Key Points
Using a sensitive method, the MYD88 (L265P) mutation is detectable in all patients with Waldenström’s macroglobulinemia, therefore representing a hallmark of the disease.
MYD88 (L265P) is also found in a substantial proportion of patients with IgM-MGUS.
Abstract
A study has shown that MYD88 (L265P) is a recurring somatic mutation in Waldenström's macroglobulinemia (WM). We developed an allele-specific polymerase chain reaction (PCR) for this mutation, and analyzed bone marrow or peripheral blood samples from 58 patients with WM, 77 with IgM monoclonal gammopathy of undetermined significance (IgM-MGUS), 84 with splenic marginal zone lymphoma (SMZL), and 52 with B-cell chronic lymphoproliferative disorders (B-CLPD). MYD88 (L265P) was detected in 58/58 (100%) patients with WM, 36/77 (47%) with IgM-MGUS, 5/84 (6%) with SMZL, and 3/52 (4%) with B-CLPD. Compared to IgM-MGUS patients with wild-type MYD88, those carrying MYD88 (L265P) showed significantly higher levels of IgM (P < .0001) and presented Bence-Jones proteinuria more frequently at diagnosis (P = .002). During follow-up, 9 patients with IgM-MGUS progressed to WM or to marginal zone lymphoma. Using a case-control approach, the risk of evolution of patients carrying MYD88 (L265P) was significantly higher than that of patients with wild-type MYD88 (odds ratio 4.7, 95% confidence interval 0.8 to 48.7, P = .047). These findings indicate that the allele-specific PCR we developed is a useful diagnostic tool for patients with WM or IgM-MGUS. In this latter condition, MYD88 (L265P) is associated with greater disease burden and higher risk of disease progression, and the mutation may therefore also represent a useful prognostic marker.
Introduction
Waldenström’s macroglobulinemia (WM) is a rare lymphoproliferative disorder characterized by the presence of a serum IgM paraprotein associated with bone-marrow (BM) infiltration by lymphoplasmacytic lymphoma (LPL).1 Familial aggregation of WM and related B-cell disorders suggest a role for genetic factors in the pathogenesis of the disease.2-4
In recent years, the genetic basis of cancer has been better understood by means of massive parallel sequencing of tumor and normal cells aimed at identifying recurrent somatic mutations.5 The application of next-generation sequencing technologies in lymphoproliferative disorders led to the identification of several disease-associated mutations, providing useful insights into their pathogenesis, and offering new approaches to diagnosis as well as potential therapeutic targets.6-12
Whole genome sequencing in WM allowed the identification of a somatic variant (T→C) at position 38182641 in chromosome 3p22.2 that results in an amino-acid change from leucine to proline (L265P) in the MYD88 gene.13 Sanger sequencing confirmed the presence of the MYD88 (L265P) mutation in tumor samples from 49/54 WM and 3/3 non-IgM secreting LPL patients. MYD88 (L265P) was absent in normal paired tissues from WM/LPL patients and in healthy donor B cells, and it was absent or rarely expressed in samples from multiple myeloma, marginal zone lymphoma (MZL) or IgM-monoclonal gammopathy of undetermined significance (IgM-MGUS) patients. A heterozygous mutation of exon 5 in the MYD88 gene has been previously reported in a substantial proportion of activated B-cell-like subtype diffuse large B-cell lymphomas,14 as well as in primary cutaneous and primary central nervous system diffuse large B-cell lymphomas15,16 and in a minority of cases of chronic lymphocytic leukemia.6,17
The single amino-acid mutation in the MYD88 gene affects the Toll/IL-1 receptor domain of adaptor protein MYD88, and it favors tumor-cell survival through IRAK1 (interleukin-1 receptor associated kinase)/IRAK4, and NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) signaling.14 The latter is a protein complex that controls inflammation, hematopoiesis, and normal lymphocyte and plasma-cell development. Its cytoplasmic form is usually inactive because of the binding of inhibitor molecules. Activated NF-κB enters the nucleus and leads to the transcription of multiple prosurvival genes, blocks apoptosis, and possibly promotes tumor development.18
We developed an allele-specific polymerase chain reaction (AS-PCR) for the MYD88 (L265P) mutation and analyzed a large series of patients with WM, IgM-MGUS, splenic marginal zone lymphoma (SMZL), and B-cell chronic lymphoproliferative disorders (B-CLPD) with the goals of: (1) assessing the prevalence of the mutation in WM, IgM-MGUS, SMZL, and B-CLPD; (2) analyzing the relationship between MYD88 (L265P) mutation and the clinical phenotype; (3) evaluating the impact of the mutation on the risk of progression from IgM-MGUS to WM or to other lymphoproliferative disorders.
