Key Points
Targeting of an anticlotting drug to activated platelets promises effective blood clot prevention without bleeding side effects.
Abstract
The ecto-nucleoside triphosphate diphosphohydrolase CD39 represents a promising antithrombotic therapeutic. It degrades adenosine 5′-diphosphate (ADP), a main platelet activating/recruiting agent. We hypothesized that delayed enrichment of CD39 on developing thrombi will allow for a low and safe systemic concentration and thus avoid bleeding. We use a single-chain antibody (scFv, specific for activated GPIIb/IIIa) for targeting CD39. This should allow delayed enrichment on growing thrombi but not on the initial sealing layer of platelets, which do not yet express activated GPIIb/IIIa. CD39 was recombinantly fused to an activated GPIIb/IIIa-specific scFv (targ-CD39) and a nonfunctional scFv (non–targ-CD39). Targ-CD39 was more effective at preventing ADP-induced platelet activation than non–targ-CD39. In a mouse carotid artery thrombosis model, non–targ-CD39, although protective against vessel occlusion, was associated with significant bleeding on tail transection. In contrast, targ-CD39 concentrated at the thrombus site; hence, a dose ∼10 times less of CD39 prevented vessel occlusion to a similar extent as high-dose non–targ-CD39, without prolonged bleeding time. An equimolar dose of non–targ-CD39 at this low concentration was ineffective at preventing vessel occlusion. Thus, delayed targeting of CD39 via scFv to activated platelets provides strong antithrombotic potency and yet prevents bleeding and thereby promotes CD39 toward clinical use.
Introduction
Mortality and morbidity of atherosclerosis is mainly caused by acute thrombotic events. Typically, the rupture of unstable atherosclerotic plaques results in adhesion and activation of platelets and the initial formation of a platelet layer. This layer of activated platelets releases dense granules containing large amounts of adenosine triphosphate, serotonin, and the potent platelet agonist adenosine 5′-diphosphate (ADP). The latter creates a microenvironment of high concentrations of ADP, which results in the amplification of platelet activation via a positive feedback mechanism (autocrine activation), as well as the recruitment and activation of additional platelets in proximity (paracrine activation). This “physiological” process, which is tailored to prevent blood loss at sites of vessel injury, can turn “pathological,” presumably caused by the strong proatherogenic surface exposed during plaque rupture. If platelet recruitment is not controlled sufficiently, more and more platelets will become activated and form aggregates, finally occluding the vessel lumen. This can result in ischemia and cell death (eg, as seen with myocardial infarction).1
Antithrombotic therapy is one of the most widely applied and most successful therapeutic interventions in modern medicine, aiming to prevent potentially deadly events. However, drugs preventing thrombosis have been a major cause of mortality and morbidity themselves because of their seemingly inherent risk to cause bleeding complications.2 Although new and more potent antithrombotic drugs are being developed, a gain in potency seems to be inherently linked to an increase in bleeding risk. This has been seen with antiplatelet drugs such as the ADP (P2Y12) receptor inhibitors clopidogrel, prasugrel, and ticagrelor. These drugs directly bind to the P2Y12 receptor and thereby inhibit primary ADP-induced platelet activation, as well as auto- and paracrine platelet activation.3 However, these drugs are also known to cause a significant increase in the rate of potentially detrimental bleeding complications.4-6
An alternative therapeutic strategy to decrease ADP-induced platelet activation is the administration of a soluble form of CD39 (solCD39), an ecto-nucleoside triphosphate diphosphohydrolase,7 which is constitutively expressed on endothelial cells and is described as a major physiological mechanism to maintain blood fluidity.8-11 CD39 works by hydrolyzing ADP rather than by inhibition of platelet ADP receptors.11 It has been shown that solCD39 administration strongly reduces ADP concentration and thereby prevents platelet activation and recruitment.12 Increased levels of CD39 have been demonstrated to be beneficial in animal models of stroke,13,14 myocardial infarction,14-16 renal and intestinal ischemia,17,18 thrombosis,19 pulmonary embolism,20 and more generally as a potent platelet inhibitor.13,14 Nevertheless, although very promising in its efficacy, CD39, as typical for the currently clinically used antithrombotic drugs, still has the tendency to cause significant concentration-dependent bleeding.10,21 Indeed, although CD39-overexpressing mice exhibit prolonged occlusion times,19 they demonstrate significant bleeding21 and are also more susceptible to bacterial infections.22
CD39 is expressed and highly functional on circulating microparticles.23 One of the potential mechanisms by which CD39 is involved in restricting thrombus growth is the accumulation of CD39-expressing microparticles in the growing clot.24,25 Because microparticles only adhere on activated platelets24,25 and the initial platelet layer typically consists of nonactivated platelets,26 a delay of CD39 accumulation during initial platelet adhesion provides a mechanism for initial hemostasis but subsequent restriction of further thrombus growth.27 We designed and tested a novel therapeutic CD39 construct that we hypothesized would mirror this naturally occurring delayed CD39 effect. Thereby, we aim for a strong antithrombotic effect via accumulation of CD39, but only once a sealing layer of platelets has formed. Therefore, major interference in hemostatic function and thus bleeding complications may be avoided. We demonstrate that activation of GPIIb/IIIa is delayed in thrombus formation and that a single-chain antibody (scFv) against the active conformation of GPIIb/IIIa represents a targeting tool for the delayed accumulation of CD39. In vitro and in vivo testing of this novel approach demonstrates strong antithrombotic potency without hemostatic disturbance. Therefore, we describe a novel antithrombotic approach, which demonstrates that the apparently inherent link between potency and bleeding complications can be overcome.
Materials and methods
Blood sampling in healthy human subjects
Blood was collected from healthy subjects who provided informed consent in accordance with the Declaration of Helsinki. All subjects were free of platelet-affecting drugs for ≥14 days. Blood sampling procedures were approved by the Ethics Committee of the Alfred Hospital, Melbourne, Australia.
Generation of CD39 constructs, production, expression, and purification
Details on scFvSCE5 and CD39 origins, the polymerase chain reaction (PCR)-based fusion, and the mammalian production and purification are provided in supplemental Methods on the Blood website.
Samples from purification steps were loaded on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions and visualized via Coomassie staining. The same samples were also stained on a western blot using an anti–Penta-His antibody coupled to horseradish peroxidase.
