B6 SJL mice. (A) CD45.2+/CD45.1− populations purified at the indicated time points were analyzed for nuclear shape (top panels) and morphology (bottom panels; bar represents 20 μm). (B) Changes in nuclear shape were examined by analyzing >2000 cells/time point after nuclear staining. Recovery rates of viable CD45.2+/CD45.1− cells relative to the originally plated cell numbers are shown on the right. (C-E) Crude BM cells from CD45.2 mice were cultured for 2 d with GM-CSF. Gr-1high/CD48− band cells were purified from these precultures and then co-cultured with BM feeder cells from CD45.1 mice in the presence of GM-CSF for an additional 6 d. (C) The starting population (left) and the CD45.2+/CD45.1− population purified from the co-culture (right) were analyzed for nuclear shape and morphology. The latter population was also examined for surface phenotype (D) and APC function to present OVA peptides to OT-II CD4 or OT-I CD8 T cells (means ± SD from triplicate cultures) (E). (F) CD15+/CD10−/CD64−/CD14− band cells purified from human BM samples were examined for the surface phenotype before (top) and after culturing for 7 d in the presence of GM-CSF, TNFα, and IL-4 (bottom). (G) The above-band cell population (top) and the CD14+ monocyte population purified from the same human BM samples (bottom) were cultured for 7 d in parallel and then examined for surface phenotype. Data are representative of 3 independent experiments.
Differentiation of band cells into neutrophil-DC hybrids in culture. (A-B) Gr-1high/CD48− band cells purified from C57BL/6 mice were cultured with GM-CSF in the presence of crude BM feeder cells from B6 SJL mice. (A) CD45.2+/CD45.1− populations purified at the indicated time points were analyzed for nuclear shape (top panels) and morphology (bottom panels; bar represents 20 μm). (B) Changes in nuclear shape were examined by analyzing >2000 cells/time point after nuclear staining. Recovery rates of viable CD45.2+/CD45.1− cells relative to the originally plated cell numbers are shown on the right. (C-E) Crude BM cells from CD45.2 mice were cultured for 2 d with GM-CSF. Gr-1high/CD48− band cells were purified from these precultures and then co-cultured with BM feeder cells from CD45.1 mice in the presence of GM-CSF for an additional 6 d. (C) The starting population (left) and the CD45.2+/CD45.1− population purified from the co-culture (right) were analyzed for nuclear shape and morphology. The latter population was also examined for surface phenotype (D) and APC function to present OVA peptides to OT-II CD4 or OT-I CD8 T cells (means ± SD from triplicate cultures) (E). (F) CD15+/CD10−/CD64−/CD14− band cells purified from human BM samples were examined for the surface phenotype before (top) and after culturing for 7 d in the presence of GM-CSF, TNFα, and IL-4 (bottom). (G) The above-band cell population (top) and the CD14+ monocyte population purified from the same human BM samples (bottom) were cultured for 7 d in parallel and then examined for surface phenotype. Data are representative of 3 independent experiments.
A key question concerned whether mature neutrophils would also acquire DC-like features. To test this, we purified Gr-1high/CD11blow immature neutrophils and Gr-1high/CD11bhigh mature neutrophils from BM using the protocol reported by Ueda et al18 The former preparation uniformly exhibited ring-shaped nuclei, resembling our original Gr-1high/CD48− band cell preparation. By contrast, most of the cells in the latter preparation contained segmented nuclei (supplemental Figure 5A-B). All 3 neutrophil preparations acquired surface expression of CD11c and MHC II while maintaining Ly6G expression (supplemental Figure 5C). No significant difference was observed among the 3 neutrophil preparations in terms of the recovery rates or the frequencies of CD11c+ or MHC II+ cells. Thus, both immature and mature neutrophils can give rise to neutrophil-DC hybrids.
Anti-Gr-1 mAb (clone RB6-8C5), which is widely used to identify neutrophils, recognizes both Ly6G (expressed exclusively by neutrophils) and Ly6C (expressed not only by neutrophils, but also by some monocytes).19 Although Ly6C is expressed by selected DC subsets, including Mo-DCs, plasmacytoid DCs (pDCs), and IFN-producing killer DCs,20-22 none of the currently recognized DC subsets has been reported to express Ly6G. Ly6G was undetectable in standard BM-derived DC (BMDC) cultures generated according to Inaba’s protocol,11 in which floating neutrophils were repeatedly discarded (supplemental Figure 6A). Likewise, Mo-DC cultures generated from CD11b+/CD11c−/Ly6G− monocytes showed no Ly6G expression (supplemental Figure 6B). Thus, retained surface expression of Ly6G serves as a unique marker for neutrophil-DC hybrids.