Patients and methods
Patients
The Ethics Committee of the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, approved these investigations. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000, and samples were obtained after subjects provided informed consent.
We studied 58 patients with WM, 77 with IgM-MGUS, 84 with SMZL, and 52 with B-CLPD. None of the cases analyzed in this study was included in the original report on the discovery of MYD88 mutation in WM.13 The diagnosis of WM and IgM-MGUS was made according to the consensus recommendations from the Second International Workshop on WM.1 In detail, patients with unequivocal BM infiltration by LPL were classified as WM, whereas patients without evidence of infiltration were classified as MGUS. Patients with equivocal evidence of BM infiltration represented by detection of clonal B cells by flow cytometry in the absence of morphologic evidence of BM infiltration, or alternatively by equivocal BM infiltrates without confirmatory phenotypic studies, were considered to have MGUS. Diagnosis of SMZL was based on morphologic and immunohistochemical findings on spleens in 20 patients and on BM biopsies coupled with flow cytometry profile on BM aspirate in 64 patients, according to the WHO 2008 classification19 and criteria defined by Matutes et al.20 Fifty-two patients were diagnosed with B-CLPD in the absence of specific phenotypic and/or genetic alterations21,22 ; in particular, a diagnosis of chronic lymphocytic leukemia or a leukemic phase of mantle cell lymphoma or follicular lymphoma was ruled out in each subject.
DNA extraction
Genomic DNA was extracted from BM mononuclear cells in 194 cases (51 WM, 74 IgM-MGUS, 27 B-CLPD, 42 SMZL) and from peripheral blood mononuclear cells in 74 cases (7 WM, 25 B-CLPD, 42 SMZL) using Puregene Blood DNA isolation kit (Qiagen, Milan, Italy) according to the manufacturer’s recommendations. In 3 cases of IgM-MGUS, genomic DNA was retrieved from archival Giemsa-stained BM slides using QuickGene DNA tissue kit S (DT-S) (Life Science) on FUJIFILM QuickGene-810 extraction platform (Fujifilm, Kyoto, Japan) according to the manufacturer's instructions.
MYD88 (L265P) sequencing and AS-PCR
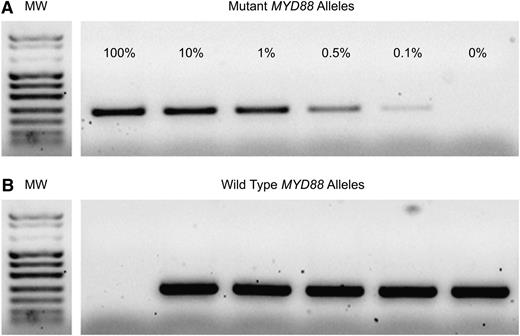
MYD88 gene exon 5 sequencing was performed to identify positive controls (c.794T>C, COSM85940) for AS-PCR. Two primers (forward, 5′-GTTGAAGACTGGGCTTGTCC-3′; reverse, 5′-AGGAGGCAGGGCAGAAGTA-3′) were used to amplify a 292 bp fragment. PCR was performed in a final volume of 50 µL containing 100 ng genomic DNA, 1× reaction buffer, 0.2 µM of each primer, 200 µM dNTPs, 2 mM MgCl2, and 2.5 U of HotStarTaq (Qiagen, Milan, Italy). PCR consisted of an initial denaturation step of 15 minutes at 94°C, followed by 35 cycles of 94°C for 30 seconds, 59°C for 30 seconds and 72°C for 60 seconds, with a final extension step of 10 minutes at 72°C. The PCR products were purified and sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit and an ABI 3500 automatic sequencer (Applied Biosystems, Foster City, CA). AS-PCR was performed to amplify the wild-type allele or MYD88 T794C transition mutation. Two different forward primers with a single base substitution at the end of the primer (FW5′-GTGCCCATCAGAAGCGcCT-3′ and FM5′-GTGCCCATCAGAAGCGcCC-3′) were