Key Points
Noninferiority of anagrelide in comparison with hydroxyurea in WHO-essential thrombocythemia, a phase 3 trial
Abstract
High platelet counts in essential thrombocythemia (ET) can be effectively lowered by treatment with either anagrelide or hydroxyurea. In 259 previously untreated, high-risk patients with ET, diagnosed according to the World Health Organization classification system, the efficacy and tolerability of anagrelide compared with hydroxyurea were investigated in a prospective randomized noninferiority phase 3 study in an a priori–ordered hypothesis. Confirmatory proof of the noninferiority of anagrelide was achieved after 6 months using the primary end point criteria and was further confirmed after an observation time of 12 and 36 months for platelet counts, hemoglobin levels, leukocyte counts (P < .001), and ET-related events (HR, 1.19 [95% CI, 0.61-2.30], 1.03 [95% CI, 0.57-1.81], and 0.92 [95% CI, 0.57-1.46], respectively). During the total observation time of 730 patient-years, there was no significant difference between the anagrelide and hydroxyurea group regarding incidences of major arterial (7 vs 8) and venous (2 vs 6) thrombosis, severe bleeding events (5 vs 2), minor arterial (24 vs 20) and venous (3 vs 3) thrombosis and minor bleeding events (18 vs 15), or rates of discontinuation (adverse events 12 vs 15 or lack of response 5 vs 2). Disease transformation into myelofibrosis or secondary leukemia was not reported. Anagrelide as a selective platelet-lowering agent is not inferior compared with hydroxyurea in the prevention of thrombotic complications in patients with ET diagnosed according to the World Health Organization system. This trial was registered at http://www.clinicaltrials.gov as #NCT01065038.
Introduction
Essential thrombocythemia (ET) is a relatively benign myeloproliferative neoplasm characterized by increased platelet production and persistently elevated platelet counts. This condition is frequently associated with major and minor vascular complications that cause increased morbidity and sometimes fatal complications.1-3 The overall estimated risk for major thrombotic and bleeding episodes in ET is 6.6% per patient year, which increases to more than 10% per year if left untreated in patients with risk factors such as age older than 60 years or a history of vascular complications.1,3,4
Patients at high risk for thrombosis or hemorrhages are therefore considered to be candidates for cytoreductive therapy.5 In a landmark trial, high-risk patients with ET were randomly assigned to receive the general cytoreductive agent hydroxyurea, or placebo. The observed platelet-lowering effect in this trial was associated with a lower rate of thrombotic complications, suggesting that high-risk patients should receive cytoreductive therapy with hydroxyurea and that platelet counts could serve as a surrogate marker for clinical complications.5,6 However, opinions differ whether high-risk groups such as asymptomatic patients older than 60 years with platelet counts of less than 1000 × 109/L or asymptomatic patients younger than 60 years but with platelet counts of more than 1500 × 109/L should be treated with platelet-lowering agents.7-10
Debate is also ongoing regarding whether a general cytoreductive (ie, leukocyte-reducing) effect, rather than a selective platelet-lowering effect, may be responsible for the reduction of thrombotic events11,12 because no clear evidence exists that a high platelet count per se is associated with major vascular complications, though there might be indirect clinical evidence that platelets are involved in microvessel symptoms. In a previous retrospective investigation, an increased leukocyte count at diagnosis of ET was associated with thrombosis during follow-up, with a relative risk of 2.3. The leukocyte-lowering effect of hydroxyurea reduced the strength of the association between leukocytosis and thrombosis in this investigation.12 The cytoreductive effect of hydroxyurea on leukocyte counts may explain the advantage of this drug with respect to the prevention of arterial thromboses vs the selective platelet-lowering compound anagrelide in the UK-PT1 trial, a randomized phase 3 trial in a cohort of high-risk patients with ET.13 On the basis of the superiority of hydroxyurea combined with aspirin vs anagrelide combined with aspirin in this trial, current guidelines for cytoreductive therapy favor hydroxyurea as first-line therapy for ET.5 However, diagnosis of ET in this trial was based on the Polycythemia Vera Study Group (PVSG) criteria14 comprising a cohort of patients with ET and with various degrees of reticulin/collagen fibrosis, which may have correlated with elevated leukocyte counts, complicating the generalization of the results.15
It is still unknown whether these recommendations can be applied to patients with ET diagnosed according to the World Health Organization (WHO) classification (WHO-ET),16,17 where in contrast to PVSG-ET,14 increased bone marrow fibrosis and an elevated leukocyte count (>11 × 109/L) are uncommon and therefore cannot be considered major thrombophilic factors.18-20 To directly assess whether selective platelet-lowering therapy with anagrelide is not inferior compared with hydroxyurea to prevent thrombohemorrhagic events in high-risk WHO-ET, we designed a prospective randomized single-blind phase 3 study to compare the efficacy, tolerability, and safety of anagrelide and hydroxyurea in a homogeneous cohort of patients with ET diagnosed according to the WHO classification.