Additional experiments were performed to exclude the possibility that hybrids might be derived from contaminants. For example, they might be generated from small numbers of highly proliferative precursors. In this regard, when CD45.2 band cells and CD45.1 BM feeder cells were labeled with carboxyfluorescein diacetate succinimidyl ester and then co-cultured in the presence of GM-CSF, significant carboxyfluorescein diacetate succinimidyl ester dilution was observed only in the CD45.2−/CD45.1+ feeder cell fraction (supplemental Figure 6C). To exclude the possibility of monocyte origin more formally, Ly6C+/Ly6G− monocytes (CD45.2) were co-cultured with CD45.1 BM feeder cells in the presence of GM-CSF. Although CD45.2+/CD45.1− cells acquired CD11c and MHC II expression as they differentiated into Mo-DCs, they showed almost no Ly6G expression (supplemental Figure 6D). In the standard BMDC culture with added GM-CSF, Inaba et al11 observed a significant APC activity as early as d 2, even before the appearance of cellular aggregates containing DCs on d 4. To eliminate such early-emerging DCs from the starting preparations, we first cultured BM cells with GM-CSF for 2 d and then purified Gr-1high/CD48− cells. The GM-CSF–primed neutrophil preparation consisted of ∼30% cells with ring-shaped nuclei and ∼70% cells with segmented nuclei (Figure 2C, left panels). When co-cultured with BM feeder cells and GM-CSF for an additional 6 d, these neutrophils also acquired oval-shaped nuclei, enlarged cell size, and dendritic processes (Figure 2C, right panels). They also expressed typical DC markers (CD11c, MHC II, and CD205) while maintaining Ly6G expression (Figure 2D). Moreover, they efficiently presented OVA peptides to both OT-II CD4 and OT-I CD8 T cells (Figure 2E). Taken together, these observations demonstrate that neutrophil-DC hybrids are indeed derived from neutrophils.
To identify a human counterpart, we cultured D15+/CD10−/CD64−/CD14− band cells23 purified from human BM samples in the presence of GM-CSF, TNFα, and IL-4, which are widely used to generate human Mo-DCs. We observed uniform surface expression of MHC II (HLA-DR) in those cultures derived from neutrophils (Figure 2F). Importantly, they maintained the expression of all tested neutrophil markers (eg, CD15, CD24, CD66b, and CD89), which were not detectable in control Mo-DCs generated in parallel from CD14+ monocytes purified from the same BM samples (Figure 2G). Both populations expressed all tested DC markers (HLA-DR, CD1c, and CD11c) but not the macrophage marker CD163. Thus, human neutrophils can also differentiate into a hybrid population characterized by dual expression of both neutrophil and DC markers.
Neutrophil-DC hybrids resemble traditional DCs in morphology and surface phenotype
When crude BM cells were cultured with GM-CSF for 6 d, Ly6G was detected in minor fractions (3.9 ± 1.9%, n = 15) of the CD11c+/MHC II+ DC populations (supplemental Figure 7). The number of hybrid cells time-dependently increased with a peak on d 9, when they accounted for roughly 3% to 5% of total cell numbers (Figure 3A filled circles). By contrast, BM cultures supplemented with Flt3L contained relatively large numbers of CD11c+ cells expressing a pDC marker B220 (CD45RA) but not Ly6G (supplemental Figure 7; Figure 3A empty circles). These findings again indicate that GM-CSF serves as the primary growth factor promoting the generation of neutrophil-DC hybrids.
Characterization of neutrophil-DC hybrids purified from crude BM culture. (A) BM cells from C57BL/6 mice were cultured with GM-CSF, Flt3L, or no added growth factor for the indicated periods, and resulting populations were examined for the expression of CD11c, Ly6G, and B220. The data show the numbers of total cells, CD11c+/Ly6G− DCs, Ly6G+/CD11c+ neutrophil-DC hybrids, and B220+/CD11c+ pDCs (means ± SD from triplicate cultures). (B-E) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs were purified on d 6 from GM-CSF–supplemented BM culture. These populations were examined under light microscopy after HEMA-3 staining (B), under differential interference contrast microscopy (C), or under confocal microscopy after staining with phalloidin and antivinculin mAb (D) or with anti-MPO mAb (E). All the images are representative of at least 3 independent experiments (bar represents 20 μm). Time-lapse images recorded under differential interference contrast microscope are shown in supplemental Video 1.
Characterization of neutrophil-DC hybrids purified from crude BM culture. (A) BM cells from C57BL/6 mice were cultured with GM-CSF, Flt3L, or no added growth factor for the indicated periods, and resulting populations were examined for the expression of CD11c, Ly6G, and B220. The data show the numbers of total cells, CD11c+/Ly6G− DCs, Ly6G+/CD11c+ neutrophil-DC hybrids, and B220+/CD11c+ pDCs (means ± SD from triplicate cultures). (B-E) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs were purified on d 6 from GM-CSF–supplemented BM culture. These populations were examined under light microscopy after HEMA-3 staining (B), under differential interference contrast microscopy (C), or under confocal microscopy after staining with phalloidin and antivinculin mAb (D) or with anti-MPO mAb (E). All the images are representative of at least 3 independent experiments (bar represents 20 μm). Time-lapse images recorded under differential interference contrast microscope are shown in supplemental Video 1.