designed. To prevent the amplification of the nonmatching primer, an additional nucleotide mismatch (A>C) was incorporated 3 bases from the 3′ termini of the allele-specific primers. The sequence of the reverse primer was the same as was used for sequencing. Mutated or wild-type sequences from peripheral blood or BM samples were specifically amplified in a noncompetitive PCR as described previously. To obtain a more specific PCR product, cycling conditions were adjusted using 65°C as the melting temperature. Healthy donors were used as negative controls. The analytical sensitivity of AS-PCR was assessed by PCR analysis of samples obtained by serial dilutions of the mutant allele in wild-type genomic DNA. AS-PCR was capable to detect 0.1% of the mutant alleles, corresponding to 10 MYD88 T794C homozygous cells (Figure 1).
MYD88 AS-PCR sensitivity assay. Serial dilutions of mutated and wild-type alleles (from 100% to 0% mutated alleles) were performed to identify the analytical sensitivity of AS-PCR. (A) Sensitivity of the method was ≥0.1%. (B) Amplification of the wild-type allele. All samples give rise to the wild-type MYD88 band, except for the 100% mutant allele sample.
MYD88 AS-PCR sensitivity assay. Serial dilutions of mutated and wild-type alleles (from 100% to 0% mutated alleles) were performed to identify the analytical sensitivity of AS-PCR. (A) Sensitivity of the method was ≥0.1%. (B) Amplification of the wild-type allele. All samples give rise to the wild-type MYD88 band, except for the 100% mutant allele sample.
DNA extracted from archival Giemsa-stained BM slides was amplified in a final volume of 50 uL containing 2 ul DNA, 1 uM of each primer, 200 uM dNTPs, 1.5 mM of MgCl2, and 1 U of AmpliTaq Gold DNA polymerase, with the reaction buffer supplied by the manufacturer (Applied Biosystems). PCR consisted of an initial denaturation step of 8 minutes at 95°C, followed by 40 cycles of 95°C for 80 seconds, 61°C for 80 seconds, and 72°C for 80 seconds, with a final extension step of 7 minutes at 72°C. All PCR were performed in duplicate. All cases analyzed with AS-PCR carrying the MYD88 (L265P) mutation were sequenced as reported previously.
Immunoglobulin heavy variable gene rearrangement
Amplification of immunoglobulin heavy chain (IGH) genes rearrangements was performed as previously described.23 All IGHV-D-J rearrangements were analyzed using the IMGT database and the IMGT/V-QUEST tool.
Statistical analysis
Numerical variables were summarized by median and range; categorical variables were described with count and relative frequency (%) of subjects in each category. Comparison of numerical variables between groups was performed with the nonparametric Wilcoxon rank sum test. Comparison of categorical variables was performed with the two-tailed Fisher exact test. Given the small number of observed evolutions, the risk of evolution was estimated with a case-control approach. Analyses were performed using Microsoft Excel and Stata 11.2 (StataCorp LP, College Station, TX) software.
Results
MYD88 mutation status in Waldenström’s Macroglobulinemia and IgM-MGUS
The clinical characteristics of 58 WM (39 asymptomatic and 19 symptomatic) and 77 IgM-MGUS patients are reported in Tables 1 and 2, respectively.
