Key Points
Glucose metabolism enhances hematopoietic stem cell formation and function in the vertebrate embryo
Glucose metabolism modulates hif1α activity via mitochondrial generation of reactive oxygen species to impact HSC-relevant gene expression
Abstract
Many pathways regulating blood formation have been elucidated, yet how each coordinates with embryonic biophysiology to modulate the spatiotemporal production of hematopoietic stem cells (HSCs) is currently unresolved. Here, we report that glucose metabolism impacts the onset and magnitude of HSC induction in vivo. In zebrafish, transient elevations in physiological glucose levels elicited dose-dependent effects on HSC development, including enhanced runx1 expression and hematopoietic cluster formation in the aorta-gonad-mesonephros region; embryonic-to-adult transplantation studies confirmed glucose increased functional HSCs. Glucose uptake was required to mediate the enhancement in HSC development; likewise, metabolic inhibitors diminished nascent HSC production and reversed glucose-mediated effects on HSCs. Increased glucose metabolism preferentially impacted hematopoietic and vascular targets, as determined by gene expression analysis, through mitochondrial-derived reactive oxygen species (ROS)–mediated stimulation of hypoxia-inducible factor 1α (hif1α). Epistasis assays demonstrated that hif1α regulates HSC formation in vivo and mediates the dose-dependent effects of glucose metabolism on the timing and magnitude of HSC production. We propose that this fundamental metabolic-sensing mechanism enables the embryo to respond to changes in environmental energy input and adjust hematopoietic output to maintain embryonic growth and ensure viability.
Introduction
Definitive hematopoietic stem cells (HSCs) are capable of both self-renewal and production of mature blood lineages for the lifetime of the vertebrate organism. Fully functional long-term repopulating HSCs first arise from hemogenic endothelium in the ventral wall of the dorsal aorta, within the murine aorta-gonad-mesonephros (AGM) region between embryonic day 10.5 (e10.5) and e11.5.1 Many transcription factors involved in HSC development have been characterized and are highly conserved among vertebrates.2 Runx1, frequently mutated in human leukemia, is required for definitive HSC formation,3-5 controlling the ability of these cells to transition from hemogenic endothelium to circulating HSCs.6 In zebrafish, runx1+ HSCs are found in an analogous location at 36 hours postfertilization (hpf) and play the same role in endothelial-to-hematopoietic transition.7,8 We have previously used this conservation of expression and function to identify novel regulators of vertebrate HSCs through an in vivo screening approach.9-11 However, it remains unknown how the various regulatory pathways identified to date form an interconnected network integrating environmental stimuli with transcriptional control to direct the timing and magnitude of HSC production.
Metabolic factors were recently shown to have an important role in HSC regulation in the adult vertebrate niche. In particular, the presence of regionalized pockets of local hypoxia within the bone marrow stimulates oxygen content-responsive hypoxia-inducible factor (Hif) family members to maintain HSC quiescence, thereby reducing their susceptibility to damage.12,13 Although the role of metabolic sensing has not been directly examined in the context of definitive hematopoiesis, several investigations imply that embryonic nutrient availability and metabolism may impact HSC fate: studies revealed significantly enhanced risks for acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML) in children exposed to chronic high glucose levels in utero14,15 ; likewise, ALL is more frequently found in children with diabetes.16 Additionally, cord blood from neonates born to diabetic mothers showed decreased platelet function17 and neutrophil mobility,18 suggesting that glucose levels can modify hematopoietic cell activity. Together, these data demonstrate that metabolic conditions can affect HSC homeostasis; however, it remains unclear whether metabolic state is involved in regulating definitive HSC production and function during embryogenesis.
Cellular respiration is dependent on nutrient availability and oxygen concentration to enable energy production and growth. As such, adequate oxygen and nutrient content are essential to embryogenesis, and mechanisms have evolved to detect and respond to metabolic insufficiencies.19 The Hif complex, which governs the embryo’s “hypoxic response,” is essential for embryogenesis: Hif1α−/− and Hif1β−/− mice die at e9.5 to e10.0, prior to the onset of definitive hematopoiesis, with prominent vascular defects and severe developmental delay.20,21 Similarly, lack of Hif1β in embryonic stem cells causes proliferative failures in vascular progenitors and primitive hematopoietic cells in vitro,22 shown through embryonic-explant cultures to result from vascular endothelial growth factor (VEGF) deficiency.23 Before establishment of convective transport, the early vertebrate embryo experiences not only local hypoxia, but also significant fluctuation in nutrients, particularly glucose, as passive diffusion of oxygen and metabolites ceases to sufficiently match embryonic growth.24 The impact of Hif regulation on oxygen consumption, cellular metabolism, and proliferation in cancer is well documented.25 However, a specific function for metabolic state and Hif1α−signaling in definitive HSC induction during embryogenesis has yet to be elucidated.
Here, we show that glucose metabolism dose-dependently influences the production of HSCs during embryogenesis. Elevated glucose levels accelerated the induction of HSCs from hemogenic endothelium, as observed by runx1 expression and ultrastructural analysis. Embryonic-to-adult transplantation studies confirmed that glucose exposure increased functional HSCs capable of sustained multilineage engraftment. Flux in the production of mitochondrial reactive oxygen species (ROS) in the AGM directly impacted HSC number downstream of glucose metabolism. Through gene-knockdown and chemical epistasis experiments, glucose metabolism-derived ROS were shown to stabilize hif1α levels in vivo. Genomic analysis confirmed the role of hif1α in metabolism-mediated HSC expansion, and demonstrated that glucose stimulated a dose-responsive induction of hif1α-target pathways, leading to increased production of HSCs from hemogenic endothelium. Our work provides direct evidence that the developing embryo responds dynamically to metabolic input by increasing HSC formation via a previously uncharacterized action of hif1α in response to ROS sensing.
Methods
Zebrafish husbandry
Zebrafish were maintained according to institutional animal care and use committee–approved protocols. Tg(−6.0itga2b(CD41):eGFP), Tg(cmyb:eGFP), Tg(fli1a:EGFP), Tg(lmo2:dsRed), Tg(−5.0tal1:EGFP(scl):eGFP), Tg(gata1:dsRed), EF1:mKO2-zCdt1(1/190)rw0405b, and EF1:mAG-zGem(1/100)rw0410h and silent heart lines were described previously.9,26 Tg(runx1P1:eGFP) was provided by P. Crosier27 , University of Auckland School of Medicine, Auckland, New Zealand, and the Tg(globin:eGFP) line was provided by L. I. Zon, Children's Hospital, Harvard Medical School, Boston, MA.
Chemical treatments and evaluation
Zebrafish embryos were exposed to compounds (see supplemental Methods and supplemental Table 1, available on the Blood website) in E3 (fish) water in multiwell plates from 5 somites (12 hpf) until 36 hpf, unless otherwise noted; glucose concentration was 1% unless indicated. In situ hybridization was performed using published protocols (http://zfin.org/ZFIN/Methods/ThisseProtocol.html) and probes. Phenotype distribution is summarized as: number altered/number scored per treatment (tx); >3 independent experiments were conducted per analysis and images were acquired using a Zeiss Axio Imager A1/Axio Cam MRC and Axiovision LE software (Carl Zeiss, Oberkochen, Germany) as previously described.9,11 Fluorescence-activated cell sorting (FACS), cell-cycle analysis, 5-bromo-2′-deoxyuridine incorporation, and western blot analysis for HIF-1α (Cayman Chemical) were previously described.9,11
Morpholino (MO) injection
MOs (listed in supplemental Methods and supplemental Table 2; GeneTools) were injected into 1-cell stage embryos as described previously,28 and effects were assessed as under the “Chemical treatments and evaluation” section.