Patients and methods
Design overview
The aim of the study as defined in the protocol was to compare anagrelide with hydroxyurea in a noninferiority trial (see also supplementary appendix A) with respect to (1) a platelet-reducing effect, hemoglobin and leukocyte reduction; and (2) prevention of ET-related complications, as defined previously,21 during 6 months, 12 months, and during follow-up of 36 months. Safety and tolerability were assessed by adverse events. After 36 months, ET-related events and safety data were collected on a yearly basis for as long as feasible.
Study population
Patients older than 18 years with ET diagnosed according to the WHO classification were screened.16 Those participants who were at risk for thrombotic or hemorrhagic events and were newly diagnosed or were treatment-naive were invited to participate in the study. At-risk inclusion criteria for patients comprised either age ≥60 years, platelet count ≥1000 × 109/L, increase of platelet count ≥300 × 109/L within 3 months, hypertension, diabetes, and/or a history of thrombohemorrhagic events.2 Histologic confirmation of the clinical diagnosis of ET as defined by the WHO classification was initially performed by local pathologists. Later, the diagnosis was reexamined in a blinded fashion by a referee panel of 2 pathologists at the pathology reference center in Cologne (Germany), where all samples were recut and stained. An additional potential thrombophilic risk factor, JAK2-V617F, was investigated in the study cohort.12 Detection of the JAK2-V617F mutation was performed using quantitative allele-specific polymerase chain reaction, as described previously.22
Random selection and interventions
Study patients were randomly assigned to receive either a non–immediate release formulation of anagrelide23 (Thromboreductin, AOP Orphan Pharmaceuticals AG, Austria) or hydroxyurea (BMS, UK) and were stratified by center and age groups (age <60 years vs >60 years). After initiation of treatment with study drugs, patients were assessed weekly for efficacy and safety in the first month. Subsequently, assessments were done at monthly intervals for up to 6 months, then every other month for up to 1 year and quarterly during follow-up until 36 months. The protocol did not require mandatory concomitant medication with acetylsalicylic acid or clopidogrel. Those patients who had already been receiving acetylsalicylic acid or clopidogrel for at least 2 weeks were permitted to remain with this concomitant therapy, if considered appropriate by the investigator.
For assessment of ET-related events or complications, predefined criteria were used to provide investigators with a standardized diagnosis tool.21 Definition of ET-related events included the following criteria (see also supplementary appendix B):
Major arterial thrombosis: stroke, myocardial infarction, peripheral arterial disease, other arterial thrombosis.
Major venous thrombosis: ileofemoral thrombosis, pulmonary embolism, splanchnic vein thrombosis, other major venous events.