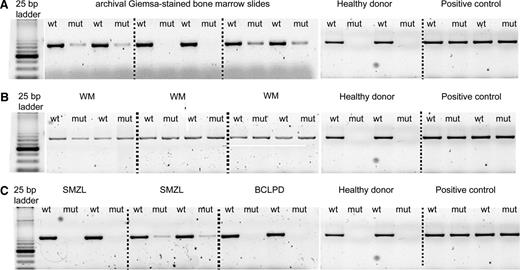
Using AS-PCR, the MYD88 (L265P) mutation was detected in 58/58 (100%) patients with WM, and in 36/77 (47%) patients with IgM-MGUS (Figure 2). Sanger sequencing detected the mutation in 25/58 (43%) patients with WM and in 3/36 (8%) patients with IgM-MGUS.
AS-PCR assay. PCR products were separated on 3% agarose gel electrophoresis, and for each sample, the wild-type (WT) and the mutant (MUT) allele were amplified. (A) Archival Giemsa-stained BM slides samples. (B) WM mutated cases presenting both the wild-type and the mutant allele. (C) Mutated and wild-type cases of SMZL and B-CLPD. A WM sample with MYD88 (L265P) mutation was used as a positive control, and a healthy donor was used as a negative control.
AS-PCR assay. PCR products were separated on 3% agarose gel electrophoresis, and for each sample, the wild-type (WT) and the mutant (MUT) allele were amplified. (A) Archival Giemsa-stained BM slides samples. (B) WM mutated cases presenting both the wild-type and the mutant allele. (C) Mutated and wild-type cases of SMZL and B-CLPD. A WM sample with MYD88 (L265P) mutation was used as a positive control, and a healthy donor was used as a negative control.
Twenty-one of 77 patients with IgM-MGUS experienced an equivocal BM infiltrate detected by immunohistochemistry without confirmatory phenotypic studies (median 5%, range 1 to 10). Nine of 21 (43%) carried the MYD88 (L265P) mutation and 12 (57%) were MYD88 wild-type. Therefore, the prevalence of the MYD88 (L265P) mutation in this subgroup of patients was similar to that observed in the whole cohort of patients.
Five of 77 patients (6%) with IgM-MGUS had a small clonal B-cell population detected by flow cytometry, without histologic evidence of BM infiltration. Three of these 5 carried the MYD88 (L265P) mutation.
The analysis of IGH rearrangement was available for 57/58 patients with WM and 71/77 patients with IgM-MGUS. A clonal IGHV-D-J rearrangement in WM and IgM-MGUS patients was demonstrated in 52/57 (91%) patients and in 48/71 (68%) patients, respectively. Among the 23 IgM-MGUS patients without a clonal IGH rearrangement, 7 carried the MYD88 (L265P) mutation and 16 were MYD88-wild-type by AS-PCR.
MYD88 mutation status in patients with splenic marginal zone lymphoma
The clinical features of the patients studied are reported in Table 3.
Using AS-PCR, the MYD88 (L265P) mutation was detected in 5/84 (6%) patients with SMZL (Figure 2). All these patients were positive also with Sanger sequencing. A serum immunofixation was available in 77/84 patients with SMZL. An IgM paraprotein was significantly more common in SMZL patients with the MYD88 (L265P) mutation (4/5, 80%) compared to those with MYD88 wild-type sequence (14/72, 19%) (P = .01). The other clinical features were similar in SMZL patients with or without the MYD88 mutation.
Histologic and immunophenotypical findings on BM biopsies and spleen tissue were overlapping in SMZL patients with or without the MYD88 (L265P) mutation, even if a more diffuse degree of plasmacytic differentiation (clumped chromatin, basophilic cytoplasm) was noticed in the former group.
MYD88 mutation status results in chronic B-cell lymphoproliferative disorders
The clinical features of these patients are reported in Table 3. By AS-PCR, the MYD88 (L265P) mutation was detected in 3/52 (6%) patients with B-CLPD (Figure 2). Sanger sequencing detected the mutation in one of these cases. Two of 3 B-CLPD patients carried a serum IgM monoclonal component, whereas the remaining B-CLPD patient carried a double (IgM and IgG) paraprotein. A serum IgM paraprotein was significantly more common in patients carrying the MYD88 (L265P) mutation (3/3, 100%) compared to those with MYD88 wild-type sequence (9/49, 18%) (P = .01)
In the 3 MYD88 (L265P)-positive B-CLPD, the BM showed an interstitial infiltrate of small to medium-sized cells, ranging from small lymphocytes to prolymphocytes, which were CD20+, CD5−/+ (1/3 cases), CD23−, CD10−, cyclinD1−, DBA.44−; no significant increase in plasma cells or mast cells was noticed.