Fluorescent microscopy
Fluorescent reporter embryos were exposed to compounds as indicated under the “Chemical treatments and evaluation” section. For H2O2 detection, embryos were treated with 5μM peroxy-fluorescein29 for 2 hours at 31°C, and for glucose detection, 1% 2-NBDG [2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose] (Cayman Chemical) was used. All embryos were imaged by fluorescence microscopy using a Zeiss Discovery V8/Axio Cam MRC and Axiovision LE software (Carl Zeiss) as previously described.9
Quantitative reverse transcription–PCR and microarray analysis
Quantitative polymerase chain reaction (qPCR) was performed on cDNA isolated from pooled embryos at 36 hpf (n = 20/variable; primers listed in supplemental Methods and supplemental Table 3) using an iQ5 RTPCR Detection System (Bio-Rad) as previously described.9 Microarray (Affymetrix) experiments were performed in triplicate and analyzed using Ingenuity Pathway software (accession number GSE43186).
Glucose uptake and biochemical assays
Embryos were incubated with 0.5 μCi/mL [3H]-2-deoxyglucose in 100μM 2-deoxyglucose for 30 to 60 minutes at 28°C, followed by scintillation counting. Total protein was determined by Bradford assay. Colorimetric glucose analysis was performed according to the manufacturer’s protocol (Cayman Chemical). Adenosine triphosphate (ATP), pyruvate, and lactate measurements were performed on lysates from 60 disaggregated embryos using standard kits according to manufacturers’ instructions (Biovision).
Electron microscopy
Electron microscopy was performed as previously described.30
Results
Glucose exposure enhances HSC formation in zebrafish embryos
Given the necessity of glucose consumption in the developing embryo, to investigate the impact of metabolic regulation on HSC formation, we upregulated nutrient input: wild-type (WT) zebrafish embryos were exposed to D-glucose (1% in fish water) from 5 somites to 36 hpf, throughout hematopoietic initiation. Compared with sibling controls, D-glucose enhanced expression of runx1 and cmyb2 in the AGM of the majority of embryos examined (Figure 1A); these effects were dose-dependent (supplemental Figure 1A), but not due to alterations in osmolarity, as indicated by exposure to the inactive enantiomer L-glucose. The impact of glucose was confirmed by fluorescent microscopy and FACS quantification in HSC-reporter lines: Tg(runx1P1:eGFP), Tg(cmyb:eGFP), and Tg(CD41:eGFP) (Figure 1B-C), and qPCR (Figure 1D). The influence of glucose was dependent on the duration, but not the timing, of exposure: treatment from 12-36 hpf (24 hours) led to greater changes than exposure preheartbeat (12-24 hpf) or postheartbeat (24-36 hpf; 12hrs), impacting the vascular HSC niche or HSC specification, respectively (supplemental Figure 1B). Consistent with that observation, glucose did not alter gross vasculogenesis by in situ hybridization or in endothelial-reporter fish (supplemental Figure 1B-C); however, qPCR revealed slight increases in endothelial gene expression (supplemental Figure 1D). The effect on HSCs was similarly not due to alterations in cardiovascular function as heartbeat onset and rate were unchanged (control: 101.2 ± 9.9, glucose: 99.8 ± 8.51, t test, P = NS, n = 9), and glucose retained the ability to increase HSCs in silent heart (sih−/−) embryos, which lack blood flow to a cardiac defect28 (supplemental Figure 1E-F). Together, these data imply glucose exposure impacts the development of phenotypic HSCs, independent of gross vascular development and function.
Glucose enhances HSC formation in the zebrafish AGM region. Zebrafish were exposed to 1% glucose from 10 somites to 36 hpf and analyzed at 36 hpf. (A) D-glucose enhanced runx1/cmyb expression by in situ hybridization (560 increased/652 scored [85.9%↑]). The metabolically inactive enantiomer, L-glucose, had no effect (n > 100 embryos/treatment [tx]). Top panels show whole embryos, while bottom panels depict AGM regions. Numbers in lower right of panels, here and following, indicate embryos with altered HSC expression (as depicted) over the total number scored. (B) Glucose exposure increased HSC number as determined by in vivo fluorescent microscopy of runx1:eGFP, cmyb:eGFP, and CD41:eGFP transgenic reporter embryos (n = 60/tx). (C) Quantification of fluorescent cell number by FACS analysis of reporter embryos supported the effects of observed by microscopy (t test, runx1: *P < .0001; cmyb/lmo2: **P < .05; CD41: ***P < .01, n = 6-9). (D) qPCR confirmed significantly enhanced expression of HSC markers following glucose exposure (20 embryos/cohort, t test, runx1, cmyb: *P < .0001; CD41: **P < .05, n = 3).
Glucose enhances HSC formation in the zebrafish AGM region. Zebrafish were exposed to 1% glucose from 10 somites to 36 hpf and analyzed at 36 hpf. (A) D-glucose enhanced runx1/cmyb expression by in situ hybridization (560 increased/652 scored [85.9%↑]). The metabolically inactive enantiomer, L-glucose, had no effect (n > 100 embryos/treatment [tx]). Top panels show whole embryos, while bottom panels depict AGM regions. Numbers in lower right of panels, here and following, indicate embryos with altered HSC expression (as depicted) over the total number scored. (B) Glucose exposure increased HSC number as determined by in vivo fluorescent microscopy of runx1:eGFP, cmyb:eGFP, and CD41:eGFP transgenic reporter embryos (n = 60/tx). (C) Quantification of fluorescent cell number by FACS analysis of reporter embryos supported the effects of observed by microscopy (t test, runx1: *P < .0001; cmyb/lmo2: **P < .05; CD41: ***P < .01, n = 6-9). (D) qPCR confirmed significantly enhanced expression of HSC markers following glucose exposure (20 embryos/cohort, t test, runx1, cmyb: *P < .0001; CD41: **P < .05, n = 3).
To further characterize the glucose-mediated effect, cell proliferation was assessed: 5-bromo-2′-deoxyuridine incorporation was increased in glucose-exposed embryos, particularly in the AGM (supplemental Figure 2A,C). Cell-cycle activity visualized in vivo using proliferation-reporter fish (red G1; green S/G2/M)26 revealed increases in proliferative cells (supplemental Figure 2B), which was corroborated by propidium iodide–based analysis (supplemental Figure 2D). These findings cannot be explained by overall enhanced growth, as whole-embryo cell counts, protein content, or expression of other mesodermal (mhc, pax2, cmlc2), endodermal (foxA3), and neural (huc) markers (supplemental Figure 2E-G) remained unchanged at 36 hpf. These data indicate that glucose exposure affects the proliferative activity of phenotypic HSCs.
Glucose specifically accelerates the onset of HSC hematopoiesis in the AGM
To investigate the mechanism behind glucose-mediated HSC enhancement, temporal expression of runx1 was evaluated. In controls, runx1 was transiently expressed in primitive erythrocytes and detected in a subset of endothelial cells by 30 hpf (Figure 2A); at 36 hpf, most hemogenic endothelial cells were runx1+. In contrast, glucose-exposed embryos had accelerated induction of runx1 expression, appearing 6 to 9 hours earlier, with an expansion of runx1+ hemogenic clusters by 30 hpf, which was corroborated by qPCR (supplemental Figure 3A). Electron microscopy of AGM sections confirmed increased budding from hemogenic endothelium in glucose-treated embryos (Figure 2B). This glucose-mediated acceleration and expansion in HSCs was runx1-dependent, as glucose exposure could not rescue cmyb expression in runx1−/− embryos31 or runx1 morphants (supplemental Figure 3B-C), Together, these data indicate that glucose elevation hastens the normal program of HSC induction from the hemogenic endothelial niche.