Minor arterial events: transitory ischemic attacks, angina pectoris, unstable angina, generalized convulsions, erythromelalgia, ocular symptoms, other peripheral arterial microcirculatory disturbances, other minor arterial events (eg, tinnitus, vertigo).24,25
Minor venous events: superficial thrombophlebitis, other minor venous events. Minor events were diagnosed based on patients’ symptoms and clinical judgment of the investigator taking patient diary notes into account.
Major bleeding events: decrease in hemoglobin level >1 g/dL or red blood cell transfusion required.
Minor bleeding events: no red blood cell transfusion required and decrease in hemoglobin level <1g/dL.
In addition, a retrospective analysis for development of post-ET myelofibrosis was performed according to recently published criteria, namely bone marrow fibrosis (grade 2-3), anemia (≥2-mg/dL decrease from baseline), leukoerythroblastic peripheral blood picture, increasing splenomegaly, increased lactate dehydrogenase levels, and development of at least 1 of 3 constitutional symptoms.26 Patients were asked to document any discomfort or noteworthy constitutional symptoms in a diary to facilitate event identification by investigators. Adverse events or drug reactions and serious adverse events were recorded using the WHO terminology and a standard operating procedure based on the International Conference on Harmonisation (ICH) guideline 377/95. The study protocol was approved by the institutional research ethics committees in all centers involved, according to the Declaration of Helsinki.
Statistical methods
A total of 4 primary criteria (percent change in platelets, hemoglobin, and leukocytes, and the total number of ET-related events) were analyzed in a confirmatory manner using a test for noninferiority. Control of the multiple level α of the study was maintained by applying the principle of an a priori–ordered hypothesis. Thus, all 4 criteria could be tested with full α-level in the defined sequence if the predecessor in the sequence turned out to be significant27 : for assessment of ET-related events, the test for platelets, hemoglobin, and leukocytes had to pass the noninferiority criterion before noninferiority for ET-related events could be performed. First-order analysis for proof of efficacy was defined as the analysis 6 months after onset of the study, which was followed by a second order of analysis after 12 months and 36 months.
A difference of 10 percentage points was defined as the lower margin of noninferiority for the 3 continuous criteria that were analyzed using the robust procedure of Su and Wei, which is based on the difference of medians.28 For all evaluations, the equivalence bound can then be confirmatorily tightened to a more narrow margin depending on the lower bound of the observed confidence interval.29 The evaluation of discontinuation rates during the first year of treatment was done using the χ2 test. ET-related events were evaluated with the patients being the observational units and the number of events being evaluated as scores. Analysis was performed using the Cochran-Mantel-Haenzel procedure with a lower noninferiority bound of OR=0.404, which equals the standardized difference d=0.5 of Cohen.30,31 In other words, noninferiority was demonstrated if the lower bound of the confidence interval was higher than OR=0.404. For event-free survival analysis for noninferiority, the Cox-Mantel log-rank test was used with a lower bound of noninferiority of a hazard ratio of 0.404. All procedures were evaluated using the statistical software package TESTIMATE. For sample size calculation, a method developed for the robust Mann-Whitney effect size measure was used because no calculation is available for the difference of medians of the Su Wei test. We defined Mann-Whitney=0.36 as the lower inferiority bound corresponding to the standardized difference d=0.5 of Cohen. Stipulating α=0.025, one-sided, and β=0.1, we obtained a total of N=184 patients. Because at the planning stage of the study only few prospective data were available on the relative effect of the 2 drugs, the study was planned as a 2-stage adaptive design with a sample size of 140 for stage I and a final sample size of 250 patients using an adaptive approach to test the hypothesis of noninferiority (see also supplementary appendix C).32 The enrolled patients were randomly assigned to the 2 treatment groups, balanced for center and age class (<60 years and ≥60 years).
The final statistical analysis plan stated that the intention-to-treat (ITT) population is to be used with missing data being imputed using the “last observation carried forward” option. The ITT population included all patients who took at least 1 dose of the trial medication and had at least 1 efficacy assessment afterward.
