Key Points
The hepcidin inhibitor NOX-H94, a structured mirror-image RNA oligonucleotide, and its in vitro and in vivo characterization are described.
First published hepcidin inhibitor that entered clinical trials for the treatment of anemia due to functional iron deficiency.
Abstract
Anemia of chronic inflammation is the most prevalent form of anemia in hospitalized patients. A hallmark of this disease is the intracellular sequestration of iron. This is a consequence of hepcidin-induced internalization and subsequent degradation of ferroportin, the hepcidin receptor and only known iron-export protein. This study describes the characterization of novel anti-hepcidin compound NOX-H94, a structured L-oligoribonucleotide that binds human hepcidin with high affinity (Kd = 0.65 ± 0.06 nmol/L). In J774A.1 macrophages, NOX-H94 blocked hepcidin-induced ferroportin degradation and ferritin expression (half maximal inhibitory concentration = 19.8 ± 4.6 nmol/L). In an acute cynomolgus monkey model of interleukin 6 (IL-6)–induced hypoferremia, NOX-H94 inhibited serum iron reduction completely. In a subchronic model of IL-6–induced anemia, NOX-H94 inhibited the decrease in hemoglobin concentration. We conclude that NOX-H94 protects ferroportin from hepcidin-induced degradation. Therefore, this pharmacologic approach may represent an interesting treatment option for patients suffering from anemia of chronic inflammation.
Introduction
Hepcidin,1-3 a small 2.8-kDa peptide, is regarded as the central mediator of iron homeostasis.4 The increased production of hepcidin during inflammatory conditions is a cornerstone of the pathogenesis of anemia of chronic inflammation (ACI).5,6 The pathophysiological effects of hepcidin are central to the development of anemia in several groups of patients, for example, those with chronic kidney disease,7 cancer,8 and Castleman disease.9 Therefore, inhibition of hepcidin represents a potentially attractive therapeutic target to improve the utilization of iron from intracellular stores in patients suffering from ACI. The efficacy of pharmaceutical hepcidin inhibition has already been demonstrated with an anti-hepcidin antibody in mice10 and by other less specific approaches with an indirect effect on hepcidin expression.11,12
NOX-H94 (sequence in the supplemental data; see the Blood Web site) is a structured mirror-image L-oligoribonucleotide, a so-called Spiegelmer,13 that binds human hepcidin (huHep) with high affinity, thereby blocking its biological function. Spiegelmers are L-enantiomeric oligonucleotides that can be evolved or designed to inhibit pharmacologically relevant target molecules, by binding them in a manner conceptually similar to antibodies, other protein-based scaffolds, or aptamers.14 Because of their nonnatural, mirror-image nature, Spiegelmers are nuclease resistant and immunologically passive (no Toll-like receptor activation, low risk for neutralizing antibodies); unlike biologicals, the production process only employs chemical manufacturing steps,15 avoiding any potential biological contamination. Data from preclinical and clinical studies generated so far suggest that Spiegelmers are well tolerated and have a benign safety profile (unpublished data). In addition to the anti–monocyte chemoattractant protein 116 and anti–stromal cell-derived factor 117 Spiegelmers, NOX-H94 is the third Spiegelmer that entered clinical development.
In the described studies, we aimed to confirm that the high binding affinity of NOX-H94 for hepcidin translates into functional inhibition of hepcidin-induced ferroportin degradation and ferritin expression in two in vitro bioassays in macrophages, and to evaluate efficacy in two cynomolgus monkey disease models.
Study design
Hepcidin-induced ferroportin degradation and ferritin expression
The effect of NOX-H94 on hepcidin-induced ferroportin degradation and ferritin expression was examined in J774A.1 mouse macrophages (DSMZ, Braunschweig, Germany) because no satisfactorily working antibodies against human ferroportin could be identified. Cells were cultivated at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium with Glutamax (Invitrogen, Karlsruhe, Germany) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin.