Glucose accelerates the onset and output of definitive hematopoiesis in the AGM. (A) Time-course analysis by in situ hybridization for runx1 in 3-hour intervals from 18 to 36 hpf revealed earlier and enhanced expression after glucose exposure (n ≥ 35/tx). (B) Electron microscopy of sagittal sections of embryos at 36 hpf revealed increased budding (arrowheads) of hemogenic endothelial cells (HSCs) from the ventral wall of the dorsal aorta (magnification: ×1200, top panels; ×10 000, bottom panels). (C) Transplantation of AGM cells from glucose-exposed CD41:eGFP embryos into irradiated adult WT recipients led to increased engraftment, as indicated by the fraction of recipients containing GFP+ cells by fluorescence microscopy 3 weeks after transplantation (t test, *P < .05, n = 28-31).
Glucose accelerates the onset and output of definitive hematopoiesis in the AGM. (A) Time-course analysis by in situ hybridization for runx1 in 3-hour intervals from 18 to 36 hpf revealed earlier and enhanced expression after glucose exposure (n ≥ 35/tx). (B) Electron microscopy of sagittal sections of embryos at 36 hpf revealed increased budding (arrowheads) of hemogenic endothelial cells (HSCs) from the ventral wall of the dorsal aorta (magnification: ×1200, top panels; ×10 000, bottom panels). (C) Transplantation of AGM cells from glucose-exposed CD41:eGFP embryos into irradiated adult WT recipients led to increased engraftment, as indicated by the fraction of recipients containing GFP+ cells by fluorescence microscopy 3 weeks after transplantation (t test, *P < .05, n = 28-31).
To assess whether glucose exposure impacted production of functional HSCs, we developed a novel zebrafish embryo-to-adult transplant model. The trunk region encompassing the AGM of CD41:eGFP transgenic reporter embryos was isolated and disaggregated; pooled cells (5 embryo equivalents) were co-injected intracardially into lethally irradiated (25 Gy) adult recipients with 200 000 WT peripheral blood (PB) cells. Fluorescent microscopy at 3 weeks postinjection revealed significantly increased numbers of recipients of glucose-treated AGM cells had GFP+ thymus, kidney marrow (KM), and spleen cells (Figure 2C; supplemental Figure 3D) compared with recipients of untreated cells; FACS analysis confirmed a twofold increase in fish showing >0.01% GFP+ KM and PB in recipients of glucose-treated cells (supplemental Figure 3E). Independent replicates revealed similar results (supplemental Figure 3F), with enhanced green fluorescent protein (eGFP) maintained at >10 weeks; CD41+ engrafted recipients exhibited equal potential for CD45:dsRed multilineage PB repopulation (supplemental Figure 3G; control: 0.125 ± 0.074, glucose: 0.171 ± .0.103, t test, P = NS, n > 15). These data show glucose-exposed embryos had increased numbers of functional HSCs.
To determine whether embryonic glucose exposure exerted sustained effects on HSCs, we examined primitive erythropoiesis and definitive hematopoietic differentiation. Glucose-exposed embryos exhibited significantly increased erythropoiesis as determined by fluorescence microscopy, FACS analysis, and qPCR (supplemental Figure 4A-C). Transient early exposure (12-36 hpf) to glucose lead to sustained elevations in CD41+ HSCs in the caudal hematopoietic tissue (CHT) at 72 hpf and KM at 144 hpf (supplemental Figure 4D-E); gene expression and FACS analysis (myeloid: lysozymeC:dsRed, myeloperioxidase:GFP; lymphoid: rag2:dsRed) during CHT and KM hematopoiesis showed no alterations in differentiation potential on a percentage basis (supplemental Figure 4F-G). However, significant increases in total embryonic cell number were found at 72 and 144 hpf, such that absolute numbers of differentiated hematopoietic cells likewise increased (supplemental Figure 4H-I). Multilineage differentiation potential was maintained at 1 month postexposure (supplemental Figure 4J). To determine whether glucose could directly enhance CHT or KM HSCs, CD41:eGFP embryos (48 hpf) or larvae (96 hpf) were exposed to 1% glucose; transient elevation increased CD41+ cells in both tissues (supplemental Figure 4K). Similarly, in adults, glucose treatment from 48 to 72 hours postirradiation injury (20 Gy) significantly increased HSPCs in the KM, as seen by FACS at 10 days postinjury9 (supplemental Figure 4L). Together, these results indicate that transient glucose elevation increases hematopoietic production, including HSC number, without impairing function or differentiation capacity.
Cellular uptake and metabolism of glucose lead to enhanced HSC number
We next sought to determine whether the glucose-mediated HSC effect was due to metabolic alterations. ATP, pyruvate, and lactate levels at 36 hpf each increased in response to exogenous glucose (Figure 3A). Enzymatic glucose determination revealed significant elevation, compared with controls at 36 hpf, after incubation with 1% glucose in the fish water (Figure 3B), increasing 1.35-fold; importantly, by 72 hpf, glucose levels dropped significantly with or without treatment, consistent with prior reports.32 Radiolabeled glucose-uptake assays confirmed these results (Figure 3C) indicating that intraembryonic glucose concentrations increase after exposure to 1% glucose; however, internal levels remain within the physiological range. Using 2-NBDG, a fluorescent glucose analog, significant enrichment of intraembryonic glucose was detected in the lmo2+ population by confocal microscopy and FACS (Figure 3D-E). MO-mediated knockdown of the glucose transporter, glut1, resulted in significantly decreased runx1/cmyb expression in both unexposed and glucose-treated embryos (Figure 3F). Conversely, glucose treatment still enhanced HSCs after MO knockdown of the insulin receptor (insr) (Figure 3F). Similarly, insulin expression was unchanged following exposure to glucose (Figure 3G) or known islet cell toxin streptozocin (supplemental Figure 5A). Consistent with prior publications placing the onset of insulin regulation in zebrafish at 48 hpf,32 these data that demonstrate cellular uptake is required for glucose-mediated effects on AGM HSCs, independent of the insulin response.
The effect of glucose is dependent on uptake rather than insulin signaling. (A) Levels of ATP, pyruvate, and lactate in whole-embryo lysates increased significantly in response to glucose (t test, ATP, pyruvate: *P < .02; lactate: **P < .05, n = 3). (B) Measurement of total embryo glucose content by enzymatic assay revealed significantly elevated glucose levels at 36 hpf after exposure to 1% glucose in the fish water from 12 to 36 hpf, which declined back to baseline by 72 hpf (analysis of variance [ANOVA], *P < .05, n = 3). (C) Glucose uptake, measured by radioisotope labeling, was significantly enhanced in the embryo after 60 minutes of exposure in the fish water (ANOVA, P = .012, n = 3). (D) Confocal microscopy of lmo2:dsRed transgenic reporter embryos at 36 hpf following exposure to 2-NBDG reveals green fluorescent glucose signal in the zebrafish aorta (arrowhead), colocalized with an increased number of lmo2+ hematopoietic cells; white dotted lines indicate the trunk of the embryo, the dashed line demarks the yolk (below) from the embryo proper (above). The inset shows GFP levels in untreated fish at the same signal amplification as 2-NBDG–treated samples (n = 5/tx). (E) FACS analysis reveals significant and selective uptake of the fluorescent glucose analog 2-NBDG (green fluorescence) by hematopoietic and endothelial lmo2+ cells (t test, *P < .05, n = 10). (F) MO knockdown of glut1 (40μM) inhibited the effect of glucose on runx1/cmyb+ HSCs. insr (40μM) knockdown did not affect runx1/cmyb expression in the presence or absence of glucose (17 normal/22). (G) Glucose exposure did not affect insulin expression prior to islet formation at 36 hpf (36 normal/36).