Determination of Vmax and Km for CD39 constructs
A malachite green phosphate assay kit from Gentaur was used to determine the enzymatic activity of targ-CD39, non–targ-CD39, and commercially available recombinant human CD39 (R&D Systems) by measuring the release of phosphate during the conversion of ADP to adenosine 5′-monophosphate (AMP). For every molecule of ADP that is converted into AMP, 1 molecule of phosphate is released. Proteins were incubated at 37°C with a series of ADP concentrations from 0 to 100 µM. The reactions were stopped at several time points from 0 to 120 minutes. The samples were measured at a wavelength of 650 nm on a Victor 3V Multi-label counter (PerkinElmer). A standard series of phosphate concentrations was used to convert raw data to the amount of AMP generated for each of the proteins. The amount of AMP generated vs time of incubation was then used to obtain the velocity of the reaction for each substrate (ADP) concentration. These velocity values were then graphed against the substrate (ADP) starting concentration to obtain Vmax and Km.
Preparation of platelet-rich, platelet-poor, and cell-free plasma
Citrated blood from volunteers was centrifuged at 180g for 10 minutes. The platelet-rich plasma (PRP) was collected and stored at 37°C. The remainder (infranatant) was centrifuged at 2500g for 10 minutes, and its supernatant was collected as platelet-poor plasma (PPP).
Flow cytometry
PRP was diluted 1:20 in phosphate-buffered saline (PBS; 100 mg/L calcium chloride, 100 mg/L magnesium chloride). To investigate the binding of scFv-CD39 constructs, the diluted PRP (45 µL) was either preincubated with a final concentration of 10 µM ADP or 5 µL of PBS for 15 minutes before addition of the constructs. The binding was then determined via a Penta-His Alexa Fluor-488–conjugated monoclonal antibody (Qiagen). To investigate the ecto-nucleoside triphosphate diphosphohydrolase efficiency, PRP was preincubated with scFv-CD39 constructs before administration of 20 µM ADP. Platelet activation status was measured by a phycoerythrin (PE)-labeled anti–P-selectin antibody (BD Bioscience). Samples were fixed using 1× Cellfix (BD Bioscience) and analyzed on a FACS Calibur (BD Bioscience).
Aggregometry
PRP and PPP were obtained by centrifugation of blood obtained from healthy volunteers. Aggregometry was performed using 600 rpm magnetic stirring on a light transmission aggregometer (AggRAMTM System; Helena Laboratories). PPP was used to calibrate for 100% aggregation and PRP for 0% aggregation. Targ-CD39 and non–targ-CD39 were preincubated in PRP (225 μL) for 3 minutes before being challenged with 20 µM ADP. Aggregometry was performed over 10 minutes after ADP addition. In the concentration-dependent series, PRP was preactivated with 2 µM ADP and incubated with scFv-CD39 constructs for 3 minutes before being incubated with 10 µM ADP, and then aggregometry was performed.
Flow chamber studies
Flow chamber studies were performed according to previously described methods.28 Microcapillary tubes (Vitrotubes; VitroCom) with a diameter of 0.2 × 2 mm were coated with collagen (20 μg/mL) overnight at 4°C and were then blocked with 1% bovine serum albumin for 1 hour at room temperature. The tubes were mounted on a microscope stage. PRP was flown through the tubes 1000 seconds−1 for 3 minutes in the flow chamber via a syringe pump (PhD 2000; Harvard Apparatus). ScFv-CD39 constructs were then flown through the capillary at 1000 seconds−1 for 3 minutes after the constructs were preincubated with an excess amount of anti–Penta-His Alexa Fluor-488 antibody for 10 minutes. All flow experiments were performed using an IX81 Olympus microscope. Platelet adhesion was visualized in real time using differential interference contrast microscopy.
Ex vivo and in vivo tests of scFv-CD39 in mice
Male C57BL/6 mice of 20 to 25 g weight were obtained from Alfred Medical Research and Education Precinct (AMREP) Animal Services. All experiments involving animals were approved by the Alfred Medical Research and Education Precinct animal ethics committee.
Animals were anesthetized with ketamine (50 mg/kg; Parnell Laboratories) and xylazine (10 mg/kg; Troy Laboratories). Body temperature was maintained at 37°C by placing mice on a heating mat to prevent hypothermia, which has been shown to activate platelets.29
Ex vivo mouse flow cytometry
Citrated blood from mice was collected through puncture of the inferior vena cava and diluted 1:50 in PBS (100 mg/L calcium chloride and 100 mg/L magnesium chloride). The diluted whole blood was incubated with 0.1 U/mL of thrombin to obtain partial platelet activation before the addition of the constructs. The activation of platelets was determined using an anti-human/mouse PE-labeled anti–P-selectin antibody (eBioscience), and binding of the constructs was determined via a Penta-His Alexa Fluor-488–conjugated monoclonal antibody. Samples were fixed using 1× Cellfix and analyzed on a FACS Calibur. During analysis, the gated platelet population was further separated into a nonactivated subpopulation and an activated subpopulation using the anti–P-selectin PE antibody. The subpopulations were then analyzed for scFv-CD39 construct binding using the anti–Penta-His Alexa Fluro-488 secondary monoclonal antibody.
Intravital microscopy
Intravital experiments were performed according to previously described methods.29 The mesentery was exteriorized through a midline abdominal incision. Mesenteric arteries, with an average size of 100 µm, were visualized with 40-fold magnification using an Olympus IX 71 microscope. A filter paper strip of 1 × 4 mm was immersed in a 7.5% ferric chloride solution for 3 seconds and then applied to the respective artery. After 4 minutes, the filter paper was removed, and the area of vessel injury was flushed with saline. The labeled scFv was administered through a jugular vein catheter at a concentration of 0.9 µg/g body weight. Photographs were taken ∼1 minute after flushing with saline and show a time point at which platelets began to attach to the injured site.
Carotid artery occlusion time model
An incision to reveal the left jugular vein was made to insert a catheter to facilitate injection of reagents. A further incision was made on the right neck to allow for careful dissection of the common right carotid artery from its connective tissue. As soon as the experimental reagents were injected, a 1 × 5-mm strip of filter paper soaked in 10% ferric chloride solution was inserted under the vessel for 3 minutes to cause an injury, which when left untreated will consistently occlude within 30 minutes. After the 3-minute injury, the filter paper was removed, the area was flushed with saline, and a Doppler flow probe (Transonic; 0.5 mm) was fitted around the site of the injury. Flow speed measurements were recorded, and time of occlusion for ≥1 minute was reported.