For the ferroportin degradation assay, macrophages were seeded in 24-well plates (7.3 × 105 cells per well), loaded with iron by addition of iron–nitrilotriacetic acid solution (100 µmol/L), and incubated overnight. Cells were incubated with huHep (100 nmol/L; Peptides International Inc., Louisville, KY) in combination with Spiegelmers (NOX-H94 or NOX-H94002, without polyethylene glycol [PEG] modification) for 3 hours, washed once, and lysed in lysis-buffer [20 mM tris(hydroxymethyl)aminomethane (Tris)/HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100] including protease inhibitors (Roche, Mannheim, Germany). Total protein concentrations were determined using the bicinchoninic acid method (Pierce BCA Protein assay Kit; Thermo Scientific, Bonn, Germany). Lysates (20 µg protein each) were mixed with sample buffer (250 mM Tris/HCl [pH 6.8], glycerol [40%], sodium dodecyl sulfate [8%], bromophenol blue [0.04%]) and incubated at 37°C for 10 minutes. Samples were then separated on 10% Novex Tris-Glycin gels (Invitrogen) and transferred on Amersham Hybond-polyvinylidene difluoride membranes (GE Healthcare, Freiburg, Germany). Equal loading and transfer were confirmed by staining with Ponceau Red solution. After blocking with 0.5% nonfat dry milk in Tris-buffered saline and 0.1% Tween-20, mouse ferroportin was detected using a rabbit anti-mouse ferroportin antibody (1:300; α diagnostics, San Antonio, TX) as primary antibody and incubated with horseradish peroxidase conjugated anti-rabbit-IgG (New England Biolabs, Frankfurt am Main, Germany) as secondary antibody. Ferroportin was finally detected using LumiGlo chemiluminescent reagent (New England Biolabs) and Hyperfilm enhanced chemiluminescence films (GE Healthcare).
For the ferritin expression assay, cells were seeded in 96-well plates (4.5 × 104 cells per well). After incubation with huHep in combination with NOX-H94 for 24 hours, cells were washed twice and lysed in M-PER buffer (Thermo Fisher Scientific, Waltham, MA) including protease inhibitors (Roche). Lysates were quantified for mouse ferritin levels by enzyme-linked immunosorbent assay (ELISA) (ICL Inc., Newberg, OR) according to the manufacturer’s instructions.
Interleukin 6 (IL-6)–induced hypoferremia and anemia
Male cynomolgus monkeys (Macaca fascicularis) were housed and handled according to the guidelines set by the animal welfare regulations enacted in Germany in the ‘Tierschutzgesetz’. Monkeys (n = 3 per group) were injected with NOX-H94 (10 mg/kg [1 mL/kg], intravenously, t = –0.5 hours) followed by a single subcutaneous injection of human IL-6 (0.3 µg/kg [1 mL/kg], 108 U/mg, t = 0 hours; Miltenyi Biotec, Bergisch-Gladbach, Germany) to induce hypoferremia. Control groups received NOX-H94 or IL-6 and the corresponding vehicle (vehicle A, 5% glucose; vehicle B, 1% autologous heat-inactivated serum). In the subchronic IL-6–induced anemia model, monkeys were injected with IL-6 (daily, 0.3 µg/kg) and NOX-H94 (daily, 10 mg/kg) for up to 7 days. Blood samples were obtained by phlebotomy from the vena cephalica or saphena magna at the indicated time points (3.2 mL per animal). Iron parameters were determined colorimetrically by Konelab 30i (Thermo Fisher Scientific, Dreieich, Germany) using Konelab kits. Blood cell parameters were determined with an ADVIA120 hematology analyzer (Siemens, Fernwald, Germany).
Results and discussion
Different Spiegelmer-based hepcidin binders were identified following a process essentially as described in Purschke et al.18 The best binding Spiegelmer NOX-H94, modified at its 5′-end with 40-kDa PEG, showed a dissociation constant of 0.65 ± 0.06 nmol/L (supplemental Figure 1A) and was selected for further in vitro and in vivo studies.
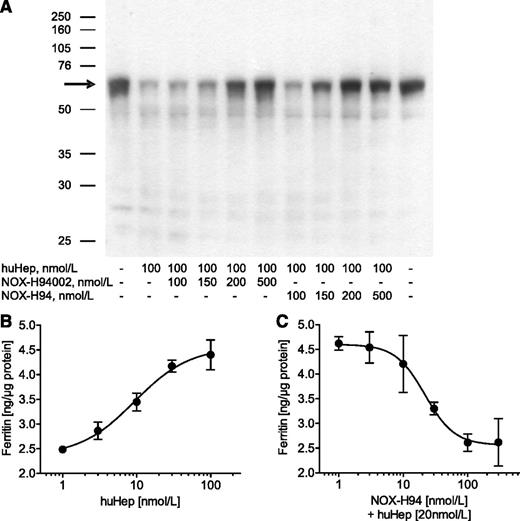
Incubation of J774A.1 macrophages with 100 nmol/L huHep clearly reduced ferroportin expression. This effect was almost completely inhibited with 200 nmol/L of the L-oligonucleotide itself (NOX-H94002) as well as with its PEGylated variant NOX-H94 (Figure 1A). Another more sensitive in vitro assay based on hepcidin-induced iron retention in macrophages showed a substantial concentration-dependent upregulation of ferritin19 with a half maximal effective concentration of 9.4 ± 1.2 nmol/L (Figure 1B). NOX-H94 inhibited the induction of ferritin expression with a half maximal inhibitory concentration of 19.8 ± 4.6 nmol/L (Figure 1C, mean ± standard error of the mean [SEM], n = 6).