The effect of glucose is dependent on uptake rather than insulin signaling. (A) Levels of ATP, pyruvate, and lactate in whole-embryo lysates increased significantly in response to glucose (t test, ATP, pyruvate: *P < .02; lactate: **P < .05, n = 3). (B) Measurement of total embryo glucose content by enzymatic assay revealed significantly elevated glucose levels at 36 hpf after exposure to 1% glucose in the fish water from 12 to 36 hpf, which declined back to baseline by 72 hpf (analysis of variance [ANOVA], *P < .05, n = 3). (C) Glucose uptake, measured by radioisotope labeling, was significantly enhanced in the embryo after 60 minutes of exposure in the fish water (ANOVA, P = .012, n = 3). (D) Confocal microscopy of lmo2:dsRed transgenic reporter embryos at 36 hpf following exposure to 2-NBDG reveals green fluorescent glucose signal in the zebrafish aorta (arrowhead), colocalized with an increased number of lmo2+ hematopoietic cells; white dotted lines indicate the trunk of the embryo, the dashed line demarks the yolk (below) from the embryo proper (above). The inset shows GFP levels in untreated fish at the same signal amplification as 2-NBDG–treated samples (n = 5/tx). (E) FACS analysis reveals significant and selective uptake of the fluorescent glucose analog 2-NBDG (green fluorescence) by hematopoietic and endothelial lmo2+ cells (t test, *P < .05, n = 10). (F) MO knockdown of glut1 (40μM) inhibited the effect of glucose on runx1/cmyb+ HSCs. insr (40μM) knockdown did not affect runx1/cmyb expression in the presence or absence of glucose (17 normal/22). (G) Glucose exposure did not affect insulin expression prior to islet formation at 36 hpf (36 normal/36).
Glycolysis and oxidative phosphorylation are required for HSC formation
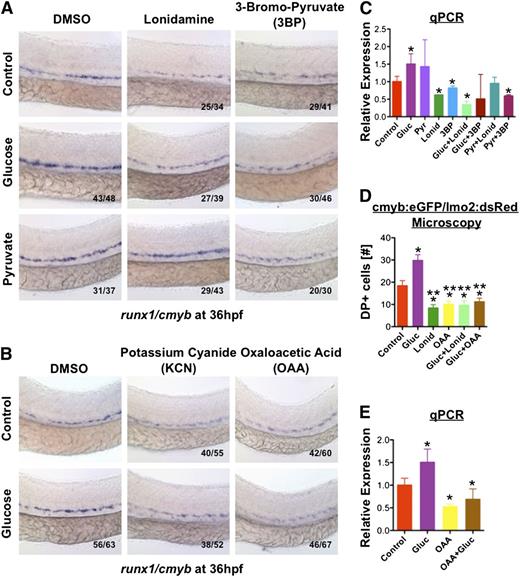
To determine whether glucose metabolism was driving the effect on HSC formation, zebrafish embryos were treated with sodium pyruvate (1%), the major metabolite derived from glucose through glycolysis, and similar inductions in HSC production were observed (Figure 4A). Conversely, treatment with lonidamine (10μM), an inhibitor of hexokinase, a central regulator of glycolysis, diminished WT HSC production and eliminated the effect of glucose. This was confirmed by exposure to 3-bromopyruvate (3BP; 20μM), a synthetic derivative of pyruvic acid which competitively inhibits hexokinase and pyruvate dehydrogenase: 3BP blocked the effects of both glucose and pyruvate, implying entry into the citric acid cycle was necessary for glucose-mediated effects on HSCs. To investigate this further, WT embryos were treated with potassium cyanide (KCN, 100μM), which blocks oxidative phosphorylation and ATP production, and oxaloacetic acid (OAA, 10μM), which inhibits succinate dehydrogenase activity. Both significantly reduced runx1/cmyb expression in the AGM and mitigated the effect of glucose (Figure 4B), as confirmed by qPCR and fluorescent analysis of double positive (yellow) HSCs in cmyb:eGFP/lmo2:dsRED embryos28 (Figure 4C-E). Together, these data indicate HSC formation is dependent on glucose metabolism.
Glucose levels influence HSC formation via increased energy metabolism. (A) Hexokinase inhibitors lonidamine (lonid; 10μM) and 3BP (20μM) decreased runx1/cymb staining and eliminated the effect of glucose on HSCs; addition of pyruvate (pyr; 1%) rescued the block in HSC formation elicited by lonid, but not 3BP, which inhibits both glycolysis and oxidative phosphorylation (n ≥ 30/tx). (B) Treatment with electron transport chain inhibitors potassium cyanide (KCN) (100μM) and OAA (50μM) decreased HSC gene expression and blocked the effect of glucose (n ≥ 50/tx). (C) Quantitative analysis of runx1 expression confirms the impact of hexokinase inhibition on mitigating the effect of glucose on HSCs (ANOVA, P < .05, n = 3). (D) Quantitative analysis of double-positive (DP) cells in the AGM by fluorescence microscopy of cmyb:eGFP;lmo2:dsRed transgenic embryos confirms the observed inhibitory effects of lonid and OAA in vivo (t test, *P < .01 vs con, **P < .001 vs gluc, n = 5). (E) The ability of OAA to eliminate the impact of glucose on runx1 expression was corroborated by qPCR (ANOVA, *P < .05, n = 3).
Glucose levels influence HSC formation via increased energy metabolism. (A) Hexokinase inhibitors lonidamine (lonid; 10μM) and 3BP (20μM) decreased runx1/cymb staining and eliminated the effect of glucose on HSCs; addition of pyruvate (pyr; 1%) rescued the block in HSC formation elicited by lonid, but not 3BP, which inhibits both glycolysis and oxidative phosphorylation (n ≥ 30/tx). (B) Treatment with electron transport chain inhibitors potassium cyanide (KCN) (100μM) and OAA (50μM) decreased HSC gene expression and blocked the effect of glucose (n ≥ 50/tx). (C) Quantitative analysis of runx1 expression confirms the impact of hexokinase inhibition on mitigating the effect of glucose on HSCs (ANOVA, P < .05, n = 3). (D) Quantitative analysis of double-positive (DP) cells in the AGM by fluorescence microscopy of cmyb:eGFP;lmo2:dsRed transgenic embryos confirms the observed inhibitory effects of lonid and OAA in vivo (t test, *P < .01 vs con, **P < .001 vs gluc, n = 5). (E) The ability of OAA to eliminate the impact of glucose on runx1 expression was corroborated by qPCR (ANOVA, *P < .05, n = 3).
