To the editor:
In the last decade, we have gained increasing insights into the antibody-independent functions of B cells. It has now become clear that apart from producing antibodies, B cells also contribute to immunity by antigen presentation, cytokine production, and a wealth of cellular interactions with other components of the immune system. In their 2012 paper, Morva et al1 report that human B cells that were activated via CD40 and Toll-like receptor 9 (TLR9) are able to regulate dendritic cells (DCs) in a cell contact–dependent fashion. In response to this article, Maddur et al2 furthermore show that the nature of the regulatory activity of B cells on DC function depends on the activation stimulus used to activate the B lymphocytes. These two papers provide important insights into the intricate mutual interactions between B lymphocytes and DCs.
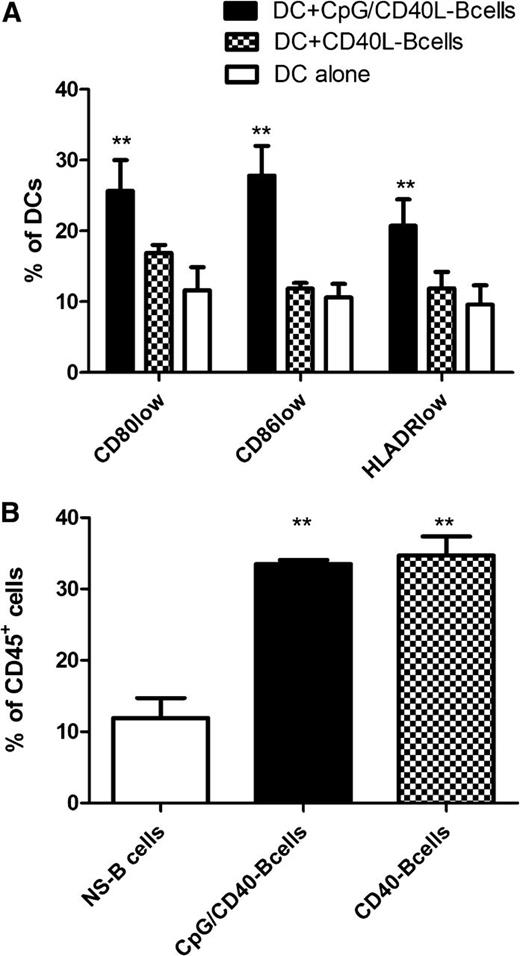
The effect of CD40 on B-cell function has been controversial. While signaling through CD40 is generally believed to have pro-inflammatory effects on B lymphocytes, leading to enhanced antigen presentation and cytokine production, some groups have found that CD40-activation can induce regulatory features in B cells.3-5 As exemplified by the results of Maddur et al,2 the type, strength, and duration of the activation stimulus has a crucial impact on the function of human B lymphocytes.2,6 Similarly, we found striking functional differences between B cells that were stimulated by CD40L compared with B cells that were stimulated by CD40L and cytosine guanine dinucleotide (CpG). The CD80lowCD86lowHLA-DRlow DC population described by Morva et al1 was increased in the cocultures with CD40L/CpG-stimulated B lymphocytes but not with CD40L-stimulated B lymphocytes (Figure 1A). Collectively, the experiments suggest that TLR9-mediated signals seem to induce a regulatory capacity in B cells and counter the pro-inflammatory effects of CD40-stimulation on B cells.
Interaction between B cells and DCs. (A) Human monocyte-derived DCs were cultured in triplicates alone or were cocultured with human CD19+ B cells, which were activated via CD40L or CD40L+CpG, at a DC/B-cell ratio of 1:4 for 48 hours. Subsequently, the cell surface expression of CD80, CD86, and HLA-DR was determined using a Gallios flow cytometer (Beckman Coulter, Krefeld, Germany). The bar charts represent the mean percentage of positive DCs ± SD. (B) To determine the effect of the initial B-cell stimulation on their survival in the coculture, we determined the percentage of remaining B cells among the total CD45+ cells after 48 hours in the coculture with DCs at a DC/B-cell ratio of 1:4. Shown are the mean percentages of CD19+ B cells ± SD. **P < .01.
Interaction between B cells and DCs. (A) Human monocyte-derived DCs were cultured in triplicates alone or were cocultured with human CD19+ B cells, which were activated via CD40L or CD40L+CpG, at a DC/B-cell ratio of 1:4 for 48 hours. Subsequently, the cell surface expression of CD80, CD86, and HLA-DR was determined using a Gallios flow cytometer (Beckman Coulter, Krefeld, Germany). The bar charts represent the mean percentage of positive DCs ± SD. (B) To determine the effect of the initial B-cell stimulation on their survival in the coculture, we determined the percentage of remaining B cells among the total CD45+ cells after 48 hours in the coculture with DCs at a DC/B-cell ratio of 1:4. Shown are the mean percentages of CD19+ B cells ± SD. **P < .01.
Importantly, even though we could confirm the findings by Morva et al1 and Maddur et al,2 there is one major caveat in the interpretation of these data. As far as one can judge from the descriptions of the methods, both research groups cocultured DCs with B cells and subsequently performed a mixed lymphocyte reaction without prior purification of the DCs. Since signaling through CD40 has anti-apoptotic effects, it could favor the survival of the CD40-activated B cells in the cocultures. Using an identical experimental setup, we found significant differences in the composition of the cell mixture after the end of the 48-hour coculture period. B cells activated by CD40L or CD40L and CpG seemed to survive longer, and they made up a higher percentage of the cells after coculture (Figure 1B). Thus, the DC/B-cell ratio in the cell mixture was 2:1 and 7:1 in the cocultures with CD40L/CpG-stimulated and unstimulated B cells, respectively. As a consequence, the mixed lymphocyte reactions with DCs exposed to CD40L/CpG-activated B cells contained a relatively higher number of B cells and a lower number of DCs. It should therefore be taken into consideration that the observed effects on T-cell activation and proliferation could be, at least in part, attributable to the “contaminating” B cells.
Authorship
Contribution: M.G.-M. and A.S.-V. contributed equally. M.G.-M. performed the experiments, analyzed the data, and wrote the manuscript. A.S.-V. and M.vB.-B. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Shimabukuro-Vornhagen, Department I of Internal Medicine, University Hospital of Cologne, Kerpenerstrasse 62, 50937 Cologne, Germany; e-mail: alexander.shimabukuro-vornhagen@uk-koeln.de.