Antihepcidin Spiegelmer inhibits hepcidin effects in vitro. (A) Ferroportin western blot analysis of lysates obtained from mouse J774A.1 macrophages after stimulation with huHep alone or in combination with different concentrations of the L-oligoribonucleotide NOX-H94002 or its PEGylated variant NOX-H94 (1 representative experiment of 9). (B) Ferritin content of lysates obtained from macrophages after stimulation with different concentrations of huHep (1 representative experiment of 3) or (C) after stimulation with 20 nM huHep and different concentrations of NOX-H94. The results from 6 independent experiments were taken to calculate a half maximal inhibitory concentration mean value. Ferritin content was analyzed with a mouse ferritin ELISA (2 lysates, each tested twice in the ELISA, mean ± standard deviation). The amount of ferritin was normalized to total protein.
Antihepcidin Spiegelmer inhibits hepcidin effects in vitro. (A) Ferroportin western blot analysis of lysates obtained from mouse J774A.1 macrophages after stimulation with huHep alone or in combination with different concentrations of the L-oligoribonucleotide NOX-H94002 or its PEGylated variant NOX-H94 (1 representative experiment of 9). (B) Ferritin content of lysates obtained from macrophages after stimulation with different concentrations of huHep (1 representative experiment of 3) or (C) after stimulation with 20 nM huHep and different concentrations of NOX-H94. The results from 6 independent experiments were taken to calculate a half maximal inhibitory concentration mean value. Ferritin content was analyzed with a mouse ferritin ELISA (2 lysates, each tested twice in the ELISA, mean ± standard deviation). The amount of ferritin was normalized to total protein.
To translate the in vitro inhibitory effects into an in vivo situation, NOX-H94 was tested for its ability to prevent the reduction of serum iron in an IL-6–induced hypoferremia model. The cross-reactivity of NOX-H94 with cynomolgus monkey hepcidin was demonstrated in competition studies (supplemental Figure 1B).
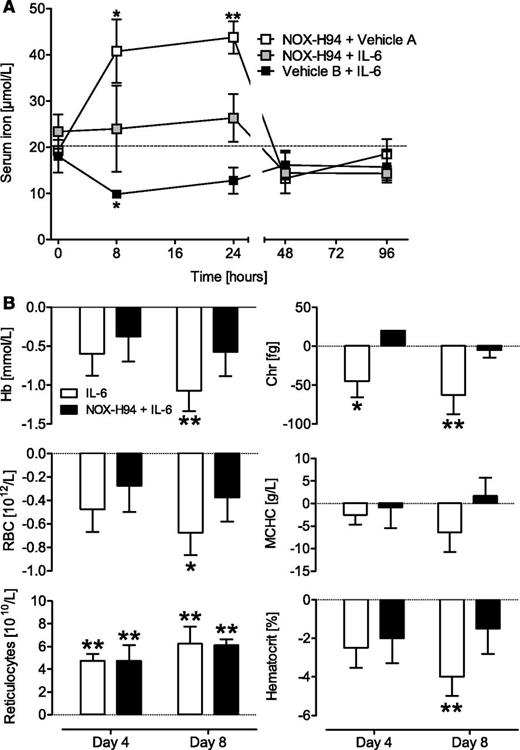
In the hypoferremia study, three treatment groups were compared: (1) NOX-H94; (2) combination of IL-6 and NOX-H94; and (3) IL-6 alone (Figure 2A). In the IL-6–treated group, serum iron dropped from 18.0 ± 3.5 µmol/L to 9.8 ± 0.2 µmol/L (P < .05) at 8 hours and returned to baseline within 48 hours. Pretreatment with NOX-H94 (10 mg/kg) prevented this decrease for up to 24 hours (predose, 8 hours, and 24 hours: 23.4 ± 3.7, 24.0 ± 9.3, and 26.3 ± 5.2 µmol/L, respectively). In the NOX-H94 control group, iron levels increased from 19.3 ± 0.4 to 40.7 ± 6.9 µmol/L at 8 hours (P < .05) and 43.3 ± 3.5 µmol/L at 24 hours (P < .01) and returned to predose levels at 48 hours.