Results
Characteristics of study population
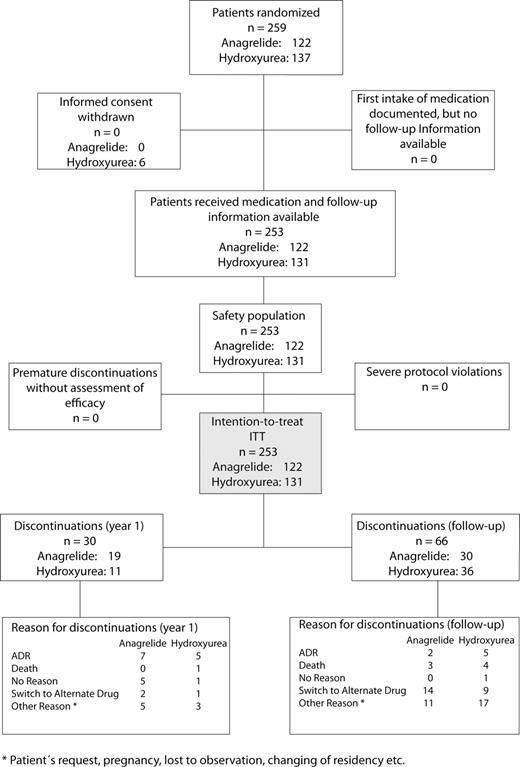
Between October 2002 and January 2006, a total of 259 patients diagnosed only with WHO-ET were assigned for treatment with anagrelide (n=122) or hydroxyurea (n=137) (Table 1 and 2). Baseline assessments showed no relevant group differences. The median duration from diagnosis to start of treatment was 25 days (range, 0-3137 days). Anagrelide was started at a dose of 1.0 mg/day (0.5 mg twice daily) during the first week. Hydroxyurea was started at a dose of 1500 mg/day. The dose of the respective study drug was increased until maintenance of the platelet count at normal (≤450 × 109/L) or close to normal levels (>450 × 109/L to 600 × 109/L). Dose reductions or a temporary interruption of dosing was performed in 15.5% and 18.9% of patients after an adverse event. Five patients assigned to the study withdrew informed consent before initiation of the treatment, and one patient was excluded after detection of a BCR-ABL translocation. A total of 253 patients remained for ITT (Figure 1). In total, 96 patients (49 in the anagrelide group vs 47 in the hydroxyurea group) discontinued study drug after a follow-up of up to 9 years. Nineteen patients in the anagrelide group (15.6%) and 11 patients in the hydroxyurea group (8.4%) discontinued the treatment before the end of the first year (P = .08). During follow-up, an additional 66 patients discontinued therapy. Among those 16 patients in the anagrelide group who were switched to hydroxyurea, conversely, 10 patients were switched from hydroxyurea to anagrelide. An additional 36 patients (16 in the anagrelide group and 20 in the hydroxyurea group) discontinued treatment for other reasons (eg, patients' request, wish for pregnancy, change of address, or lost to follow-up). The reason for discontinuation in another group of 27 patients was related to adverse events (9 anagrelide vs 10 hydroxyurea) or death (3 anagrelide and 5 hydroxyurea). Lack of efficacy was a reason for discontinuation in 5 patients in the anagrelide group and in 2 patients in the hydroxyurea group.
The blinded reevaluation of the bone marrow biopsy specimens obtained from 235 patients confirmed that a very homogeneous ET patient cohort was included in the study. When the original diagnosis of ET according to the WHO classification made in each center was compared with diagnoses made by the central bone marrow histology review panel, concordance of the diagnosis WHO-ET was achieved in 82.5% of these bone marrow biopsy specimens. Only 19 patients were rediagnosed as having early-prefibrotic primary myelofibrosis (PMF) with or without a minor increase in fibers (PMF-0/1) on a 3-graded scale,33 and another 16 patients were rediagnosed as having ETs with a PV-like pattern (Table 3). In 199 of the 253 patients, a retrospective analysis of the JAK2-V617F mutational status could be carried out, and in 107 patients (53.8%), the JAK2-V617F mutation was detected. The JAK2-V617F allele burden was low, ranging in a quantitative assay from 2.22% to 35.30% (median, 9.24%), and only 2 of 107 patients testing positive for JAK2-V617F had an allele burden of more than 25%. Patients with WHO-ET had a marginally lower allele burden compared with patients who were reclassified as having PMF-0/1 or ET-like PV (Table 3).