Tail bleeding time
Tail bleeding times were performed as previously described.30 An incision to reveal the left jugular vein was made to insert a catheter to facilitate scFv-CD39 injections. Three minutes after injection of constructs, the tail was transected 5 mm from the tip and immediately submersed in 37°C saline. Time until blood flow ceased for >1 minute was reported.
Results
Generation, purification, and enzymatic activity of the scFV-CD39 fusion molecules targ-CD39 and non–targ-CD39
To achieve targeting and enrichment of therapeutics on activated platelets, the scFv SCE5 was used. This scFv was previously generated by scFv phage display selecting for binding to activated GPIIb/IIIa and depleting for binding to nonactivated GPIIb/IIIa.31 ScFvSCE5, which provides the targeting specificity of the fusion protein, has been shown to bind specifically to activated platelets but not to nonactivated platelets.31
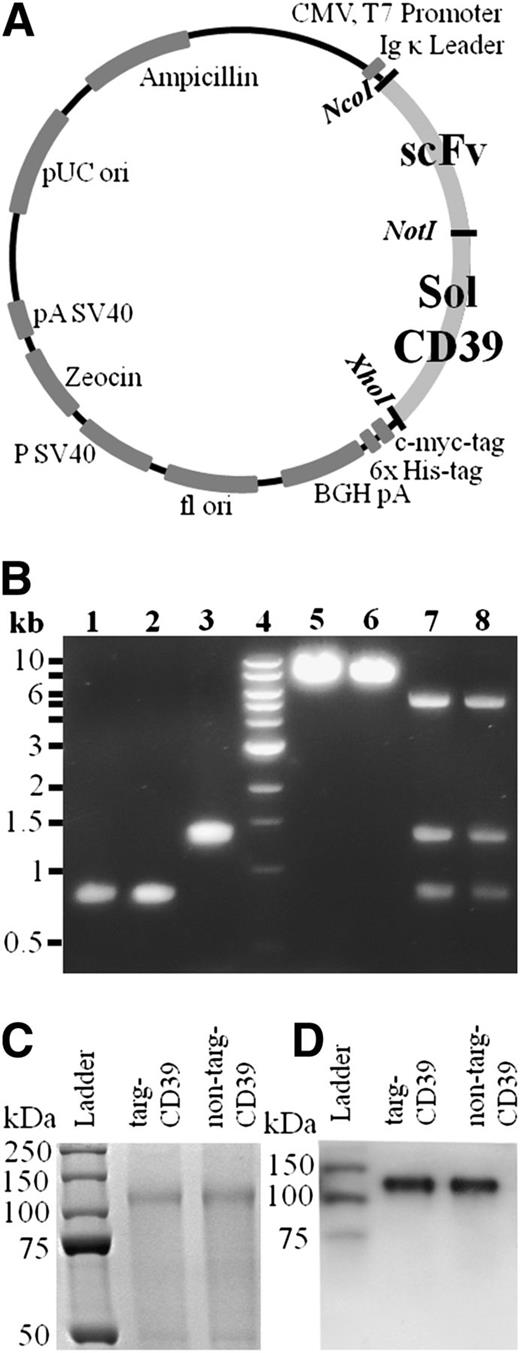
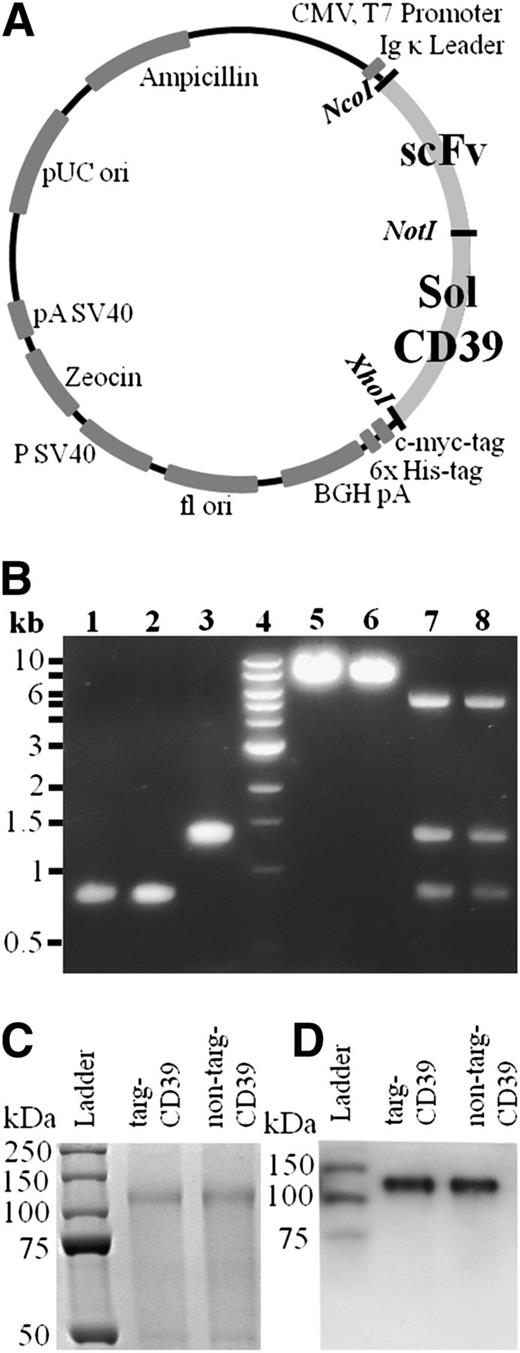
CD39 was modified via PCR using pcDNA3/CD3917,21,32 as a template to obtain a truncated form, which was flanked by NcoI and XhoI restriction enzyme recognition sites (Figure 1A), cut by these restriction enzymes, and then ligated into a vector containing the scFvs (SCE5 or a nonbinding scFv). After confirmation of successful cloning by control digests (Figure 1B) and PCR sequencing, the DNA was transfected into HEK293F cells and cultured for 8 days. Supernatant was purified using a nickel agarose column with strong affinity toward the 6xHis-tag, included on the C terminus of the scFv-CD39 constructs.
