“How poor are they that have not patience! What wound did ever heal but by degrees?” (William Shakespeare, Othello [II.iii.376-377])
In this issue of Blood, Stefater III and colleagues present a molecular mechanism for restraining blood vessel growth during wound repair.1
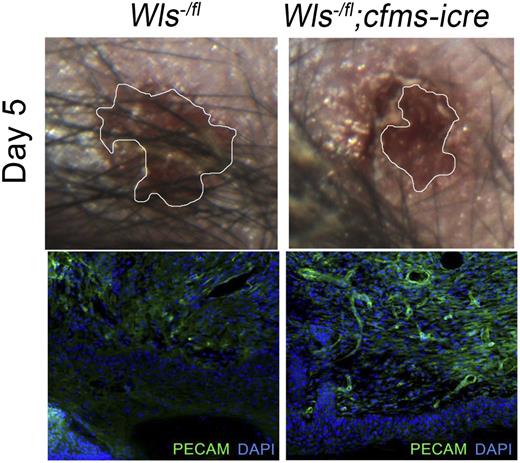
Macrophage Wnts suppress wound repair and angiogenesis. Wound area in control and homozygous null Wls animals at day 5 after initial injury (top panels) and immunostaining for wound vasculature (PECAM) in control and null animals (bottom panels). See the complete Figure 1 in the article by Stefater et al that begins on page 2574.
Macrophage Wnts suppress wound repair and angiogenesis. Wound area in control and homozygous null Wls animals at day 5 after initial injury (top panels) and immunostaining for wound vasculature (PECAM) in control and null animals (bottom panels). See the complete Figure 1 in the article by Stefater et al that begins on page 2574.
The repair of damaged tissue is an essential response to life’s slings and arrows, but the coordination of the many pathways involved in this process is a delicate balance that remains incompletely understood. The existence of such a pathway seems at first paradoxical; after all, re-perfusion of damaged tissue is an essential step in the healing process, and one might therefore think that inhibition of revascularization would impede recovery. However, as Shakespeare anticipates, the process of wound healing is stepwise, and the relegation of angiogenesis to the appropriate healing phase may be essential in assuring the correct and timely re-establishment of vascular networks. This study by Stefater et al1 not only lends support to this idea, but also implicates macrophages as mediators of this initial restraint, and attributes their function to the activity of the Wnt-Calcineurin-Flt1 axis.
Wound healing proceeds through 3 distinct phases: inflammation, proliferation, and remodeling.2 During the inflammatory phase, the recruitment of neutrophils, macrophages, and mast cells highlights the need for a robust immune response to ward off potential infections. Macrophages not only mediate this inflammation, but are also implicated in the guidance of the angiogenic response,3 part of the subsequent proliferative phase. However, there is a great deal of speculation as to the mechanisms underlying the macrophage contribution to angiogenesis; indeed, there is not even a consensus in the literature on whether macrophages are pro-angiogenic,4,5 anti-angiogenic,6 or not influential at all.7
Stefater et al1 probe the function of distinct signaling pathways in macrophage angiogenic regulation, using genetic tools to knockout genes of interest specifically in the monocyte/macrophage lineage. Previously, this approach helped them to establish that noncanonical Wnt signaling controls the expression of the anti-angiogenic protein sFlt-1 by retinal macrophages, referred to as “retinal myeloid cells.” This regulation restricts vascular sprouting in the deep layers of the developing retina, and exposes a heretofore unappreciated role for macrophages in the inhibition of endothelial cell growth.8 Subsequently, they used full-thickness dermal wounding of mice with defective myeloid Wnt signaling, and discovered that wound revascularization progressed more rapidly, although their macrophage content remained the same.1 They reasoned that macrophages must provide a growth-restraining signal downstream of Wnt, and once again a likely candidate was sFlt-1. Stefater et al1 elegantly dissect this pathway by demonstrating that noncanonical Wnt signaling utilizes NFAT-calcineurin to induce sFlt1/VEGFR-1 in cultured myeloid cell lines, and that loss of calcineurin signaling in myeloid/macrophage lineage phenocopies the loss of Wnt signaling in their wound healing model.
This work contributes to an emerging understanding of macrophages as not just participants, but coordinators of early wound healing. They are among the first cells to arrive at the wound site, and serve in the inflammatory stage to mediate the passive immune response and delay the onset of angiogenesis. In the following proliferative phase, they appear to encourage angiogenesis by the production of proangiogenic factors, and possibly by the mechanical facilitation of anastomosis, as seen in physiological settings.9 It is unclear whether these varied roles are performed by different subpopulations of macrophages, or whether they represent evolving functions within a constant population.
Given that robust angiogenesis is associated with rapid wound closure, it might seem that the initial inflammatory phase is superfluous or even counterproductive; however, there are significant theoretical advantages to the postponement on vascular reperfusion. In nature (and particularly outside of the relatively sterile environment of a research facility), the initial inflammatory phase is likely an essential step in warding off infection. Also, as Stefater et al1 speculate, increased early angiogenesis “might also make the wound weaker and more susceptible to a second injury during repair.”
Of course, the ability to speed wound closure is of great potential medical utility, and merits both further investigation of the macrophage Wnt-Calcineurin-sFlt-1 pathway and examination of other signaling modalities that may feed into this axis. For instance, Notch signaling controls sFlt1/vascular endothelial growth factor A receptor Flt1 (VEGFR-1) gene expression in cultured macrophages, and Notch provides an intrinsic signal required for macrophage recruitment during wound healing in vivo.10
Macrophage function, and particularly production of sFlt-1, clearly represents a key point of regulation in the transition between the degrees of wound healing. Further elucidation of the cellular and molecular dynamics in this environment, using elegant genetic approaches such as the ones seen in these studies, will both greatly increase our understanding of the physiological response to injury, and pave the way for therapeutic interventions that improve patient recovery.
Conflict-of-interest disclosure: The author declares no competing financial interests.