Clinical phenotype and risk of evolution of IgM-MGUS according to the MYD88 (L265P) mutation status
Compared to IgM-MGUS patients with wild-type MYD88, those carrying the MYD88 (L265P) mutation showed significantly higher levels of IgM (P < .0001), lower levels of IgG (P = .04) and IgA (P = .04), and a higher incidence of Bence-Jones proteinuria at diagnosis (P = .002) (Table 2).
The median follow-up of IgM-MGUS patients was 12 months (range: 6 to 202). Nine of the patients experienced evolution to WM (7 cases) or to MZL (2 cases). Of the 7 patients who progressed to WM, 6 carried the MYD88 (L265P) mutation, and 1 was MYD88 wild-type at the time of diagnosis of MGUS. In this latter patient, the MYD88 (L265P) mutation was not detectable also at the time of progression. Of the 2 patients who progressed to MZL, 1 had the MYD88 (L265P) mutation, and 1 was MYD88 wild-type at diagnosis. Therefore, our data do not indicate a different pattern of evolution in IgM-MGUS patients with or without the MYD88 mutation. Using a case-control approach, the risk of evolution of patients with MYD88 (L265P) was significantly higher compared to that of patients with wild-type MYD88 sequence (OR 4.7, 95% confidence interval: 0.8 to 48.7, P = .04).
Discussion
In this study, we developed an AS-PCR for detecting the MYD88 (L265P) mutation, and employed it for analyzing 271 patients with WM, IgM-MGUS, or other mature B-cell neoplasms. In the original study that described the mutation,13 targeted Sanger resequencing detected MYD88 (L265P) in approximately 90% of patients with WM and in only 10% of those with IgM-MGUS. The higher sensitivity of AS-PCR compared with Sanger sequencing allowed us to detect the mutation in all patients with WM, either symptomatic or asymptomatic, indicating that MYD88 (L265P) is a hallmark of the disease.
In keeping with previous studies,13,24,25 we detected the mutation in 6% of patients with SMZL. Interestingly, most of these SMZL patients carrying MYD88 (L265P) had a serum IgM monoclonal component, compared with only 19% of those with wild-type MYD88 sequence. The differential diagnosis between WM and SMZL is often challenging because of their overlapping clinical characteristics.26 Immunophenotyping and molecular cytogenetics are useful to distinguish WM from SMZL.27,28 In addition, we previously demonstrated that BM histology of SMZL is characterized by sinusoidal infiltration and a more frequent nodular pattern, whereas WM usually has an interstitial BM localization.28 The findings of the present study indicate that the MYD88 (L265P) mutation can discriminate between WM and SMZL in the vast majority of cases. However, further studies are needed to characterize the minority of SMZL patients that carry MYD88 (L265P).
Patients with various lymphoproliferative disorders may have an IgM paraprotein, and these cases are difficult to distinguish from WM. We found the MYD88 (L265P) mutation in 6% of patients with B-CLPD, all carrying an IgM monoclonal component. Interestingly, only 18% of MYD88 wild-type B-CLPD patients had an IgM paraprotein. These clinical and molecular similarities suggest that at least some cases of B-CLPD might be considered within the spectrum of WM, indicating that a search of the MYD88 (L265P) mutation should be included in the diagnostic work-up of unclassifiable chronic B-cell proliferations.
WM is often preceded by an IgM-MGUS, which is characterized by the presence of an IgM monoclonal protein without evidence of BM infiltration by lymphoma.1 In this study, the MYD88 (L265P) mutation was identified by AS-PCR in nearly half the patients with IgM-MGUS. By comparison, using Sanger sequencing, we were able to detect the MYD88 (L265P) mutation in only 8% of these patients, in keeping with the original report that detected MYD88 (L265P) in 10% of subjects with IgM-MGUS.13 The presence of a clonal IgH rearrangement in nearly 70% of the IgM-MGUS samples analyzed indicates that an adequate number of clonal B cells were likely present in most of these samples.