Inhibition of IL-6–induced hypoferremia and anemia by NOX-H94 treatment in cynomolgus monkeys. (A) Treatment with IL-6 induced a nonsustained hypoferremia. The serum iron decrease was prevented by 10 mg/kg NOX-H94 (vehicle A, 5% glucose; vehicle B, 1% autologous heat-inactivated serum). In the control group without IL-6, NOX-H94 treatment induced a temporal hyperferremia (n = 3 per group, mean ± SEM). Statistical analysis was performed with the unpaired t test comparing the different time points of each group with the respective predose values. The dotted line indicates the mean predose serum iron concentration. (B) Repeated subcutaneous injections of human IL-6 (0.3 µg/kg, daily) reduced hemoglobin concentration (Hb), erythrocyte count (RBC), reticulocyte hemoglobin content (Chr), hematocrit, and mean corpuscular hemoglobin concentration (MCHC). This effect was ameliorated in NOX-H94–treated (10 mg/kg, daily) animals (mean ± SEM, n = 4 per group, individual levels describe the difference compared with predose levels). Statistical analysis was performed with Dunnett’s multiple comparison test. *P < .05; **P < .01.
Inhibition of IL-6–induced hypoferremia and anemia by NOX-H94 treatment in cynomolgus monkeys. (A) Treatment with IL-6 induced a nonsustained hypoferremia. The serum iron decrease was prevented by 10 mg/kg NOX-H94 (vehicle A, 5% glucose; vehicle B, 1% autologous heat-inactivated serum). In the control group without IL-6, NOX-H94 treatment induced a temporal hyperferremia (n = 3 per group, mean ± SEM). Statistical analysis was performed with the unpaired t test comparing the different time points of each group with the respective predose values. The dotted line indicates the mean predose serum iron concentration. (B) Repeated subcutaneous injections of human IL-6 (0.3 µg/kg, daily) reduced hemoglobin concentration (Hb), erythrocyte count (RBC), reticulocyte hemoglobin content (Chr), hematocrit, and mean corpuscular hemoglobin concentration (MCHC). This effect was ameliorated in NOX-H94–treated (10 mg/kg, daily) animals (mean ± SEM, n = 4 per group, individual levels describe the difference compared with predose levels). Statistical analysis was performed with Dunnett’s multiple comparison test. *P < .05; **P < .01.
IL-6 is only one of several factors contributing to ACI. A central role of IL-6 was shown in Castleman disease, where treatment with an IL-6–receptor antibody downregulated hepcidin and improved ACI.9 IL-6–induced anemia may therefore be regarded as a potential model of Castleman disease that reflects the main characteristics of ACI. In this subchronic IL-6–induced anemia model, NOX-H94 (10 mg/kg) ameliorated the hemoglobin reduction produced by IL-6 but was not able to block it completely (Figure 2B). On day 4, no effect of the treatment was observed. On day 8, the hemoglobin concentration was reduced by 1.1 ± 0.3 mmol/L (P < .01) in the IL-6 group, in contrast to the NOX-H94 group in which no significant reduction (0.6 ± 0.3 mmol/L) compared with baseline was observed. In this model, NOX-H94–treated animals also exhibited a smaller reduction in reticulocyte hemoglobin content, erythrocyte counts, hematocrit, and mean corpuscular hemoglobin concentration on day 8. No additional effect of hepcidin inhibition was observed for reticulocyte counts, which increased in both groups (Figure 2B). This increase is regarded as a response to the anemia and an attempt of the system to compensate for the hemoglobin reduction, which was also seen in other anemia models.6,10
IL-6 was also discussed to increase plasma volume and to induce a dilutional anemia.20 This effect may contribute to the anemia in both treatment groups but cannot explain the effect of NOX-H94 on hemoglobin. Regarding the rapid hemoglobin decrease in IL-6–induced anemia, sequestration of erythrocytes21 was discussed as a possible explanation. Another study postulated a shortened life span of erythrocytes in anemia of chronic disease.22 This was supported by the observation of a hepcidin-mediated decreased expression of the antiapoptotic protein pBad,23 indicating how hepcidin could be involved in erythroid progenitor proliferation and survival. In contrast, normal survival rates were observed for erythrocytes in hepcidin-overexpressing transgenic mice.6
Earlier studies already indicated that a relatively high dose of a hepcidin antibody was necessary to reverse the effects of inflammation on iron metabolism and erythropoiesis.10 One hypothesis to explain this observation is an assumed high synthesis rate of hepcidin. Initial data from our hypoferremia study indicate a saturation of NOX-H94 at 48 hours after treatment and would support this hypothesis (supplemental Figure 2). How a temporary hepcidin blockade and increased availability of iron translates into a benefit for the patients will be investigated in a clinical phase 2 trial.