Vector map, generation, and purification of scFv-CD39 constructs. (A) Gene map of scFv-CD39 constructs in the pSectag2A vector for mammalian expression. The restriction enzymes used to insert the constructs are NotI, AscI, and XhoI. (B) Electrophoresis with 1% agarose gel. Lanes 1-3: molecular cloning of constructs using PCR amplification and double digest. (1) scFvSCE5, AscI and NotI (821 bp); (2) scFvMutMA2, AscI and NotI (821 bp); (3) solCD39, NotI and XhoI (1326 bp). Lane 4: DNA ladder. Lanes 5 and 6: single control digests of cloned constructs in pSectag2A. (5) targ-CD39, XhoI (7247 bp); (6) non–targ-CD39, XhoI (7247 bp). Lanes 7 and 8: triple control digests of cloned constructs in pSectag2A. (7) targ-CD39, AscI, NotI, and XhoI (821 bp for scFvSCE5, 1326 bp for solCD39, 5100 bp for pSectag2A); (8) non–targ-CD39, AscI, NotI, and XhoI (821 bp for scFvMutMA2, 1326 bp for solCD39, 5100 bp for pSectag2A) (C) 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis of scFv-CD39 visualized via Coomassie staining. (D) Western blot analysis of scFv-CD39s using a horseradish peroxidase–coupled anti–6xHis-tag antibody that binds to the constructs’ 6xHis-tags.
Vector map, generation, and purification of scFv-CD39 constructs. (A) Gene map of scFv-CD39 constructs in the pSectag2A vector for mammalian expression. The restriction enzymes used to insert the constructs are NotI, AscI, and XhoI. (B) Electrophoresis with 1% agarose gel. Lanes 1-3: molecular cloning of constructs using PCR amplification and double digest. (1) scFvSCE5, AscI and NotI (821 bp); (2) scFvMutMA2, AscI and NotI (821 bp); (3) solCD39, NotI and XhoI (1326 bp). Lane 4: DNA ladder. Lanes 5 and 6: single control digests of cloned constructs in pSectag2A. (5) targ-CD39, XhoI (7247 bp); (6) non–targ-CD39, XhoI (7247 bp). Lanes 7 and 8: triple control digests of cloned constructs in pSectag2A. (7) targ-CD39, AscI, NotI, and XhoI (821 bp for scFvSCE5, 1326 bp for solCD39, 5100 bp for pSectag2A); (8) non–targ-CD39, AscI, NotI, and XhoI (821 bp for scFvMutMA2, 1326 bp for solCD39, 5100 bp for pSectag2A) (C) 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis of scFv-CD39 visualized via Coomassie staining. (D) Western blot analysis of scFv-CD39s using a horseradish peroxidase–coupled anti–6xHis-tag antibody that binds to the constructs’ 6xHis-tags.
Protein purity was analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis via Coomassie staining (Figure 1C), and specificity of scFv-CD39 constructs was shown by western blotting via the use of an anti–Penta-His antibody coupled to horseradish peroxidase (Figure 1D).
To allow for comparison of the targeted and nontargeted construct, the enzymatic activity of CD39 was determined. Activity of the scFv-CD39 constructs was assessed via a bioluminescence-based assay, as well as a malachite green assay based on free phosphate measurement. The concentration of targ-CD39 and non–targ-CD39 were adjusted after purification until both the bioluminescence and the malachite green assays indicated that their activities were matched. This was achieved by doubling the concentration used of non–targ-CD39 in relation to targ-CD39 for all of the following assays. Commercially available CD39 with a known enzymatic activity was used as a comparison for the enzymatic activities of non–targ-CD39 and targ-CD39 constructs (supplemental Figure 1; supplemental Table 1).
In vitro proof of targeting of targ-CD39
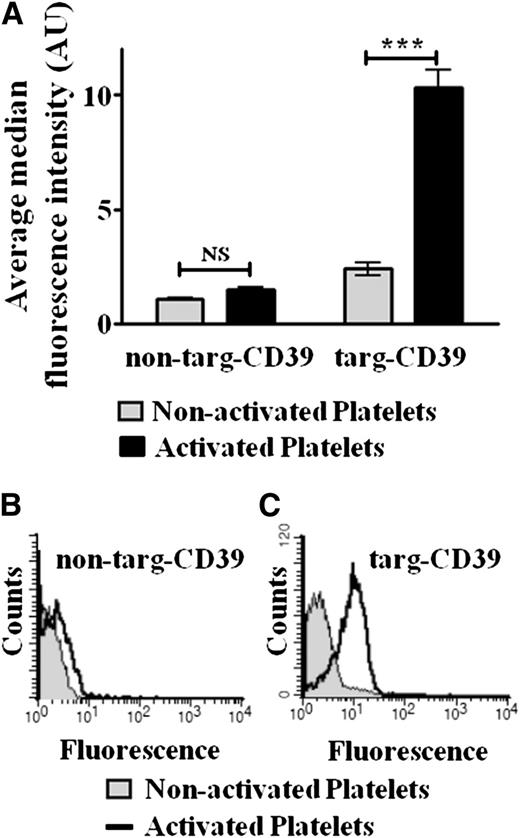
Binding of targ-CD39 to activated human platelets was evaluated by flow cytometry: 0.1 μg/mL of targ-CD39, 0.2 μg/mL of non–targ-CD39 (both activity matched), and 0.038 μg/mL of scFvSCE5 (equimolar amount of scFvSCE5 as in targ-CD39) were tested. Targ-CD39 binds selectively to activated platelets but not to nonactivated platelets (n = 3; **P < .01; Figure 2A,C). Flow cytometry also revealed significantly more binding of targ-CD39 to activated platelets compared with the non–targ-CD39 control (n = 3; ***P < .001; Figure 2A), which demonstrated only background binding to activated platelets. Neither targ-CD39 nor non–targ-CD39 showed significant binding to resting nonactivated platelets (Figure 2A). Non–targ-CD39 also does not show binding to activated platelets (Figure 2A-B).
Flow cytometric assay of scFv-CD39 binding to human platelets, detected with an Alexa Fluor 488–coupled anti–Penta-His antibody that binds to the constructs’ 6xHis-tag. (A) Quantitative comparison. Bar graphs depict the median fluorescence intensity values of 3 independent experiments (mean ± standard error of the mean; ***P < .001). These assays were analyzed with 2-way repeated-measures analysis of variance with the Bonferroni posttest. (B and C) Representative fluorescence histograms. Binding of (B) non–targ-CD39 and (C) targ-CD39 to activated platelets vs nonactivated platelets is shown.