Mitochondrial production of ROS induce HSC expansion
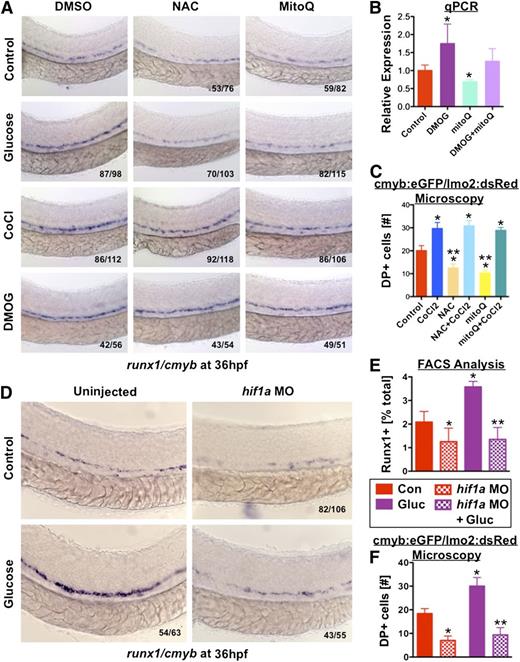
Glucose metabolism and oxidative phosphorylation generates mitochondrial ROS.33 To determine whether enhanced ROS generation in glucose-exposed embryos impacted HSC formation, embryos were directly exposed to ROS: hydrogen peroxide (H2O2, 0.05%) enhanced HSC formation and rescued OAA-mediated HSC loss (Figure 5A). Other ROS enhancers (propylthiouracil, L-buthionine sulphoximine, and paraquat) had similar effects (supplemental Figure 5B). Incubation with N-acetylcysteine (NAC; 10μM), a clinically used nonspecific ROS inhibitor, reduced runx1/cmyb expression and diminished glucose-mediated HSC increases, as confirmed by qPCR and fluorescent microscopy (Figure 5B-C); vitamin C, Euk134, and MitoQ (10μM), a specific inhibitor of mitochondrially derived H2O2, showed similar results (supplemental Figure 5C). The role of mitochondrial-derived H2O2 was confirmed genetically by MO knockdown of peroxiredoxin 1 (prdx1), an antioxidant enzyme that metabolizes H2O2 to H2O, where significant increases in HSCs were observed as determined by in situ hybridization and analysis of runx1:eGFP and CD41:eGFP embryos (Figure 5D-G). Incubation with PeroxyFluor2 (PF2; green),29 which specifically detects H2O2, revealed enhanced activity in the AGM of glucose-treated lmo2:dsRed embryos, as indicated by colocalization (yellow) (supplemental Figure 5D), while MitoQ prevented H2O2 generation in response to glucose; these results were quantified by FACS, where a 2.3-fold increase in H2O2-induced fluorescence was seen in glucose-exposed lmo2:Red+ cells (Figure 5H). These data indicate that heightened H2O2 generated by increased mitochondrial metabolism works as the intermediary for glucose-mediated regulatory effects on HSCs.
ROS produced by mitochondrial activity cause HSC expansion. (A) Treatment with the antioxidant NAC (10μM) reduced HSC formation and blocked the effect of glucose. Addition of exogenous hydrogen peroxide (0.05% H2O2) rescued HSC formation in OAA-treated embryos (n > 40/tx). (B) qPCR for runx1 expression confirmed the inhibitory impact of NAC exposure (ANOVA, P < .05, n = 3). (C) Quantitative analysis of double-positive (DP) cells in the AGM of cmyb:eGFP;lmo2:dsRed transgenic embryos by fluorescence microscopy confirms the inhibitory impact of NAC on HSC formation in vivo (t test, *P < .02 vs con, **P < .001 vs gluc, n = 5). (D-F) Morpholino knockdown of the endogenous metabolic antioxidant enzyme, peroxiredoxin (prdx1, 25μM) increased HSC formation as determined by (D) runx1/cmyb in situ hybridization, and observed in (E) runx1:eGFP and (F) CD41:eGFP transgenic zebrafish (n > 20/tx). (G) Quantification of runx1:eGFP and CD41:eGFP+ cells in prdx1 morphants by FACS revealed significant changes compared with controls (runx1: control 2.65% ± 1.38%; prdx1 MO 4.06% ± 1.02%, t test, *P = .023, n ≥ 9; CD41: control 0.22 ± 0.11%; prdx1 MO 0.533 ± 0.16%; t test, **P < .001, n = 10). (H) PF2 fluorescence intensity quantified by FACS in lmo2:dsRed endothelial cells revealed significantly increased ROS production after 1% glucose exposure (t test, *P < .0001, n = 4).
ROS produced by mitochondrial activity cause HSC expansion. (A) Treatment with the antioxidant NAC (10μM) reduced HSC formation and blocked the effect of glucose. Addition of exogenous hydrogen peroxide (0.05% H2O2) rescued HSC formation in OAA-treated embryos (n > 40/tx). (B) qPCR for runx1 expression confirmed the inhibitory impact of NAC exposure (ANOVA, P < .05, n = 3). (C) Quantitative analysis of double-positive (DP) cells in the AGM of cmyb:eGFP;lmo2:dsRed transgenic embryos by fluorescence microscopy confirms the inhibitory impact of NAC on HSC formation in vivo (t test, *P < .02 vs con, **P < .001 vs gluc, n = 5). (D-F) Morpholino knockdown of the endogenous metabolic antioxidant enzyme, peroxiredoxin (prdx1, 25μM) increased HSC formation as determined by (D) runx1/cmyb in situ hybridization, and observed in (E) runx1:eGFP and (F) CD41:eGFP transgenic zebrafish (n > 20/tx). (G) Quantification of runx1:eGFP and CD41:eGFP+ cells in prdx1 morphants by FACS revealed significant changes compared with controls (runx1: control 2.65% ± 1.38%; prdx1 MO 4.06% ± 1.02%, t test, *P = .023, n ≥ 9; CD41: control 0.22 ± 0.11%; prdx1 MO 0.533 ± 0.16%; t test, **P < .001, n = 10). (H) PF2 fluorescence intensity quantified by FACS in lmo2:dsRed endothelial cells revealed significantly increased ROS production after 1% glucose exposure (t test, *P < .0001, n = 4).
Glucose-induced ROS activates hif1α to mediate effects on HSC formation
Elevations in ROS stabilize the transcription factor Hif1α by inhibiting prolyl hydroxylases, which target it for ubiquitination and destruction by von Hippel-Lindau protein at normoxia.34 To determine whether Hif-mediated responses to ROS contributed to glucose-dependent HSC elevation, embryos were exposed to cobalt chloride (CoCl2, 500μM), a hypoxia mimetic that interferes with hif1α/vhl interactions, which significantly enhanced runx1/cmyb expression and rescued antioxidant-mediated HSC reduction (Figure 6A). The prolyl hydroxylase inhibitor dimethyloxallyl glycine (DMOG; 50μM) produced similar results (Figure 6A-C). Hif1α is expressed in dorsal aorta of the zebrafish embryo35 ; MO knockdown of hif1a severely decreased HSC formation and blunted the effect of glucose (Figure 6D-F). To verify the importance of hif1α in coordinating the biological response in HSC production to fluctuations in environmental glucose levels, we examined genome-wide expression changes in pooled cohorts of glucose-treated and control embryos at 36 hpf by microarray and ingenuity pathway analysis. “Hif1α signaling” scored as the most significantly regulated canonical pathway (supplemental Figure 6A). Despite the use of whole-embryo fractions, the top networks and functions associated with genetic responses to glucose were statistically enriched for hematopoietic targets (supplemental Figure 6B-C); connectivity maps of regulated genes illustrate the node around Hif1a, integrated with key regulators of hematopoietic development, cell cycle, and metabolism (supplemental Figure 6D). Together, these analyses indicate that glucose-mediated metabolic enhancement at this stage of embryogenesis primarily impacts hematopoiesis via hif1α.