To perform more comprehensive analyses, we purified Ly6G+/CD11c+/MHC II+ hybrids from GM-CSF–supplemented BM cultures together with 2 additional leukocyte populations emerging in the same cultures: Ly6G−/CD11c+/MHC II+ traditional DCs and Ly6G+/CD11c−/MHC II− neutrophils. All 3 sorted populations were >95% pure in post-sort analyses (supplemental Figure 8). Although both adherent and floating cells were kept in our BM cultures, most of the hybrids (99.4 ± 0.5%, n = 3), traditional DCs (99.1 ± 0.9%), and neutrophils (99.1 ± 0.9%) were recovered as floating cells. The hybrids were indistinguishable from traditional DCs in morphology, as they both exhibited oval-shaped nuclei and lamellar dendritic processes (Figure 3B). As recently demonstrated for Mo-DCs,24 the hybrids and traditional DCs both showed a “probing” motion of dendrites in time-lapse imaging under differential interference contrast microscopy (Figure 3C; supplemental Video 1). For comparison, we generated macrophages in M-CSF–supplemented BM culture and harvested tightly adherent cells by scraping according to a standard protocol.25 Those macrophages showed numerous cytoplasmic vacuoles (supplemental Figure 9A), rapidly adhered to and spread on the chamber coverglass during the 2-h preincubation period before imaging, and exhibited amoeba-like, slow movement of cell bodies (supplemental Video 1). The CD11b+/F4/80+ macrophage population expressed CD11c and MHC II at low levels and showed no detectable expression of any tested neutrophil markers (supplemental Figure 9B). Neutrophil-DC hybrids and traditional DCs both showed clusters of podosome-like structures containing both F-actin and vinculin at the ventral surface (Figure 3D), corroborating previous reports for immature DCs.26,27 None of these features was observed in the neutrophil preparations. Conversely, MPO was detected in hybrids and neutrophils but not in Ly6G− traditional DCs (Figure 3E). These morphological and phenotypic features clearly distinguish neutrophil-DC hybrids and traditional DCs from neutrophils and macrophages emerging in BM cultures.
To identify unique marker(s) of neutrophil-DC hybrids, we next examined the expression of >50 surface molecules (supplemental Figure 10). Ly6G+/CD11c+ hybrids and Ly6G−/CD11c+ traditional DCs both expressed DC markers (MHC II and CD1d), selected adhesion molecules (CD11a, CD18, and CD54), and co-stimulatory molecules (CD80 and CD86) at comparable levels. Although CD206 was detected in fractions of both populations, neither of them expressed Langerin (CD207), DC-SIGN (CD209), c-kit (CD117), or Flt3 (CD135). Ly6G+/CD11c+ hybrids and Ly6G+/CD11c− neutrophils uniformly displayed 7/4 (a neutrophil marker) and Ly6C. Consistent with the reports on dual expression of 7/4 and Ly6C by “inflammatory” monocytes,28,29 these 2 molecules were detected on some traditional DCs. In essence, neutrophil-DC hybrids were virtually indistinguishable from traditional DCs in terms of surface phenotype, except uniform expression of Ly6G and 7/4.
Neutrophil-DC hybrids exhibit dual functionality as professional phagocytes and APCs
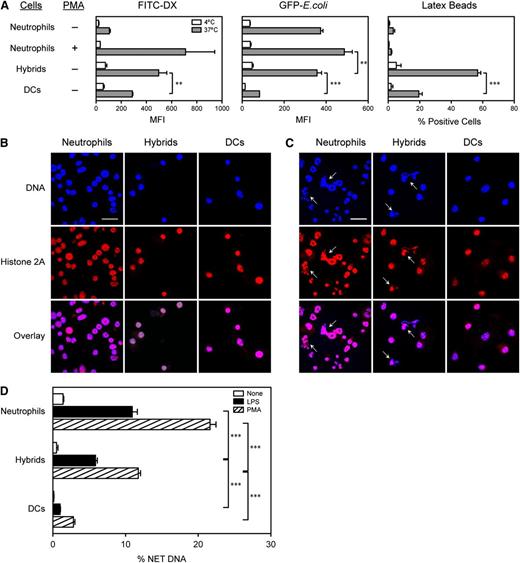
Ly6G+/CD11c+ neutrophil-DC hybrids emerging in GM-CSF–supplemented BM culture showed potent endocytotic capacity to incorporate FITC-DX, GFP-expressing live E. coli, and latex beads (Figure 4A). In fact, they were significantly more efficient than Ly6G− traditional DCs emerging in the same culture. Hybrids were roughly comparable to phorbol myristic acetate (PMA)-activated or nonactivated neutrophils in the uptake of DX or live bacteria, respectively. Corroborating a previous report,30 neutrophils failed to incorporate noncoated latex particles. Thus, hybrids resemble neutrophils in the ability to capture soluble and particulate materials and living bacteria from extracellular spaces.