Flow cytometric assay of scFv-CD39 binding to human platelets, detected with an Alexa Fluor 488–coupled anti–Penta-His antibody that binds to the constructs’ 6xHis-tag. (A) Quantitative comparison. Bar graphs depict the median fluorescence intensity values of 3 independent experiments (mean ± standard error of the mean; ***P < .001). These assays were analyzed with 2-way repeated-measures analysis of variance with the Bonferroni posttest. (B and C) Representative fluorescence histograms. Binding of (B) non–targ-CD39 and (C) targ-CD39 to activated platelets vs nonactivated platelets is shown.
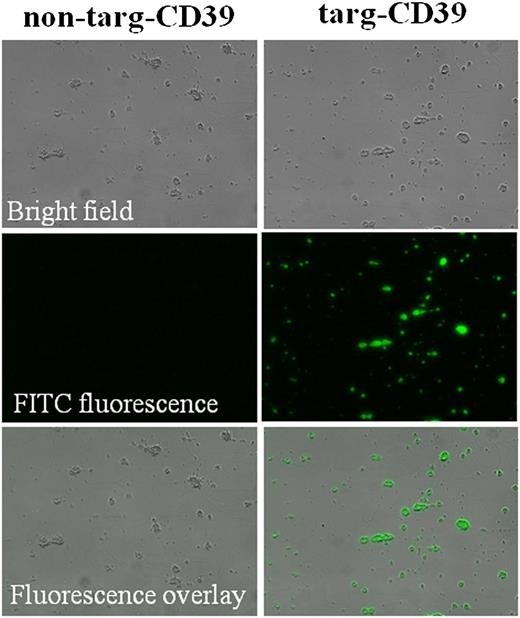
To confirm that targ-CD39 is able to bind to platelet-rich thrombi, ex vivo platelet microthrombi were created in a flow chamber setup, where platelets were flown over collagen fibrils, which results in platelet adhesion and consequent formation of adherent microthrombi. The scFv-CD39 constructs (targ-CD39 at 5 μg/mL and non–targ-CD39 at 10 μg/mL to be activity matched) were then added under physiological flow speed (1500 seconds−1) and visualized with an anti–Penta-His Alexa Fluor-488 antibody. Targ-CD39 shows specific binding to activated platelets on microthrombi, whereas non–targ-CD39 demonstrates no visible binding to platelet microthrombi (Figure 3).
Flow chamber adhesion assay investigating binding of non–targ-CD39 and targ-CD39 to microthrombi. Platelet microthrombi were generated on a collagen matrix. Non–targ-CD39 and targ-CD39 were flown over these microthrombi, and binding was assessed by fluorescence microscopy. The scFv-CD39 constructs were detected with an Alexa Fluor-488–coupled anti–Penta-His secondary monoclonal antibody that binds to the constructs’ 6xHis-tag.
Flow chamber adhesion assay investigating binding of non–targ-CD39 and targ-CD39 to microthrombi. Platelet microthrombi were generated on a collagen matrix. Non–targ-CD39 and targ-CD39 were flown over these microthrombi, and binding was assessed by fluorescence microscopy. The scFv-CD39 constructs were detected with an Alexa Fluor-488–coupled anti–Penta-His secondary monoclonal antibody that binds to the constructs’ 6xHis-tag.
In vitro antithrombotic effects of targ-CD39
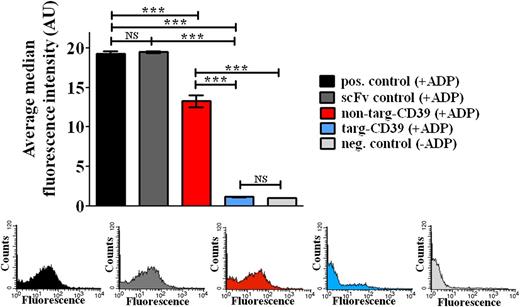
To analyze the antithrombotic efficacy of the targ-CD39 construct vs the non–targ-CD39 construct, we investigated their inhibitory effects on ADP-induced platelet activation. P-selectin expression assessed in flow cytometry was used as a measure of platelet activation: 0.1 μg/mL of targ-CD39 and 0.2 μg/mL of non–targ-CD39, both activity matched, were tested. Targ-CD39 demonstrated strong inhibition of ADP-induced platelet activation compared with non–targ-CD39 (n = 3; ***P < .001; Figure 4), although non–targ-CD39 also showed a small inhibitory effect (n = 3; *P < .05). Targ-CD39 is therefore shown to be significantly more potent at preventing platelet activation compared with non–targ-CD39. ScFvSCE5 (0.038 μg/mL of scFvSCE5, equimolar amount of scFvSCE5 as in targ-CD39) alone was not significantly different from the positive control.
Comparison of targ-CD39, non–targ-CD39, and scFvSCE5 alone in inhibition of platelet activation as assessed by P-selectin expression in flow cytometry. P-selectin expression was determined with a PE-labeled anti–P-selectin antibody. Bar graphs depict the median fluorescence intensity values of 3 independent experiments (mean ± standard error of the mean; ***P < .001). These assays were analyzed with 2-way repeated-measures analysis of variance with the Bonferroni posttest. Representative fluorescence histograms of P-selectin expression are shown in the same order and color as above.
Comparison of targ-CD39, non–targ-CD39, and scFvSCE5 alone in inhibition of platelet activation as assessed by P-selectin expression in flow cytometry. P-selectin expression was determined with a PE-labeled anti–P-selectin antibody. Bar graphs depict the median fluorescence intensity values of 3 independent experiments (mean ± standard error of the mean; ***P < .001). These assays were analyzed with 2-way repeated-measures analysis of variance with the Bonferroni posttest. Representative fluorescence histograms of P-selectin expression are shown in the same order and color as above.