Nearly a quarter of the IgM-MGUS patients showed an equivocal BM infiltrate by immunohistochemistry without confirmatory phenotypic studies. These patients were classified as IgM-MGUS according to the diagnostic criteria proposed at the Second International Workshop on WM. Langdren et al performed Sanger sequencing in 9 patients with IgM-MGUS and found the MYD88 (L265P) mutation in 5 of them (56%). All patients carrying the MYD88 (L265P) mutation exhibited either clonal plasma cells or clonal lymphocytes in their BM. The authors suggest that these cases represent a precursor to WM rather that a transformation from IgM-MGUS to WM.29
In our series of IgM-MGUS patients, the presence of the MYD88 (L265P) mutation was associated with greater disease burden at diagnosis, consisting of higher levels of IgM, deeper suppression of polyclonal IgG and IgA fractions, and higher incidence of Bence-Jones proteinuria. These observations might suggest that MYD88 (L265P) is not a founding mutation in IgM-MGUS but rather a genetic lesion associated with disease progression. Alternatively, we could hypothesize the existence of two distinct subtypes of IgM-MGUS, the first carrying the MYD88 (L265P) mutation from the onset and having a higher propensity to evolve to WM or other lymphoproliferative disorders, and the second without the mutation and with a more indolent course.
Finally, the interpretation of IgM-MGUS patients without a detectable MYD88 (L265P) mutation should also consider the sensitivity of the method, which may be unable to detect the mutation in the presence of very small B-cell clones. If this was the case, the percentage of IgM-MGUS patients carrying the MYD88 (L265P) mutation could be even higher using AS-PCR on sorted CD19+ B cells. However, CD19+ sorting is a cumbersome procedure that is not applicable in a routine diagnostic setting.
The risk of progression from IgM-MGUS to WM or to other lymphoproliferative disorders is approximately 1.5% per year.30 Thus far, there are not reliable prognostic models able to identify IgM-MGUS patients at risk of evolution. The size of the IgM paraprotein has been reported as a prognostic factor for progression in different studies, whereas the prognostic significance of hemoglobin level, lymphocytosis, and serum albumin level is more controversial.31-33 In the original report on the MYD88 (L265P) mutation, one of the two MYD88-positive IgM-MGUS patients experienced evolution to LPL. We performed a case-control study in IgM-MGUS patients and found that the MYD88 (L265P) mutation was associated with a significantly higher risk of progression to WM or to other lymphoproliferative disorders. Although the retrospective case-control design of the study precludes firm conclusions, our observation suggests that the MYD88 mutation may have prognostic significance in IgM-MGUS, and it supports prospective studies in this field.
In conclusion, the AS-PCR for MYD88 (L265P) mutation that we developed represents a useful tool that allows a clear-cut diagnosis of WM and its differentiation from other lymphoproliferative disorders with overlapping features. In IgM-MGUS, the MYD88 (L265P) mutation is associated with higher risk of progression and therefore may represent also a useful prognostic marker.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniela Capello for helpful discussions.
This work was supported by grants from AIRC (My First AIRC Grant 2011 #11415) and Fondazione IRCCS Policlinico San Matteo to L.A.
Authorship
Contribution: M.V., L.A., and M.C. designed the research; S.Z. developed the allele-specific PCR and performed molecular investigations; E.B., G.C., and M.P. reviewed histologic diagnosis; R.R. extracted DNA from bone marrow biopsies; S.R., A.C., E.O., M.B., M.G., S.M., V.F., L.M., and M.L.G. collected clinical data; C.P. performed statistical analysis. All authors critically revised the manuscript and approved the version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marzia Varettoni, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, 27100 Pavia, Italy; e-mail: m.varettoni@smatteo.pv.it; and Luca Arcaini, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, 27100 Pavia, Italy; e-mail: luca.arcaini@unipv.it.
References
Author notes
M.V. and L.A. equally contributed to this study.
There is an Inside Blood commentary on this article in this issue.