Neutrophil-DC hybrids retain functionality as professional phagocytes. (A) Cells harvested from GM-CSF–supplemented BM culture (d 6) were incubated for 30 min at 4°C or 37°C with FITC-DX, live E. coli expressing GFP, or fluorescent beads and then examined for the mean fluorescence intensity (MFI) or percent bead+ cells within the Ly6G+/CD11c− neutrophil, Ly6G+/CD11c+ hybrid, and Ly6G−/CD11c+ traditional DC populations. Some samples were tested after a 2-h pretreatment with PMA (100 nM). (B-D) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were examined for NET formation. The purified samples were incubated for 2 h with phosphate-buffered saline alone (B) or PMA (C) on cover slips and then stained for DNA and histone 2A. Arrows indicate NET-forming cells (bar represents 20 μm). The same images are shown at a higher magnification in supplemental Figure 11. (D) The purified samples were incubated for 2 h with phosphate-buffered saline alone, LPS, or PMA and then examined for the magnitudes of NET formation by measuring DNA release (means ± SD from triplicate cultures). Data are representative of 2 independent experiments. **P < .01, ***P < .001 between the indicated samples.
Neutrophil-DC hybrids retain functionality as professional phagocytes. (A) Cells harvested from GM-CSF–supplemented BM culture (d 6) were incubated for 30 min at 4°C or 37°C with FITC-DX, live E. coli expressing GFP, or fluorescent beads and then examined for the mean fluorescence intensity (MFI) or percent bead+ cells within the Ly6G+/CD11c− neutrophil, Ly6G+/CD11c+ hybrid, and Ly6G−/CD11c+ traditional DC populations. Some samples were tested after a 2-h pretreatment with PMA (100 nM). (B-D) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were examined for NET formation. The purified samples were incubated for 2 h with phosphate-buffered saline alone (B) or PMA (C) on cover slips and then stained for DNA and histone 2A. Arrows indicate NET-forming cells (bar represents 20 μm). The same images are shown at a higher magnification in supplemental Figure 11. (D) The purified samples were incubated for 2 h with phosphate-buffered saline alone, LPS, or PMA and then examined for the magnitudes of NET formation by measuring DNA release (means ± SD from triplicate cultures). Data are representative of 2 independent experiments. **P < .01, ***P < .001 between the indicated samples.
NET formation is another unique feature of neutrophils.13 In the absence of exogenous stimuli, none of the 3 leukocyte populations formed NETs, defined as extracellular fibrous structures containing both DNA and chromatin proteins (eg, histones) (Figure 4B). Within 2 h after exposure to PMA, NET structures were observed with neutrophils and hybrids but not with traditional DCs (Figure 4C; supplemental Figure 11). We quantified the magnitude of NET formation by digesting the DNA scaffold of NETs with an endonuclease and measuring DNA release into supernatants.14 Once again, no significant DNA release was observed in any population in the absence of stimuli (Figure 4D). Neutrophils showed robust DNA release after exposure to PMA or LPS. Neutrophil-DC hybrids also responded to both stimuli, albeit at smaller magnitudes. This finding is striking, because NET formation has been reported only for neutrophils,13 eosinophils,31 and mast cells.32 Traditional DCs showed only marginal DNA release after stimulation. In summary, neutrophil-DC hybrids maintain the intrinsic functionality of neutrophils to serve as professional phagocytes.
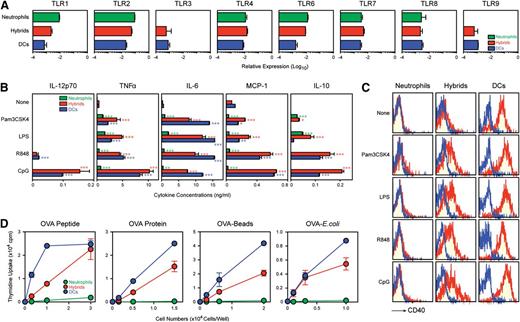
An equally important question concerns whether hybrids can also function as professional APCs. Ly6G+/CD11c+ hybrids were almost comparable with Ly6G−/CD11c+ traditional DCs in the Toll-like receptor (TLR) mRNA expression profiles (Figure 5A). The 2 populations were also comparable with each other in their functional responses to TLR agonists, including Pam3CSK4 (TLR2), LPS (TLR4), R848 (TLR7/8), and CpG oligonucleotide (TLR9). Upon stimulation with CpG oligonucleotide, for example, both populations secreted IL-12p70, TNFα, IL-6, MCP-1, and IL-10 (Figure 5B). Other TLR agonists also triggered cytokine/chemokine production by both populations. Moreover, all tested TLR agonists elevated surface expression of CD40 by the hybrids (Figure 5C). By contrast, Ly6G+/CD11c− neutrophils showed only modest responses to the tested TLR agonists.
Neutrophil-DC hybrids acquire functionality as APCs. (A) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were examined for TLR mRNA expression profiles by quantitative polymerase chain reaction (means ± SD from triplicate samples). (B-C) The same 3 purified populations were cultured for 24 h with phosphate-buffered saline alone or each of the indicated TLR agonists. The samples were then examined for cytokine release measured by the cytometric bead array system (means ± SD from triplicate cultures) (B) and surface expression of CD40 (C). (D) The same 3 purified populations were pulsed for 60 min with the indicated form of OVA antigen and then co-cultured with OVA-specific CD4 T cells from OT-II TG mice (means ± SD from triplicate cultures). *P < .05, **P < .01, ***P < .001 compared with control group treated with PBS alone. Data are representative of at least 2 independent experiments.