Efficacy
Noninferiority of anagrelide was evaluated with the criteria platelet count, hemoglobin level, leukocyte count, and ET-related events applying the principle of an a priori–ordered hypothesis in the above-stated order with a cutoff period of 3 years. There was no relevant difference in platelet-lowering effect between anagrelide and hydroxyurea throughout all 3 stages of evaluation (Figure 2). Median hemoglobin levels decreased slightly in both groups. Although this effect was more pronounced in anagrelide-treated patients, there was only a small median difference. In the anagrelide-treated patient group, leukocyte counts remained constant at a level of ∼9 × 109/L throughout the entire study period and did not increase, whereas in hydroxyurea-treated patients, the leukocyte counts were markedly decreased after 3 months and remained decreased throughout the entire study period. No statistically significant difference was observed in the frequency of ET-related major and minor thrombohemorrhagic events between the 2 treatment groups, with hazard ratios (HRs) and 95% confidence intervals (CIs) of 1.19 (95% CI, 0.61-2.30) at 6 months, 1.03 (95% CI, 0.57-1.81) after 12 months, and 0.92 (95% CI, 0.57-1.46) at 36 months (Figure 2). After long-term observation of up to 6 years, there was no difference in platelet counts, hemoglobin level, and leukocyte counts compared with the 12- and 36-month data, and 63.9% of the anagrelide-treated patients still remained free of ET-related thrombotic or bleeding events vs 67.4% in the hydroxyurea-treated group, again showing no difference between groups (HR, 0.92; 95% CI, 0.57-1.50). When the events were differentiated into major and minor (Table 4), no apparent difference was observed for major clinical events, with 14 events or 3.32% per patient-year in the anagrelide group vs 16 events or 3.42% per patient-year in the hydroxyurea group (Table 4A). Also, no significant difference was seen in minor events (Table 4B), with 45 minor events (10.6% per patient-year) in the anagrelide group vs 38 minor events (8.1% per patient-year) in the hydroxyurea group. There were slightly more major (5 vs 2) and minor (18 vs 15) bleeding events in patients receiving anagrelide. In total, 32 patients had at least 1 bleeding event (4 of them had multiple bleeding events); of these, 19 patients were receiving anagrelide and 13 were receiving hydroxyurea. Nine of the 19 anagrelide-treated patients with bleeding events were receiving additional aspirin therapy, whereas only 3 of the 13 hydroxyurea-treated patients with bleeding events had additional aspirin. No apparent difference in event-free survival was seen when the patient cohort was subdivided into JAK2-V617F–positive vs JAK2-V617F–negative patients. In JAK2-V617F–positive patients, the occurrence of thrombotic events was equally distributed in both treatment groups.
Course of platelet counts, hemoglobin levels, and leukocyte counts with time, up to 36 months. Data are expressed as medians ± quartiles. P values indicate proof of noninferiority (see statistical section).
Course of platelet counts, hemoglobin levels, and leukocyte counts with time, up to 36 months. Data are expressed as medians ± quartiles. P values indicate proof of noninferiority (see statistical section).