Hif1α activity induced by elevated ROS mediates the effect of glucose metabolism on HSCs. (A) Exposure to CoCl2 (500μM) or the hif1α prolyl hydroxylase inhibitor DMOG (500μM) expanded runx1/cmyb expression and rescued the impairment of HSC formation induced by antioxidants NAC and mitoQ (n > 50/tx). (B) qPCR demonstrated the effect of DMOG in enhancing runx1 expression and rescuing the effect of mitoQ exposure on HSCs (ANOVA, *P < .05, n = 3). (C) Fluorescent analysis of double-positive (DP) AGM cells in cmyb:eGFP;lmo2:dsRed transgenic embryos confirmed the positive impact of CoCl2 on HSC formation in vivo that rescued the inhibitory effects of the antioxidants NAC and mitoQ (t test, *P < .01 vs con, **P < .001 vs CoCl2, n = 5). (D) hif1a-MO knockdown decreased runx1/cmyb expression and blocked the effect of glucose on HSCs (43↓/55). (E) FACS quantification of runx1:eGFP+ cells following hif1a-MO knockdown revealed reduced HSC formation in the presence and absence of glucose (t test, *P < .001 vs con, **P < .001 vs gluc, n = 12). (F) Quantitative analysis of fluorescence microscopy images of cmyb:eGFP;lmo2:dsRed reporter embryos revealed the negative impact on HSCs of loss of hif1α function by morpholino knockdown could not be rescued by glucose exposure (t test, *P < .002 vs control, **P < .0001 vs glucose, n = 5).
Hif1α activity induced by elevated ROS mediates the effect of glucose metabolism on HSCs. (A) Exposure to CoCl2 (500μM) or the hif1α prolyl hydroxylase inhibitor DMOG (500μM) expanded runx1/cmyb expression and rescued the impairment of HSC formation induced by antioxidants NAC and mitoQ (n > 50/tx). (B) qPCR demonstrated the effect of DMOG in enhancing runx1 expression and rescuing the effect of mitoQ exposure on HSCs (ANOVA, *P < .05, n = 3). (C) Fluorescent analysis of double-positive (DP) AGM cells in cmyb:eGFP;lmo2:dsRed transgenic embryos confirmed the positive impact of CoCl2 on HSC formation in vivo that rescued the inhibitory effects of the antioxidants NAC and mitoQ (t test, *P < .01 vs con, **P < .001 vs CoCl2, n = 5). (D) hif1a-MO knockdown decreased runx1/cmyb expression and blocked the effect of glucose on HSCs (43↓/55). (E) FACS quantification of runx1:eGFP+ cells following hif1a-MO knockdown revealed reduced HSC formation in the presence and absence of glucose (t test, *P < .001 vs con, **P < .001 vs gluc, n = 12). (F) Quantitative analysis of fluorescence microscopy images of cmyb:eGFP;lmo2:dsRed reporter embryos revealed the negative impact on HSCs of loss of hif1α function by morpholino knockdown could not be rescued by glucose exposure (t test, *P < .002 vs control, **P < .0001 vs glucose, n = 5).
Hif1α transcriptional activity mediates the effect of glucose on HSCs
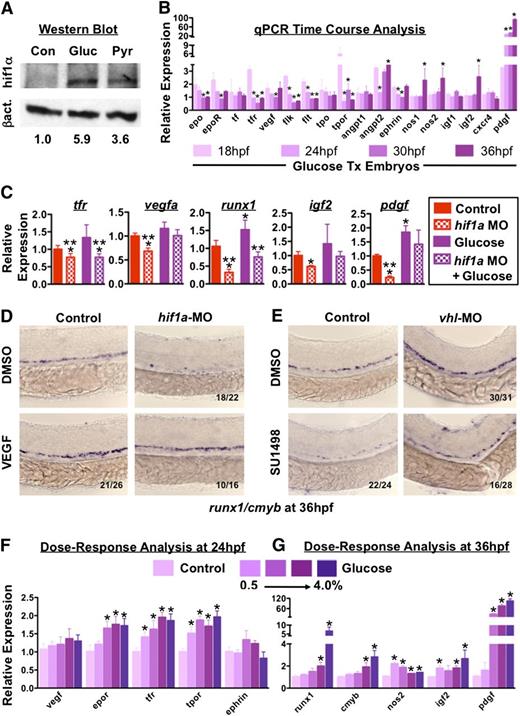
During hypoxia, Hif1α affects the transcriptional regulation of metabolic enzymes to control oxygen consumption and induces expression of vascular and erythrocyte-specific genes to boost available oxygen content.36 To test whether hif1α coordinates nutrient availability with embryonic HSC output, we examined alterations in hif1α levels following glucose exposure. Expression of hif in the AGM was corroborated (supplemental Figure 7A), and western blot analysis showed increased hif1α protein levels in response to incubation with glucose or pyruvate (Figure 7A). Likewise, expression analysis showed increased levels of glut1 and that of central glycolytic regulators in glucose-treated embryos, similar to prior reports37 (supplemental Figure 7B-C). To assess transcriptional variations in hif1α targets potentially mediating effects on hematopoiesis in glucose-treated embryos, qPCR analysis was conducted on embryos at fixed time points from 18 to 36 hpf; consistent with published effects on erythropoiesis,38 erythroid-specific genes such as erythropoietin (epo), epo receptor (epor), transferrin (tf), and tfr increased in response to glucose at early time points (Figure 7B). These effects were likely due to hif1α-mediated regulation, as embryos injected with a dominant-negative hif1a construct (dnhif1a),39 that can reduce runx1+ HSCs in vivo (supplemental Figure 7D-E), did not display glucose-mediated enhancement in erythropoiesis as determined by O-dianisidine (heme) staining (supplemental Figure 7F, n ≥ 27). As HSCs are derived from arterial endothelium, we next examined previously characterized endothelial hif1α targets,40 such as vegf, its receptors, flk1 and flt1, following glucose exposure and found that the majority were similarly upregulated (Figure 7B). Additional hematopoietic regulators linked with Hif activity41 were also assayed for developmental responses to glucose and each, with the exception of cxcr4, showed upregulation, particularly at 36 hpf during HSC budding and proliferation (Figure 7B). In chemical/genetic epistasis analysis for a cohort of glucose-upregulated genes, hif1a-MO knockdown reduced gene expression and blunted the effect of glucose (Figure 7C). To confirm that this glucose-ROS-hif1α gene regulatory cascade affected HSCs, we performed a knockdown-rescue experiment with the classical Hif-target VEGF: exogenous human VEGF (10μM) increased runx1/cmyb+ expression and ameliorated hif1a MO-mediated reductions (Figure 7D). Conversely, MO-mediated knockdown of vhl that increased HSCs was blunted by SU1498, a VEGFR inhibitor (Figure 7E). However, while the vegfaa-MO likewise decreased runx1/cmyb expression, it failed to completely block the effect of glucose (supplemental Figure 7G), suggesting that it is coordinate upregulation of several hematopoietic factors by hif1α that impacts HSC development downstream of glucose metabolism (supplemental Figure 6A-C). Significantly, in the presence of increasing glucose concentrations, the expression of glucose/hif1α-responsive genes dose-dependently increased (Figure 7F-G). Together, these experiments show hif1α coordinates the heightened metabolic response to elevated glucose levels with enhanced production of hematopoietic cells through the combined modulation of relevant target pathways active in the AGM.