Neutrophil-DC hybrids acquire functionality as APCs. (A) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were examined for TLR mRNA expression profiles by quantitative polymerase chain reaction (means ± SD from triplicate samples). (B-C) The same 3 purified populations were cultured for 24 h with phosphate-buffered saline alone or each of the indicated TLR agonists. The samples were then examined for cytokine release measured by the cytometric bead array system (means ± SD from triplicate cultures) (B) and surface expression of CD40 (C). (D) The same 3 purified populations were pulsed for 60 min with the indicated form of OVA antigen and then co-cultured with OVA-specific CD4 T cells from OT-II TG mice (means ± SD from triplicate cultures). *P < .05, **P < .01, ***P < .001 compared with control group treated with PBS alone. Data are representative of at least 2 independent experiments.
We next compared the same 3 populations for their APC capacities to present OVA antigen in 4 different forms, ie, MHC II-restricted OVA peptide, soluble OVA protein, OVA protein immobilized on latex beads, and OVA-expressing E. coli. After antigen pulsing, Ly6G+/CD11c+ hybrids, Ly6G−/CD11c+ traditional DCs, and Ly6G+/CD11c− neutrophils were co-cultured with OVA-specific CD4 T cells isolated from OT-II TG mice (Figure 5D). Neutrophil-DC hybrids showed a remarkable ability to present all tested forms of OVA antigen. In terms of relative potency, traditional DCs were roughly 3-fold more efficient than the hybrids, estimated based on the numbers of APCs required to induce a comparable level of T-cell proliferation. Neutrophils failed to induce significant T-cell proliferation. With regard to cross-presentation, OVA protein-pulsed hybrids, which triggered a robust (up to 15 000 cpm) proliferation of OT-II CD4 T cells, induced only a modest (<500 cpm) proliferation of CD8 T cells from OT-I TG mice. Because the 2 T-cell populations showed similar levels of proliferation after stimulation with OVA peptide-pulsed neutrophil-DC hybrids (Figure 2E), we have concluded that hybrids exhibit only a limited capacity to cross-present exogenous antigen. Both hybrids and traditional DCs induced significant production of IFNγ and IL-17 (but not IL-4) by CD4 T cells, suggesting their ability to drive T-cell differentiation into Th1 and Th17 cells (supplemental Figure 12). They failed to promote the generation of regulatory T cells as assessed by intracellular staining for FoxP3 (data not shown). In essence, neutrophil-DC hybrids can function not only as professional phagocytes but also as APCs, triggering activation and differentiation of CD4 T cells.
Unique properties that distinguish neutrophil-DC hybrids from traditional DCs
Using the Affymetrix Mouse Genome 2.0 Array, we examined the gene expression profiles of neutrophil-DC hybrids and Ly6G− traditional DCs purified from the same BM cultures. Working with the most advanced Affymetrix MoGene 1.0ST Array system, Miller et al33 recently identified the “core DC transcripts” that are expressed by many conventional or classical DC (cDC) populations, but not macrophage populations, isolated from various lymphoid and nonlymphoid tissues in mice. We observed that most of these transcripts were expressed by both neutrophil-DC hybrids and Ly6G− traditional DCs (supplemental Figure 13). We extracted the core DC gene expression profiles from the publicly available, Affymetrix 2.0 Array-based datasets for various DC and macrophage populations. Hierarchical clustering analysis showed neutrophil-DC hybrids most closely resembled the Ly6G− traditional DC counterpart purified from the same BM culture and also conventional BMDCs generated using Ianaba’s protocol.11 Hybrids and all tested DC populations were clustered into a group, whereas all macrophage populations were clustered into an independent group. Thus, it appears reasonable to suggest that neutrophil-DC hybrids belong to the DC lineage in terms of core DC gene expression profiles.
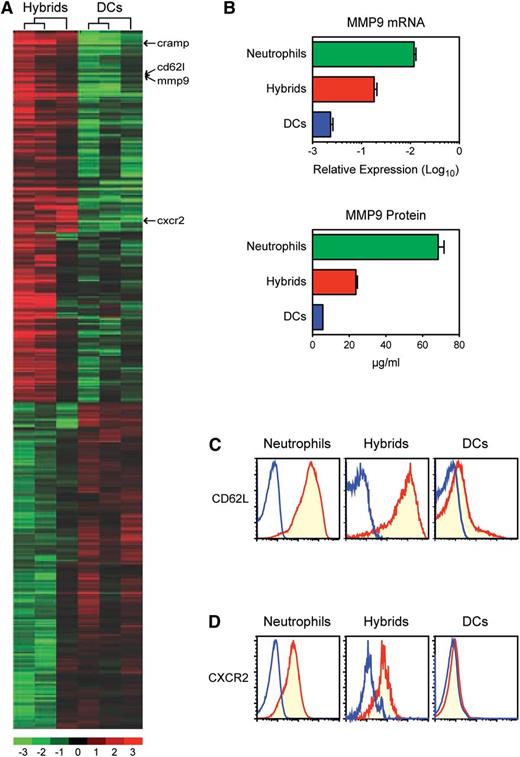
Neutrophil-DC hybrids were distinguishable from Ly6G− traditional DCs by preferentially expressing >170 genes (Figure 6A). This cluster included MMP9, CD62L, and CXCR2, which are all known to be abundantly produced by neutrophils.1 Consistent with our GeneChip data, neutrophil-DC hybrids produced significantly higher levels of MMP9 mRNA and MMP9 protein than did traditional DCs (Figure 6B). Moreover, only the former also uniformly displayed CD62L proteins at high levels (Figure 6C). CXCR2 surface expression was observed only on neutrophil-DC hybrids (Figure 6D). CXCR2 appeared to be a neutrophil-specific marker, because its expression was detectable almost exclusively within the Ly6G+ neutrophil fraction among CD45+ hematopoietic cells in peripheral blood and spleen (supplemental Figure 14). As expected, Ly6G+/CD11c−/MHC II− neutrophils isolated from BM cultures produced MMP9, CD62L, and CXCR2 at high levels (Figure 6B-D). Thus, neutrophil-DC hybrids maintain several unique features of neutrophils.