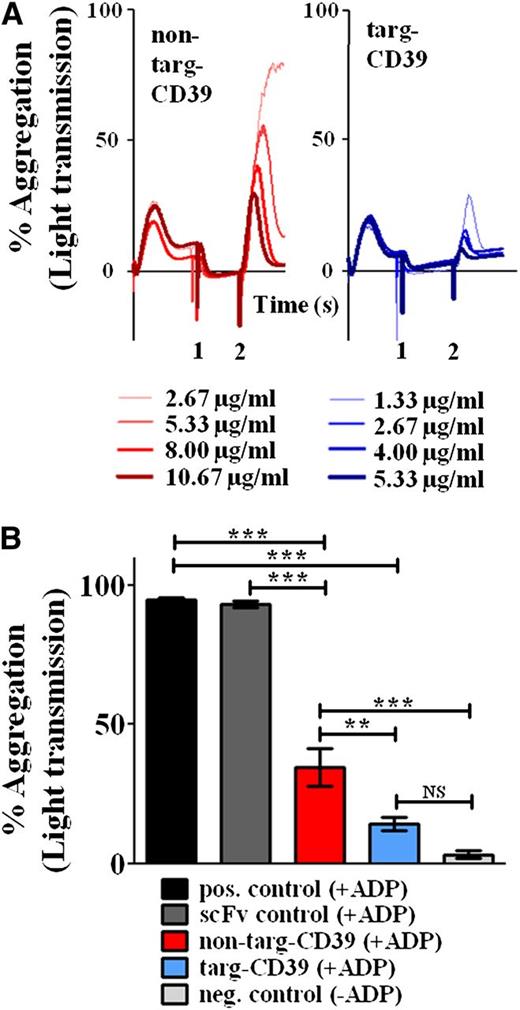
To analyze the ability of targ-CD39 to inhibit platelet aggregation, light transmission aggregometry was performed: 5 μg/mL of targ-CD39 and 10 μg/mL of non–targ-CD39, both activity matched, were tested. Although both non–targ-CD39 and targ-CD39 were able to significantly inhibit aggregation, the inhibitory effect of targ-CD39 was significantly more efficient than equimolar concentrations of non–targ-CD39 (n = 3; **P < .01; Figure 5B), whereas scFvSCE5 (2 μg/mL of scFvSCE5, equimolar amount of scFvSCE5 as in targ-CD39) alone was not significantly different from the positive control.
Comparison of non–targ-CD39, targ-CD39, and controls in inhibiting platelet aggregation measured by light transmission aggregometry. (A) Representative aggregometry traces. ScFv-CD39 constructs were administered after preactivation of platelets with 2 µM ADP at time point 0. After 3 minutes (time point 1), scFv-CD39 constructs were added at various, activity-matched concentrations (eg, 1.33 μg/mL matches 2.66 μg/mL). Samples were challenged with 20 µM ADP at time point 2 (3 minutes after time point 1). (B) Quantitative comparison. Bar chart showing the percent light transmission after 10 minutes of incubation with scFv-CD39 constructs or the equimolar amount of the scFv control. These assays were analyzed with 1-way repeated-measures analysis of variance with the Bonferroni posttest (n = 3; **P < .01; ***P < .001).
Comparison of non–targ-CD39, targ-CD39, and controls in inhibiting platelet aggregation measured by light transmission aggregometry. (A) Representative aggregometry traces. ScFv-CD39 constructs were administered after preactivation of platelets with 2 µM ADP at time point 0. After 3 minutes (time point 1), scFv-CD39 constructs were added at various, activity-matched concentrations (eg, 1.33 μg/mL matches 2.66 μg/mL). Samples were challenged with 20 µM ADP at time point 2 (3 minutes after time point 1). (B) Quantitative comparison. Bar chart showing the percent light transmission after 10 minutes of incubation with scFv-CD39 constructs or the equimolar amount of the scFv control. These assays were analyzed with 1-way repeated-measures analysis of variance with the Bonferroni posttest (n = 3; **P < .01; ***P < .001).
To further characterize the differences between targ-CD39 and non–targ-CD39, an aggregometry assay was chosen, in which platelets were first preactivated with a low dose of ADP (2 µM) before addition of the constructs, thereby allowing the binding of the scFv-CD39 construct to activated GPIIb/IIIa. This experiment mimics the clinical situation of a developing thrombus. Subsequently, a large dose of ADP (20 µM) was given to demonstrate the inhibitory effects of the constructs. In this assay, we demonstrated that targ-CD39 is several times more potent at inhibiting platelets compared with non–targ-CD39 (Figure 5A). A concentration-dependent increase in inhibition was observed for both targ-CD39 and non–targ-CD39; however, at all 4 tested concentrations, the targ-CD39 was more potent than equimolar concentrations of controls (Figure 5A).
Unique targeting properties of scFvSCE5 in mouse platelets in vitro and in vivo
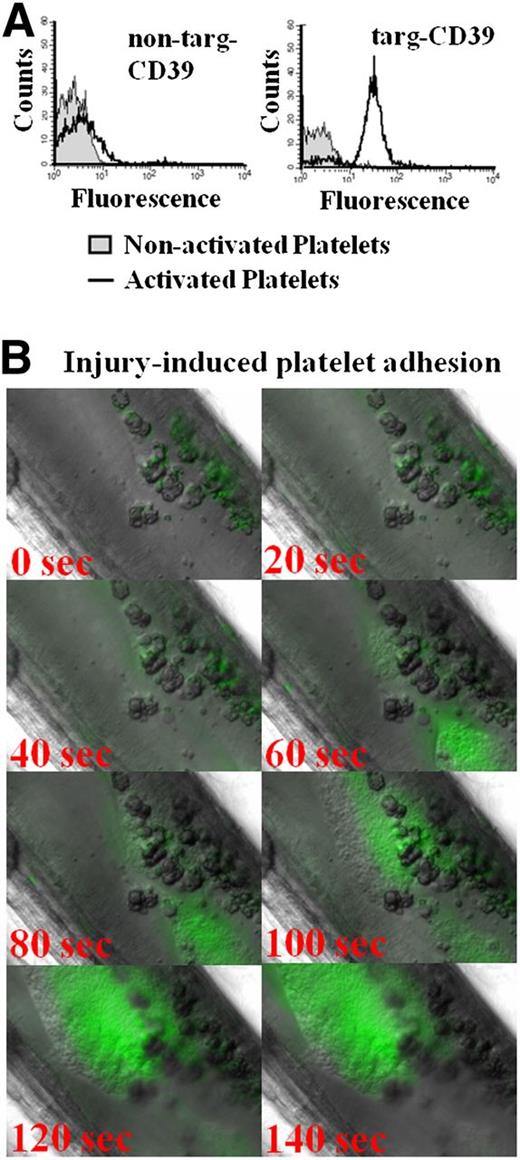
ScFvSCE5 was originally selected for its binding to human platelets. However, it demonstrates a cross-reactivity to mouse platelets, which fortunately allows for in vivo testing in mouse models of thrombosis and hemostasis. In vitro testing of the fusion constructs demonstrates selective binding of targ-CD39 to activated but not to nonactivated mouse platelets, whereas the non–targ-CD39 did not significantly bind to either (Figure 6A).