Downstream targets of hif1α are temporally regulated to modulate HSC induction to match nutrient availability. (A) Western blot analysis of whole-embryo homogenates showed increased hif1α levels (top band) following exposure to glucose or pyruvate compared with untreated controls. β-actin is shown as a loading control (bottom band). Normalized quantification is below. (B) Analysis of temporal variation of gene expression from 18 to 36 hpf in glucose-treated embryos normalized pairwise to controls at each time point revealed enhanced induction of erythropoietic genes at early time points, and later induction of genes involved in definitive HSC development in response to glucose (t test vs 18 hpf, *P < .05, n = 3). (C) qPCR analysis indicated that functional hif1a is required to mediate the full effect of glucose exposure on hematopoietic-relevant targets (t test, * vs control, ** vs glucose, P < .05, n = 3). (D-E) Epistasis analysis demonstrated that (D) exogenous VEGF (10μM) could partially rescue a hif1a-MO knockdown on HSCs. (E) Elevations in runx1/cmyb expression mediated by loss of the negative hif1α regulator vhl by MO knockdown can be partially blocked if VEGF signaling is inhibited using SU1498 (10μM, n > 15/tx). (F) qPCR analysis following exposure to increasing glucose concentrations (0.5%-4%) in the fish water revealed a dose-dependent increase in genes involved in vascular and erythroid formation (ANOVA, P < .05, n = 3) at 24 hpf. (G) qPCR at 36 hpf following exposure to increasing glucose concentrations demonstrated increased expression of runx1, cmyb, and hif1α targets associated with definitive hematopoiesis (nos2, igf2, pdgf; ANOVA, P < .05, n = 3).
Downstream targets of hif1α are temporally regulated to modulate HSC induction to match nutrient availability. (A) Western blot analysis of whole-embryo homogenates showed increased hif1α levels (top band) following exposure to glucose or pyruvate compared with untreated controls. β-actin is shown as a loading control (bottom band). Normalized quantification is below. (B) Analysis of temporal variation of gene expression from 18 to 36 hpf in glucose-treated embryos normalized pairwise to controls at each time point revealed enhanced induction of erythropoietic genes at early time points, and later induction of genes involved in definitive HSC development in response to glucose (t test vs 18 hpf, *P < .05, n = 3). (C) qPCR analysis indicated that functional hif1a is required to mediate the full effect of glucose exposure on hematopoietic-relevant targets (t test, * vs control, ** vs glucose, P < .05, n = 3). (D-E) Epistasis analysis demonstrated that (D) exogenous VEGF (10μM) could partially rescue a hif1a-MO knockdown on HSCs. (E) Elevations in runx1/cmyb expression mediated by loss of the negative hif1α regulator vhl by MO knockdown can be partially blocked if VEGF signaling is inhibited using SU1498 (10μM, n > 15/tx). (F) qPCR analysis following exposure to increasing glucose concentrations (0.5%-4%) in the fish water revealed a dose-dependent increase in genes involved in vascular and erythroid formation (ANOVA, P < .05, n = 3) at 24 hpf. (G) qPCR at 36 hpf following exposure to increasing glucose concentrations demonstrated increased expression of runx1, cmyb, and hif1α targets associated with definitive hematopoiesis (nos2, igf2, pdgf; ANOVA, P < .05, n = 3).
Discussion
During vertebrate embryogenesis, the cardiovascular and hematopoietic systems are the first to be fully functional, and developmental impairments are associated with embryonic lethality. Although extensive research has identified pathways controlling HSC production, the mechanisms integrating these factors to precisely regulate timing, localization, and magnitude of de novo HSC formation and expansion are not well understood. Here, we identified glucose metabolism as the inductive trigger, and hif1α as a fundamental integrator of nutrient availability and hematopoietic production via sensing of metabolism-mediated ROS production. Our results suggest that beyond its role in detecting oxygenation status and adjusting consumption and cellular activity, hif1α is the primary rheostat of nutrient levels and resulting proliferative state of the vertebrate embryo. Coordinating glucose metabolism to HSC production via hif1α not only allows appropriate responses to environmental deficits, but also equally ensures adequate supply of blood to prepare the developing organism to endure fluctuations in biological demands during accelerated tissue growth in congruence with heightened metabolic input.
While embryonic development is impaired by deprivations of either oxygen or nutrient levels, several clinical studies highlight potential therapeutic implications of our investigations. In newborns, abnormally high birth weight is most commonly associated with gestational diabetes and caused by persistently elevated glucose levels in placental circulation.42 Sustained glucose elevations during embryogenesis have potential far-reaching consequences for the hematopoietic system: meta-analysis of 18 clinical studies revealed that for each kilogram gain in birth weight, the ALL risk increases 14% and the AML risk increases 29%.15 Furthermore, a birth weight of >4000 g doubled leukemia risk in children under 2 years of age,14 ALL incidence was elevated in diabetic children,16 and mortality risk in patients with AML coordinately increases with glucose levels.43 Similarly, HIF1α dysregulation due to VHL loss is associated with pathological overproduction of erythroid cells in zebrafish, mice, and humans.44,45 In this study, we focused on short-term consequences of increased glucose exposure on hematopoietic induction and function: physiologically relevant glucose elevation led to significantly elevated HSC production without short-term disturbances in function. However, while not yet examined in prospective studies, one could speculate that sustained glucose elevations, or accompanying increases in insulin, may lead to gross HSC hyperproliferation, influencing replication fidelity and differentiation capacity toward leukemogenesis.
The direct physiological consequences of fluctuating energy supply and metabolism during the early phases of embryonic development have not been studied in detail. Prior to implantation and establishment of placental oxygen and nutrient exchange, the mammalian embryo is relatively hypoxic and nutrient-starved, receiving dietary supply from extraembryonic milieu via passive diffusion. Similarly, zebrafish embryos depend on passive diffusion of maternally derived yolk stores for embryonic growth prior to establishment of circulation. In mice, 14C-labeling studies revealed glucose as the major source of energy via glycolysis from e6 to e946 ; aerobic energy production increases exponentially in conjunction with establishment of unidirectional circulation at e9.5.47 Zebrafish studies reveal that endogenous glucose levels increase within a similar embryonic time window,32 indicating that progressive depletion of maternal energy stores and a shift toward glucose-dependent energy metabolism is coincident with the timing of de novo HSC production in vertebrates. The data presented here imply that this increase in metabolic activity is an intrinsic component controlling hematopoietic production, including HSC induction, providing not only the energy to “bud,” proliferate, and eventually migrate, but also to sustain transcription of required hematopoietic programs through hif1α. These results may at first appear to be in contrast to investigations of the bone marrow niche, where Hif is directly responsible for maintenance of HSC quiescence12 and ROS is considered a DNA-damaging danger.48 However, more recent investigations have demonstrated requirements for ROS in recovery after marrow injury49 and during expansion of myeloid precursors.50 Together, these studies show Hif1α-mediated responses to environmental stimuli are a fundamental mechanism of HSC regulation throughout organismal lifetime.