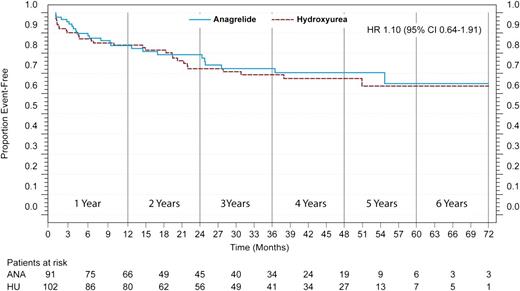
A subgroup analysis supports the findings of the noninferiority of anagrelide for prevention of ET-related events in a descriptively significant sense in patients with “true-ET.” In this subgroup, only patients who were rediagnosed with “true-ET” were included in the analysis. In the Cox-Mantel log-rank test for noninferiority the HRs are situated close to the benchmark of equality, with an HR of 1.10 (95% CI, 0.64-1.91) (Figure 3), with the lower bound of the CI situated well above the benchmark (HR, 0.404).
Event-free survival for ET-related events for patients who were rediagnosed as having WHO-ET (“true-ET”). The HR (95% CI) is presented after an observation time of 6 years.
Event-free survival for ET-related events for patients who were rediagnosed as having WHO-ET (“true-ET”). The HR (95% CI) is presented after an observation time of 6 years.
Tolerability and safety
The adverse events were equally distributed between the treatment groups; a total of 1063 adverse events were recorded, with 68 being serious adverse events. Adverse events leading to drug-related discontinuation again were equally distributed: 9 patients in the anagrelide group and 10 patients in the hydroxyurea group were withdrawn because of adverse events (Figure 1). However, regarding which organs were affected, the safety profile of the drugs differed. Cardiovascular side effects (ie, hypertension, palpitations, and tachycardia) were observed significantly more frequently in the anagrelide group, whereas leukocytopenia (grades 1 and 2) and minor infections (grade 1) were significantly more frequent in the hydroxyurea group (Table 5). In 2 patients in the anagrelide group and in 1 patient in the hydroxyurea group, development of post-ET myelofibrosis was reported. Because follow-up bone marrow biopsies were not available for these patients, clinical parameters (anemia and a decrease of hemoglobin levels by > 2 g/dL; development or increase of splenomegaly by > 5 cm; and development of leukoerythroblastic peripheral blood count, elevated lactate dehydrogenase levels, and constitutional symptoms) were taken as surrogate parameters for transformation into myelofibrosis.26 None of the 253 patients in the study (except for the 3 patients reported above), who were treated for up to 9 years comprising a total observation period of 730 patient-years, fulfilled more than 1 criterion for transformation into post-ET myelofibrosis. Transformation into myelodysplastic syndrome or secondary leukemia was not observed during the entire observation period.
Discussion
Our current investigation shows that anagrelide was not inferior in the prevention of thromboembolic and bleeding events compared with hydroxyurea in patients diagnosed with WHO-ET. In addition, our study confirms previously published data that anagrelide does not induce disease progression to myelofibrosis34 or acute leukemia in WHO-ET and, in the absence of aspirin, does not provoke bleeding complications in this subgroup of myeloproliferative neoplasms. Our data also show that anagrelide can be considered a safe drug, despite some cardiovascular adverse effects that can usually be managed by dose reductions.35
Previously, it was shown that leukocytosis and a higher degree of reticulin fibrosis add prognostic significance to existing risk factors for arterial thrombosis in PVSG-ET.12,15,36,37 These findings are confirmed by a recent investigation in prefibrotic PMF where leukocytosis has been shown to be an important risk factor for arterial thrombosis.38 The present results demonstrate that these observations may not be relevant for ET diagnosed according to the WHO classification, which is usually characterized by a near-to-normal leukocyte count. In contrast to the UK-PT1 trial, which showed that hydroxyurea combined with aspirin was more effective in the prevention of arterial events than anagrelide combined with aspirin, our study revealed a similar rate of major and minor arterial events and major bleeding events in both treatment groups.13 This difference in the study outcomes may be explained by several differences in the design of the 2 studies: (1) Our patients were enrolled at diagnosis or in a previously untreated status. In contrast, all participants were included into the UK-PT1 trial, including those receiving hydroxyurea, necessitating a withdrawal from