In vitro and in vivo targeting of scFvSCE5 in mouse platelets. (A) Flow cytometric detection of scFv-CD39 constructs using an Alexa Fluor-488–coupled anti–Penta-His antibody that binds to the constructs’ 6xHis-tags. Representative histograms showing binding of targeted or nontargeted CD39 constructs as assessed by a secondary anti–Penta-His Alexa Fluor-488 antibody. Nonactivated and activated platelets were differentiated by gating with a PE-labeled anti–P-selectin antibody. (B) Intravital microscopy of delayed binding of scFvSCE5 to adhering platelets. Representative images of the formation of a platelet layer induced by ferric chloride injury in the mesenteric artery. ScFvSCE5 binding was detected by an anti–Penta-His Alexa Fluor-488 antibody. Time point 0 depicts time after 4 minutes of ferric chloride–induced injury and 1 minute after flushing with saline when platelets began to attach to the injured site.
In vitro and in vivo targeting of scFvSCE5 in mouse platelets. (A) Flow cytometric detection of scFv-CD39 constructs using an Alexa Fluor-488–coupled anti–Penta-His antibody that binds to the constructs’ 6xHis-tags. Representative histograms showing binding of targeted or nontargeted CD39 constructs as assessed by a secondary anti–Penta-His Alexa Fluor-488 antibody. Nonactivated and activated platelets were differentiated by gating with a PE-labeled anti–P-selectin antibody. (B) Intravital microscopy of delayed binding of scFvSCE5 to adhering platelets. Representative images of the formation of a platelet layer induced by ferric chloride injury in the mesenteric artery. ScFvSCE5 binding was detected by an anti–Penta-His Alexa Fluor-488 antibody. Time point 0 depicts time after 4 minutes of ferric chloride–induced injury and 1 minute after flushing with saline when platelets began to attach to the injured site.
To confirm our hypothesis that the delay in GPIIb/IIIa activation on platelet adhesion would provide the opportunity of a delayed targeting toward the growing thrombus, a ferric chloride injury model of the mouse mesenteric arteries was performed and visualized by intravital microscopy. A highly specific binding of the scFvSCE5 to the growing thrombus (Figure 6B) was shown, whereas the control (anti–Penta-His Alexa Fluor-488 antibody) showed no binding (data not shown). Indeed as hypothesized, we found a significant delay of 20 to 40 seconds between the attachment of platelets to areas of injury and the binding of the scFvSCE5 to these platelets. This confirms a delayed activation of GPIIb/IIIa after initial platelet adhesion and also indicates the unique suitability of scFvSCE5 as a tool for delayed targeting, allowing for the built up of an injury-sealing platelet layer before the therapeutic agent becomes enriched to its effective concentration.
In vivo antithrombotic effects of targ-CD39
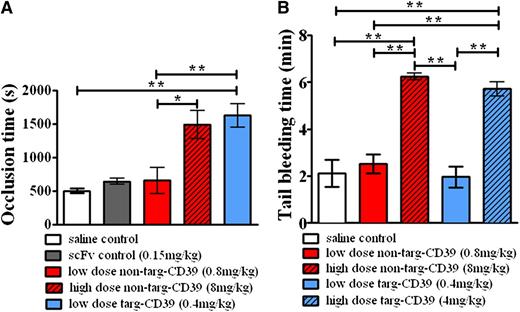
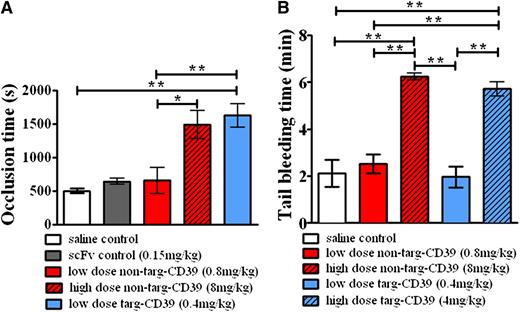
To demonstrate the advantage of using activated-platelet targeted CD39 as an antithrombotic treatment in vivo, we choose a mouse thrombosis model based on a ferric chloride–induced injury of the carotid artery. Targ-CD39 showed a significant prolongation of the vessel occlusion time compared with saline control, whereas the equimolar dose of non–targ-CD39 showed no significant difference in vessel occlusion time compared with saline control (Figure 7A). To demonstrate that the improved effect of targ-CD39 is not caused by the scFv part of the construct, but rather caused by the enrichment of CD39 at the growing thrombus, we also used a scFvSCE5-only control. This showed no significant prolongation compared with the saline control (Figure 7A). To achieve a similar occlusion time prolongation as targ-CD39, a high dose of non–targ-CD39, equivalent to CD39 activity 10 times greater than targ-CD39, was required (Figure 7A). Overall, these data indicate that the benefits observed with the targ-CD39 are because of the targeting of the CD39 moiety to activated platelets.
Effects of CD39 targeting on thrombus formation and hemostasis. (A) Comparison of occlusion time measurements of ferric chloride–induced thrombosis in carotid arteries of mice administered non–targ-CD39, targ-CD39, saline, or single-chain antibody (scFv) controls (n = 8 each) at an equimolar amount in regard to the scFv component in the targ-CD39 group. (B) Comparison of bleeding time in mice determined by tail transection between non–targ-CD39, targ-CD39, and saline (n = 5 each). Doses administered were activity matched: eg, low-dose non–targ-CD39 of 0.8 mg/kg corresponds to low-dose targ-CD39 of 0.4 mg/kg. Measurements were analyzed with a Gehan-Breslow-Wilcoxon survival analysis and subsequently represented as bar graphs (*P < .05; **P < .01).
Effects of CD39 targeting on thrombus formation and hemostasis. (A) Comparison of occlusion time measurements of ferric chloride–induced thrombosis in carotid arteries of mice administered non–targ-CD39, targ-CD39, saline, or single-chain antibody (scFv) controls (n = 8 each) at an equimolar amount in regard to the scFv component in the targ-CD39 group. (B) Comparison of bleeding time in mice determined by tail transection between non–targ-CD39, targ-CD39, and saline (n = 5 each). Doses administered were activity matched: eg, low-dose non–targ-CD39 of 0.8 mg/kg corresponds to low-dose targ-CD39 of 0.4 mg/kg. Measurements were analyzed with a Gehan-Breslow-Wilcoxon survival analysis and subsequently represented as bar graphs (*P < .05; **P < .01).