Identification of unique properties that distinguish neutrophil-DC hybrids from traditional DCs. (A) Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids and Ly6G−/CD11c+/MHC II+ traditional DCs purified from the same GM-CSF–supplemented BM cultures (d 6) were compared for global gene expression profiles. The heat map was created from 3 independent pairs to show the genes that are predominantly expressed (>2-fold and P < .05). The whole-gene datasets have been deposited in Gene Expression Omnibus with accession number GSE28408. (B-D) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs were examined for MMP9 mRNA expression by quantitative polymerase chain reaction and MMP9 protein elaboration by enzyme-linked immunosorbent assay (means ± SD from triplicate samples) (B) and for surface expression of CD62L (C) and CXCR2 (D). Data are representative of 3 independent experiments.
Identification of unique properties that distinguish neutrophil-DC hybrids from traditional DCs. (A) Ly6G+/CD11c+/MHC II+ neutrophil-DC hybrids and Ly6G−/CD11c+/MHC II+ traditional DCs purified from the same GM-CSF–supplemented BM cultures (d 6) were compared for global gene expression profiles. The heat map was created from 3 independent pairs to show the genes that are predominantly expressed (>2-fold and P < .05). The whole-gene datasets have been deposited in Gene Expression Omnibus with accession number GSE28408. (B-D) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ hybrids, and Ly6G−/CD11c+/MHC II+ traditional DCs were examined for MMP9 mRNA expression by quantitative polymerase chain reaction and MMP9 protein elaboration by enzyme-linked immunosorbent assay (means ± SD from triplicate samples) (B) and for surface expression of CD62L (C) and CXCR2 (D). Data are representative of 3 independent experiments.
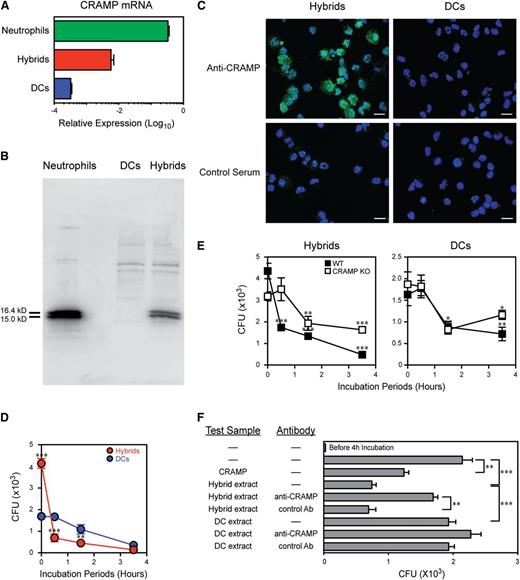
The above cluster also included CRAMP, a murine homolog of human cathelicidin LL-37, which is produced predominantly by neutrophils.12 Neutrophil-DC hybrids expressed 10-fold higher CRAMP mRNA compared with traditional DCs (Figure 7A). Western blotting revealed that hybrids produce CRAMP polypeptides (Figure 7B), corresponding to the forms of CRAMP reported in murine neutrophils and spleen samples.34 CRAMP proteins were also detected within the cytoplasm of the hybrids (Figure 7C). In contrast, no CRAMP protein expression was detected in traditional DCs. As expected, neutrophils produced CRAMP at high levels (Figures 7A-B). Because CRAMP has been shown to mediate bacterial killing in neutrophils,35 we hypothesized that those hybrid cells equipped with phagocytic machinery, NET formation, and CRAMP production might exhibit bactericidal activities. To test this, we allowed the hybrids or traditional DCs to incorporate viable E. coli for 60 min and then eliminated free or surface-bound bacteria via a brief exposure to kanamycin. At this point (time 0), hybrids had ingested twice as many E. coli as traditional DCs. Moreover, the former rapidly killed the internalized bacteria in the subsequent 30-min period, whereas bacterial killing occurred more slowly in the latter population (Figure 7D). Importantly, the hybrids from CRAMP-knockout mice10 failed to exhibit this rapid bactericidal activity, whereas CRAMP-deficient traditional DCs were comparable with the wild-type counterpart (Figure 7E). Whole-cell extracts of neutrophil-DC hybrids contained limited amounts of CRAMP (1-2 μg/106 cells) as estimated in western blotting experiments. For comparison, the addition of a small amount (8 μM or 30 μg/mL) of a synthetic CRAMP140-172 peptide to cultured E. coli resulted in ∼50% reduction in colony-forming unit values. Similar activity was detected in the extracts from the hybrids but not from traditional DCs, and this activity was significantly inhibited by neutralizing antibodies against CRAMP (Figure 7F). Thus, CRAMP-mediated, rapid, bacterial killing represents another distinctive feature distinguishing neutrophil-DC hybrids from traditional DCs.