hydroxyurea and transition to anagrelide, which may have resulted in an increase in leukocyte counts. This may explain the higher rate of arterial events in some patients treated with anagrelide in the UK-PT1 trial who were previously treated with hydroxyurea. (2) However, the different diagnostic criteria that have been applied (PVSG-ET vs WHO-ET) may be considered the most important reason for the different outcomes in the 2 studies, as the thrombotic risk in WHO-ET may be different than that observed in PVSG-ET because of different hematologic phenotypes at presentation.39-41 In this context, it is remarkable that a considerable fraction of patients in the UK-PT1 trial presented initially with higher levels of so-called reticulin fibrosis. Because the extent of reticulin fibrosis correlates with leukocyte counts and arterial thrombosis, it is possible that in PVSG-ET unselective cytoreduction with hydroxyurea could be superior to a selective thrombocyte-reductive therapy with anagrelide, which is not able to decrease leukocyte counts.13,15 Finally, major bleeding events associated with thrombocytosis might be specific to early-prefibrotic PMF vs WHO-ET, and it was shown that low-dose aspirin exacerbates this bleeding tendency.42 Therefore, differences in bleeding complications in the patient groups treated with anagrelide between the UK-PT1 trial and our study may, again, be explained by the different diagnostic criteria (PVSG-ET vs WHO-ET) used in the 2 studies. (3) In addition, the restrictive use of aspirin in our study may have been responsible for the decreased bleeding rate in anagrelide-treated patients because it is also known that bleeding complications associated with aspirin may be provoked in ET, especially when aspirin is combined with anagrelide.21,43 On the other hand, the restrictive use of aspirin in our study may explain the somewhat higher rates of thrombosis in both treatment groups (anagrelide vs hydroxyurea: 3.32% vs 3.42% per patient-year) compared with previous randomized studies, where the rate of thrombosis was shown to be ∼2% per patient-year. This trend emphasizes that aspirin may account for some protective effect against thrombosis in ET.
The strength of our present data indicates for the first time in a prospective randomized study that a distinction between WHO-ET and early-prefibrotic PMF may be important for the choice of cytoreductive therapy; therefore, our study can be considered a validation of the WHO classification, with a differentiation between WHO-ET and early-prefibrotic PMF. Although the patient cohort included in our study was broadly similar to that of previous studies, we did not observe any relevant increase of reticulin fibrosis in our very homogeneous cohort of patients with WHO-ET. Also, the median leukocyte count remained constant below the threshold level of 11 × 109/L for increased thrombosis risk in WHO-ET throughout the entire treatment period, even in anagrelide-treated patients. This observation is important, in particular, for the low rate of arterial thrombotic events in anagrelide-treated patients in our study vs the anagrelide-treated patients in the UK-PT1 trial. In addition, our cohort of patients with WHO-ET was characterized by a low JAK2-V617F allele burden, which confirms the results of a significantly lower JAK2-V617F allele burden in WHO-ET vs early-prefibrotic PMF reported in a cohort of patients primarily defined by bone marrow morphologic features.44
Controversy persists concerning whether the histologic bone marrow criteria proposed by the WHO for the classification of ET, particularly the discrimination between WHO-ET vs early-prefibrotic PMF, is reproducible.18 Contrasting 1 group,45 other investigators confirmed their ability to discriminate between WHO-ET and the thrombocythemic manifestations of early-prefibrotic PMF.41,46-49 The result of our study, including the blinded reevaluation of bone marrow specimens diagnosed as WHO-ET by a large number of pathologists, underscores the reproducibility of relevant parameters proposed by the WHO classification.16,17 The noninferiority design might be considered a weakness of our study. However, this design allowed us to exclude the inferiority of anagrelide vs hydroxyurea in WHO-ET, and to evaluate the superiority of one of these 2 compounds would not have been feasible (see also supplementary appendix A).