The rationale for the initial induction of definitive HSCs within the AGM and continuous shift in sites of hematopoiesis during development remains elusive. We hypothesize that embryonic hematopoietic niches correlate with activated HIF1α status and nutrient-rich environments. In de novo sites of HSC production, such as hemogenic endothelium, true hypoxia (low O2) may serve as one of several factors that stimulate the onset of the hematopoietic program. However, following the initiation of convective flow or gluconeogenesis, stabilization of HIF1α-transcriptional activity via metabolism/ROS-mediated “hypoxic response” allows for simultaneous maintenance of hematopoietic gene programs and enhanced energy production for proliferation. The conservation of the aortic endothelium as the first site of definitive HSC induction supports this model: hemogenic endothelium from which HSCs are derived is situated centrally in the developing vertebrate embryo, during the time window when oxygen and nutrient delivery by diffusion become growth-limiting. The HIF complex with its well-characterized role in regulating oxygen consumption19 and hematovascular expression in vitro is ideally suited to detect this deficit to modify the initiation and maintenance of hematopoietic gene programs. Significant developmental delay and embryonic lethality prevent definitive conclusions concerning the requirement for Hif1α in HSC formation in mammalian models; here, through chemical and genetic modulation, we demonstrated hif1α is essential to embryonic HSC induction, allowing the embryo to respond dynamically to environmental changes, ensuring postnatal viability.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.ll
Acknowledgments
The authors thank P. Liu and R. Sood (National Institutes of Health, Bethesda, MD) for the runx1 mutant line, P. Crosier and K. Crosier (University of Auckland, Auckland, New Zealand), for runx1P1:eGFP transgenic fish, and L. I. Zon (Children’s Hospital, Boston, MA) for globin:eGFP transgenic fish. Electron microscopy was performed with support from S. Hagen and A. Calhoun (Beth Israel Deaconess Medical Center [BIDMC]/Harvard Digestive Disease Center Imaging Facility, Boston, MA). The authors thank P. Li (Children’s Hospital, Boston, MA) for zebrafish transplantation advice. FACS and microarray analyses were conducted with support of the BIDMC/Harvard Stem Cell Institute (HSCI) FACS facility and the Children’s Hospital Genomics core facilities.
This work was supported by the Harvard Stem Cell Institute Blood Program (T.E.N.) and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5K01DK080226 (T.E.N.) and 1R01DK090311 (W.G.).
Authorship
Contribution: J.M.H., V.E., G.M.F., L.J.H., K.J.C., and C.C.C. performed embryo exposures, FACS, qPCR, and in situ hybridizations; J.M.H. and A.G.C. conducted ROS and fluorescence experiments; M.K.G. performed MO injections and electron microscopy; M.C. conducted western blot and glucose-content experiments; T.K. and M.G.V.H. completed glucose-uptake studies; P.M.E., S.A.R., B.C.D., C.J.C., M.P.M., and B.H.P. provided probes, reagents, or zebrafish mutants; J.M.H., V.E., W.G., and T.E.N. designed experiments and evaluated results; W.G. and T.E.N. wrote the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Goessling, MD, PhD, Genetics Division, Brigham and Women’s Hospital, NRB458, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: wgoessling@partners.org; and Trista E. North, PhD, Department of Pathology, Beth Israel Deaconess Medical Center, CLS 638, 3 Blackfan Circle, Boston, MA 02115; e-mail: tnorth@bidmc.harvard.edu.
References
Author notes
J.M.H. and V.E. contributed equally.
G.M.F. and L.J.H. contributed equally.
W.G. and T.E.N. contributed equally.

![Figure 1. Glucose enhances HSC formation in the zebrafish AGM region. Zebrafish were exposed to 1% glucose from 10 somites to 36 hpf and analyzed at 36 hpf. (A) D-glucose enhanced runx1/cmyb expression by in situ hybridization (560 increased/652 scored [85.9%↑]). The metabolically inactive enantiomer, L-glucose, had no effect (n > 100 embryos/treatment [tx]). Top panels show whole embryos, while bottom panels depict AGM regions. Numbers in lower right of panels, here and following, indicate embryos with altered HSC expression (as depicted) over the total number scored. (B) Glucose exposure increased HSC number as determined by in vivo fluorescent microscopy of runx1:eGFP, cmyb:eGFP, and CD41:eGFP transgenic reporter embryos (n = 60/tx). (C) Quantification of fluorescent cell number by FACS analysis of reporter embryos supported the effects of observed by microscopy (t test, runx1: *P < .0001; cmyb/lmo2: **P < .05; CD41: ***P < .01, n = 6-9). (D) qPCR confirmed significantly enhanced expression of HSC markers following glucose exposure (20 embryos/cohort, t test, runx1, cmyb: *P < .0001; CD41: **P < .05, n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/13/10.1182_blood-2012-12-471201/4/m_2483f1.jpeg?Expires=1768006675&Signature=3NL6WnT4o4w2sxk4F0AcKzSKLOKXm6pVJFCV9U3fJSJK91qTlEfjYIE7qFnCfWHBLPhgKlwnYdLewTgWHP8ZOVZCCMFsO7Lq9es8B8xt9e1GzULxyMyItJE9jw1HKUsO-habrtuHDdokvw1J1E1QAWMe2XUpg3DhTw2xA9-~RnuC4hasZUsDyAUqmQR-s1xv-abUyraNchAlau4iwKY5kH1RRM~QSCbsi93waYdy~3jCtF4D0LungECMcheQQ8r4OjrFvT9D77BP-eSmPitF1ftRRbMSh7Pbdaiby2~YX3WDO-x-iLvLApP5FrjONtNwhBj49RpFEdg88kmQf6TBnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. The effect of glucose is dependent on uptake rather than insulin signaling. (A) Levels of ATP, pyruvate, and lactate in whole-embryo lysates increased significantly in response to glucose (t test, ATP, pyruvate: *P < .02; lactate: **P < .05, n = 3). (B) Measurement of total embryo glucose content by enzymatic assay revealed significantly elevated glucose levels at 36 hpf after exposure to 1% glucose in the fish water from 12 to 36 hpf, which declined back to baseline by 72 hpf (analysis of variance [ANOVA], *P < .05, n = 3). (C) Glucose uptake, measured by radioisotope labeling, was significantly enhanced in the embryo after 60 minutes of exposure in the fish water (ANOVA, P = .012, n = 3). (D) Confocal microscopy of lmo2:dsRed transgenic reporter embryos at 36 hpf following exposure to 2-NBDG reveals green fluorescent glucose signal in the zebrafish aorta (arrowhead), colocalized with an increased number of lmo2+ hematopoietic cells; white dotted lines indicate the trunk of the embryo, the dashed line demarks the yolk (below) from the embryo proper (above). The inset shows GFP levels in untreated fish at the same signal amplification as 2-NBDG–treated samples (n = 5/tx). (E) FACS analysis reveals significant and selective uptake of the fluorescent glucose analog 2-NBDG (green fluorescence) by hematopoietic and endothelial lmo2+ cells (t test, *P < .05, n = 10). (F) MO knockdown of glut1 (40μM) inhibited the effect of glucose on runx1/cmyb+ HSCs. insr (40μM) knockdown did not affect runx1/cmyb expression in the presence or absence of glucose (17 normal/22). (G) Glucose exposure did not affect insulin expression prior to islet formation at 36 hpf (36 normal/36).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/13/10.1182_blood-2012-12-471201/4/m_2483f3.jpeg?Expires=1768006675&Signature=LTAsDoTvYzl68cIcKB-SUnILaEpVwi~eYTG87vc52KxDhSzJ8nhB~AwhsP2m736wO6bvQRbDnMAeEqlQny93CypTqdfqPEjsbZBQ8hfo4c35bQUdpwAtxFAOUTFajJYQ9d-9Rq75JJt0zGR9S~ihfdMCzxaLIv6mj6S4G3HXBymOBqK4AMLrhL4Y6wuCtKi2t~2Uqf7qbOJPCfMQOG7xNADSrCM-sRlwlX4sl-KiZc161CvDUfB3QipAx3z9wTIOAPdWFmNmq6LfHHyvHwenRdR1FYDCNHQWa~L92r6cbJE6F79iRDC~UDGC-Tq3vnGRYXplBGuZi7P-Mu-35PktXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)