Neutrophil-DC hybrids kill bacteria rapidly by a CRAMP-mediated mechanism. (A-C) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ hybrids, and/or Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were compared for CRAMP mRNA expression by quantitative polymerase chain reaction (means ± SD from triplicate samples) (A) and for CRAMP protein production by western blotting (B) and immunofluorescence staining (bar represents 20 μm) (C). (D) The above hybrid and traditional DC populations were incubated for 60 min with live E. coli. After killing extracellular bacteria by exposure to kanamycin (time 0), the samples were incubated for an additional 0.5-3.5 h to test intracellular bacterial killing (means ± SD from triplicate samples). (E) Neutrophil-DC hybrids (left panel) and traditional DCs (right panel) purified from wild-type mice or CRAMP-deficient mice were examined for intracellular bacterial killing activities (means ± SD from triplicate samples). (F) E. coli were cultured for 4 h with a synthetic CRAMP peptide or whole protein extracts from neutrophil-DC hybrids or traditional DCs in the presence or absence of neutralizing anti-CRAMP antibodies or control antibodies. The starting bacterial number before culturing is shown in the top bar (means ± SD from triplicate bacterial cultures). *P < .05, **P < .01, ***P < .001 between the indicated samples (F) or compared with traditional DCs (E) or to the starting bacterial numbers at time 0 (E). Data are representative of at least 2 independent experiments.
Neutrophil-DC hybrids kill bacteria rapidly by a CRAMP-mediated mechanism. (A-C) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11c+/MHC II+ hybrids, and/or Ly6G−/CD11c+/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture (d 6) were compared for CRAMP mRNA expression by quantitative polymerase chain reaction (means ± SD from triplicate samples) (A) and for CRAMP protein production by western blotting (B) and immunofluorescence staining (bar represents 20 μm) (C). (D) The above hybrid and traditional DC populations were incubated for 60 min with live E. coli. After killing extracellular bacteria by exposure to kanamycin (time 0), the samples were incubated for an additional 0.5-3.5 h to test intracellular bacterial killing (means ± SD from triplicate samples). (E) Neutrophil-DC hybrids (left panel) and traditional DCs (right panel) purified from wild-type mice or CRAMP-deficient mice were examined for intracellular bacterial killing activities (means ± SD from triplicate samples). (F) E. coli were cultured for 4 h with a synthetic CRAMP peptide or whole protein extracts from neutrophil-DC hybrids or traditional DCs in the presence or absence of neutralizing anti-CRAMP antibodies or control antibodies. The starting bacterial number before culturing is shown in the top bar (means ± SD from triplicate bacterial cultures). *P < .05, **P < .01, ***P < .001 between the indicated samples (F) or compared with traditional DCs (E) or to the starting bacterial numbers at time 0 (E). Data are representative of at least 2 independent experiments.
Discussion
The present study has unveiled a pathway in which neutrophils differentiate into a previously unrecognized leukocyte population exhibiting dual features of neutrophils and DCs. Neutrophils are generally regarded as the first defender against tissue injury and infection. Under pathological conditions, neutrophils home to inflammatory sites using chemokine receptors (eg, CXCR2) migrate across the endothelial cell lining using selectins (eg, CD62L) and integrins (eg, CD11a/CD18 and CD11b/CD18) and penetrate through the basement membrane by elaborating proteases (eg, MMP9). After reaching the sites of injury or infection, neutrophils perform their assigned defensive tasks: to recruit monocytes by releasing preformed granule proteins (eg, cathelicidin) and secreting chemokines (eg, MCP-1), to produce powerful antiseptics in an MPO-dependent manner, to entrap extracellular microorganisms by NET formation, to engulf and kill bacteria intracellularly, and to remodel damaged tissues by digesting tissue debris.1 Remarkably, neutrophil-DC hybrids conserve many of these essential molecules and activities of neutrophils. At the same time, they also exhibit many features that are typically reserved for DCs, including a probing motion of dendritic processes, podosome formation, cytokine production upon stimulation with TLR agonists, and APC function to present various forms of antigens to naïve T cells. In essence, this dual functionality signifies neutrophil-DC hybrids derived from neutrophils.