Overall, the treatment with NOX-H94 was well tolerated as no influence on the body weight and no signs of local or systemic intolerance were noted for any of the animals treated daily with NOX-H94 (10 mg/kg, intravenously) and with IL-6 (0.3 µg/kg, subcutaneously) for 7 consecutive days. Furthermore, in vitro studies demonstrated that the L-RNA Spiegelmer NOX-H94 even at high concentrations (100 µM) does not elicit innate immune responses through Toll-like receptor activation (data not shown), a side effect frequently observed with certain types of natural and synthetic nucleic acids in the natural D-configuration.
The aim of the current work was to characterize NOX-H94 in preclinical experiments. We have demonstrated that NOX-H94 is a high-affinity huHep binder and inhibits ferroportin degradation in two in vitro models. In cynomolgus monkeys, we confirmed the rapid induction of hepcidin and hypoferremia in response to IL-6 that had been reported in human volunteers receiving IL-6 or endotoxin infusions24,25 and demonstrated that NOX-H94 can reverse hypoferremia in this in vivo model of acute systemic inflammation. Repeated administration of IL-6 has been reported to induce anemia in several studies.20,21 Our results suggest that NOX-H94 may attenuate the inflammation-induced decrease in hemoglobin, but a larger study would be needed to further substantiate this observation. Strikingly, the clearest differences were observed for reticulocyte hemoglobin content. This could be explained by improved supply of iron for hemoglobin synthesis in reticulocytes, as a result of NOX-H94–mediated hepcidin neutralization.
We conclude that NOX-H94 may provide a new therapeutic option for the treatment of ACI. To enable a phase 2a study in anemic myeloma and lymphoma patients, two human clinical trials are currently being conducted to evaluate safety and pharmacokinetics and to determine if the described effects of NOX-H94 during systemic inflammation can be reproduced in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Turner and the NOXXON chemistry group for synthesizing the Spiegelmers, and Johannes Hoos and Stéphanie Vauléon for providing analytical services. The technical assistance of Lisa Bauer and Verena Dolata is gratefully acknowledged. The authors also thank Laboratory of Pharmacology and Toxicology, GmbH & Co. KG (Hamburg, Germany) for the excellent performance of animal studies, and Hepcidinanalysis.com (Nijmegen, The Netherlands) for the determination of hepcidin concentrations in plasma samples.
This project was funded in part by the European Union European Regional Development Fund/Programm zur Förderung von Forschung, Innovationen und Technologien.
Authorship
Contribution: F.S. conceived of studies, established models, interpreted data, and prepared the manuscript; L.T.v.E. made intellectual comments on the study and aided substantially in interpretation of data as well as in preparation of the manuscript; D.Z. conceived of and conducted experiments, established assays, interpreted data, and prepared the manuscript; S.S. identified the compound, conducted experiments, and interpreted data; K.B. conceived of and conducted experiments, established assays, interpreted data, and prepared the manuscript; C.M. identified the compound, established assays, conceived of and conducted experiments, interpreted data, and prepared the manuscript; W.G.P. conducted experiments, established assays, and interpreted data; M.H. designed studies, interpreted data, and prepared the manuscript; S.Z. established assays, interpreted data, and prepared the manuscript; D.E. interpreted data and prepared the manuscript; F.M. conceived of studies, interpreted data, and prepared the manuscript; P.P. made intellectual comments on the study and aided substantially in interpretation of data as well as preparation of the manuscript; and S.K. designed and evaluated Spiegelmers, interpreted data, and prepared the manuscript.
Conflict-of-interest disclosure: F.S., D.Z., S.S., K.B., C.M., W.G.P., M.H., S.Z., D.E., F.M., and S.K. are or had been employees of NOXXON Pharma AG. F.S., S.S., K.B., C.M., F.M., and S.K. are inventors on a patent describing hepcidin-binding nucleic acids including NOX-H94. The remaining authors declare no competing financial interests.
Correspondence: Frank Schwoebel, NOXXON Pharma AG, Max-Dohrn-Strasse 8-10, 10589 Berlin, Germany; e-mail: fschwoebel@noxxon.com.