Our study is the first prospective randomized phase 3 trial in ET applying the WHO classification. Because significant differences exist in the clinical behavior between WHO-ET and ET diagnosed according to the PVSG criteria, recommendations for treatment derived from PVSG-diagnosed ET cohorts may not be applicable to patients with WHO-ET. An elevated leukocyte count, besides elevated platelet counts, may therefore not be considered an additional thrombophilic factor in our patient cohort with WHO-ET. This is in contrast to PVSG-ET or early-prefibrotic PMF, where leukocytosis represents one of the most prominent risk factors for arterial events.37,38 This may explain why, in our study, the selective thrombocyte-lowering effect of anagrelide is sufficient to also prevent ET-related arterial thromboses. Our study suggests that in patients with WHO-ET, anagrelide represents a nonchemotherapeutic alternative to hydroxyurea as first-line therapy for the prevention of thrombotic complications.
Appendix: study group members
The members of the ANAHYDRET Study Group, in addition to the authors, include the following investigators: G. Gastl (Innsbruck, Austria), B. Gisslinger (Vienna, Austria), L. Muellauer (Vienna, Austria), E. Schlögl (Vienna, Austria); K. Indrak (Olomouc, Czech Republic), R. Pytlik (Prague, Czech Republic); J. Briere (Paris, France), J.J. Kiladjian (Paris, France); M. Beykirch (Munich, Germany), B. Gaede (Hannover, Germany), M. Griesshammer (Minden, Germany), M. Herbrik-Zipp (Weingarten, Germany), H.J. Hurtz (Halle, Germany), G. Jacobs (Saarbrücken, Germany), S.H.Jäcki (Tübingen, Germany), U. Keilholz (Berlin, Germany), J. Mezger (Karlsruhe, Germany), P. Schlag (Würzburg, Germany), F. Schriever (Dorfen, Germany); T. Masszi (Budapest, Hungary); Italy: S. Sacchi (Modena, Hungary); R. Griniptì (Kaunas, Lithuania), I. Tavoriene (Vilnius, Lithuania); A. Dmoszynska (Lublin, Poland), A. Hellman (Gdansk, Poland), W.W. Jedrzejczak (Warszawa, Poland), T. Robak (Lodz, Poland), A. Skotnicki (Kraków, Poland); Y.T. Goh (Singapore, Singapore); P. Cernelc (Ljubljana, Slovenia).
The online version of this article contains a data supplement.
Presented in parts in abstract form at the 50th annual meeting of the American Society of Hematology, December 7, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The trial was sponsored by AOP Orphan Pharmaceuticals AG, Austria. The database was set up and the data were analyzed by an independent Clinical Research Organization (idv Gauting, Munich, Germany). We thank Helen Pickersgill for edition of the manuscript. We also thank Bettina Gisslinger for performing the JAK2 assays and Renate Schoder for data and manuscript administration.
Authorship
Contributions: A steering committee (H.G., P.E.P., and J.T.) was involved in designing and conducting the trial. The manuscript was written by a writing committee (H.G., P.E.P., J.T., and R.K.) headed by the coordinating principal investigator (H.G.). J.T. and H.M.K. reviewed bone marrow slides and performed together with M.G. J.H. and M.P. researched and revised the manuscript.
Conflict-of-Interest disclosure: All authors declare no conflict-of-interest and no competing financial interests. All authors or their institutions received remunerations for participating in the study from AOP Orphan Pharmaceuticals AG, Austria, in accordance with the cooperation agreement. The company was explicitly not permitted to exert any influence on the analysis of the study results or on the interpretation of the data.
A complete list of the members of the ANAHYDRET Study Group appears in “Appendix.”
Correspondence: Heinz Gisslinger, MD, Medical University of Vienna, Department of Internal Medicine I, Division of Hematology and Blood Coagulation, Währinger-Gürtel 18-20, A-1090 Vienna, Austria; e-mail: heinz.gisslinger@meduniwien.ac.at.