DCs are extremely heterogeneous in terms of origin, tissue distribution, phenotype, and function. They are generally divided into cDCs and pDCs, with the former further subdivided into those that are found in epithelial tissues, those that normally reside in lymphoid tissues, and those migrating from peripheral tissues to lymphoid tissues. All cDC populations show typical DC-like morphology, express CD11c and MHC II at high levels, and exhibit potent APC capacity.36,37 By contrast, pDCs are round-shaped leukocytes expressing CD11c and MHC II at low levels; they are characterized by expression of B220, Ly6C, and PDCA1 (CD317) and secretion of large amounts of type I IFN.38 In addition to the above populations found in the steady state, novel DC subsets appear during infection and inflammation; they include TNF/inducible nitric oxide synthase-producing DCs,39 Ly6C+ Mo-DCs,20 and CD209+/CD206+ Mo-DCs.24 To the best of our knowledge, no DC subset has been shown to express Ly6G or CXCR2, extrude NET-like structures, or kill bacteria in a cathelicidin-dependent mechanism. Neutrophil-DC hybrids did not express any of the markers for lymphoid DCs (CD8), migratory DCs (CD103), Mo-DCs (CD209), Langerhans cells (LCs) (CD207), or pDC (B220 and CD317). Thus, they are distinguishable from any of the currently recognized DC populations.
Several DC precursor populations have been identified in BM.40 The macrophage and DC progenitors41 give rise to monocytes and to the common DC progenitors that can generate both cDCs and pDCs but not other lineages.42,43 Common DC progenitors can also produce the precursors for cDCs, which acquire characteristic features of fully differentiated DCs upon homing to peripheral tissues.42,44 In addition to this pathway of steady-state DC development, Ly6C+ “inflammatory” monocytes can also differentiate into various DC subsets, including Mo-DCs and TNF/ inducible nitric oxide synthase-producing DCs during inflammation.24,39 The present study has unveiled an additional pathway: neutrophils acquire DC-like properties while maintaining the key functionality as phagocytes. Flt3L is generally regarded as a key growth factor indispensable for steady-state DC development in BM from nonmonocytic precursors,45,46 while GM-CSF is required for generation of CD103+ migratory DCs in the intestine and skin.47 Moreover, GM-CSF promotes monocyte differentiation toward Mo-DCs under inflammatory conditions.24 In this regard, we observed that neutrophil differentiation into hybrids was supported by GM-CSF but not Flt3L. Thus, 2 professional phagocyte populations, ie, neutrophils and monocytes, may both acquire DC-like properties upon recruitment to GM-CSF-rich inflammatory lesions.
Neutrophil origin of neutrophil-DC hybrids is supported by the following observations. First, our starting band cell preparations were extremely pure and uniform in post-sort analyses, surface phenotype, and morphology. Second, band cells that originally showed ring-shaped nuclei acquired oval-shaped nuclei as they differentiated into the hybrids. Finally, FACS-purified monocytes failed to acquire the characteristic phenotype of the hybrids during their differentiation into Mo-DCs. These results imply that maturation into polymorphonuclear neutrophils represents one, but not the only, differentiation option for band cells.
It is important to compare our findings with the previous reports demonstrating the acquisition of APC-like properties by neutrophils. In many of those studies, neutrophils purified from human peripheral blood were found to express APC surface markers when cultured with multiple cytokines, such as GM-CSF, IFNγ, TNFα, IL-4, and IL-15.5-9 Murine neutrophils purified from thioglycollate-induced peritonitis lesions or from chronic colitis lesions have been shown to present OVA protein or OVA peptide to CD4 T cells from OT-II TG mice, respectively.48,49 Murine neutrophils purified from BM acquired the ability to cross-present OVA protein to CD8 T cells after short-term culture in the presence of GM-CSF.50 However, cellular identity of those unusual neutrophils showing APC-like properties has remained unclear due to the unavailability of specific markers. By using Ly6G, CD11c, and MHC II, we have been able to identify and purify neutrophil-DC hybrids for comprehensive analyses of morphology, transcription profile, surface phenotype, and function. Our results now demonstrate that neutrophils can give rise to a previously unrecognized hybrid population characterized by dual phenotype and functionality of neutrophils and DCs. We interpret our findings to imply extraordinary plasticity of neutrophils, as well as their potential contributions to both innate and adaptive arms of the host immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sean Linkes for technical assistance and Dr. Casey T. Weaver for providing the pnir15.OVA-E.coli strain.
This work was supported by grants from the National Institutes of Health and the Bill and Melinda Gates Foundation.
National Institutes of Health
Authorship
Contribution: H.M. and S.G. performed most of the experiments, analyzed the data, and drafted the manuscript; R. L., T.O., and Y.Y. made significant contributions to the morphological and functional analyses; N.M. played a major role in identification of a human counterpart; P.F.K., T.M., and R.L.G. provided key materials and reagents; B.J.C. assisted cell sorting; and A.T. designed the overall study, interpreted the data, and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akira Takashima, Department of Medical Microbiology and Immunology, University of Toledo College of Medicine, 3000 Arlington Ave, Toledo, OH 43614-5806; e-mail: akira.takashima@utoledo.edu.
References
Author notes
H.M. and S.G. contributed equally to this work.