Finally, as bleeding complications determine clinical suitability of a new antithrombotic drug, we investigated tail bleeding time in mice. The concentration of targ-CD39, which was highly effective in preventing carotid artery occlusion, did not demonstrate any bleeding time prolongation (n = 5; Figure 7B). In contrast, the concentration of the non–targ-CD39 construct, which was necessary to cause a significant prolongation of the occlusion time, resulted in a strong concentration-dependent prolongation of the bleeding time (Figure 7B; supplemental Figure 2). The high dose of targ-CD39 caused bleeding time prolongations comparable to that of the high-dose non–targ-CD39 (Figure 7B). These data confirm the unique advantages of the newly described therapeutic approach using low-dose activated-platelet targeted CD39. This therapeutic strategy promises effective inhibition of thrombus formation without associated bleeding risk.
Discussion
In this study, we genetically fused CD39 to a single-chain antibody (scFv) that specifically binds to the active conformation of GPIIb/IIIa, which is only exposed with a noticeable delay on platelets after their binding to injured vessel areas. As our data indicate, this approach is a highly effective strategy for enrichment of CD39 at developing clots. In vitro assays confirmed specific binding of this construct (targ-CD39) to activated platelets and showed a significantly higher antithrombotic potency compared with its nontargeted control (non–targ-CD39). In vivo mouse models of thrombosis and bleeding demonstrate targ-CD39’s capability to protect against thrombosis without causing bleeding time prolongation when administered at a low dose. Non–targ-CD39, however, was not sufficient in protecting against thrombosis at the same dose. The dose required for the nontargeted CD39 to prevent thrombosis caused significant bleeding time prolongation. Hence, targeting CD39 to activated platelets represents a novel promising strategy to break the link between efficient antithrombotic potency and associated bleeding risk.
CD39 has attracted major interest as a new pharmacologic strategy both for its antithrombotic and anti-inflammatory potential, particularly as it was initially not expected to cause bleeding problems. The working hypothesis was that the initial layer after vessel injury could be formed by nonactivated platelets,26,33 which would then become activated by their binding to the vessel surface, and that the platelet agonist ADP mainly plays a secondary role to amplify further platelet activation and stabilize forming platelet aggregates.3 Hence, CD39 was thought not to affect the initial “seal” but only prevent further growth of thrombi.34 Although our data confirm effective inhibition of ADP’s role as a secondary platelet agonist by CD39, our in vivo data clearly shows that nontargeted CD39 causes bleeding problems in parallel to its antithrombotic effects, which is consistent with the observed antithrombotic effects and bleeding time prolongation seen in CD39 overexpressing mice.19,21 A potential explanation for this finding is that, in addition to preventing the paracrine effects of ADP that result in the recruitment of platelets to the growing clot, high concentrations of solCD39 also inhibit the autocrine effect of ADP,3 which acts as a positive feedback loop by further amplifying platelet activation. This inhibition of the autocrine feedback loop could prevent platelets from activating themselves strongly enough to form a sufficiently stable “sealing” plug.
Selective targeting to activated platelets via scFvs ensures a selective enrichment of the fusion construct at a growing thrombus.35 The activated GPIIb/IIIa receptor has proven itself as a suitable epitope for targeting activated platelets, both diagnostically and therapeutically.28,36-39 The GPIIb/IIIa complex, also known as integrin αIIbβ3, is the most abundant protein expressed on the platelet surface (60 000-80 000 copies per platelet). In addition, the conformational change, which GPIIb/IIIa undergoes on platelet activation from a low-affinity fibrinogen binding state to a high-affinity state, allowed for the selection of a scFv that only binds to the active form and hence only binds to activated platelets but not to nonactivated platelets in circulation.31,35 ScFvs in general have several advantages over full-sized antibodies: the latter, because of their quaternary structure, cannot be simply fused to an effector protein via molecular biology methods.40 Instead, they are mostly chemically coupled, a process often accompanied by significant loss of the binding affinity toward the target epitope, as well as activity on the effector protein side. ScFvs, however, only consist of the variable region of the heavy and light chain linked via a short peptide. Hence, they can be fused to their effector protein with standard molecular biology techniques without significant loss of function.41 Furthermore, the small size of a scFv reduces the chance of immunogenic responses and may allow for a greater number of fusion molecules binding to the thrombus as well.42,43
Targ-CD39’s unique ability to effectively prevent vessel occlusion without increasing the bleeding tendency can be explained by the specific targeting of CD39 to developing clots, which allows targ-CD39 to be efficacious at a low systemic concentration. This concentration is well below that shown to cause bleeding. As indicated by the fluorescence staining in Figure 6, anti-GPIIb/IIIa scFv targeting only begins at the site of thrombus formation after the initial plug has been established. Once a sealing platelet layer is formed, targ-CD39 is enriched at the growing thrombus, effectively preventing the ADP-induced autocrine and paracrine activation of platelets. As the thrombus grows, more targ-CD39 is concentrated at the thrombus site, and hence further platelet activation and thrombus growth is prevented. Additionally, our intravital microscopy experiments (Figure 6) show that there is a distinct delay between platelets forming an initial layer and the activation of GPIIb/IIIa. Therefore, this delay would allow platelets to form a sufficient sealing layer, because the autocrine platelet-activating function of ADP is not inhibited before the epitope for the targeted CD39 construct becomes available for enriching CD39 at the growing thrombus.
Besides CD39’s antithrombotic effects, it also has significant anti-inflammatory effects.21,44 Initially, it was assumed that this anti-inflammatory effect was caused by adenosine, which is created by the CD39/CD73 cascade, where CD39 hydrolyses adenosine triphosphate to ADP and ADP to AMP, and CD73, which is expressed on endothelial cells, converts AMP to adenosine.45 It is this additional adenosine created by administration of extra CD39, the rate-limiting step in the CD39/CD73 cascade, that was thought to account for the anti-inflammatory effects. Recently, however, Rajakumar et al46 proposed that AMP and/or the decrease of ADP itself may be a cause of CD39’s anti-inflammatory effects. In addition to these direct anti-inflammatory effects, inhibition of platelet function by CD39 may also have a potent anti-inflammatory effect, as there is increasing evidence that platelets are early and central players in inflammatory reactions.47,48 Therefore, the described targ-CD39 construct may be an attractive therapeutic approach not only in thrombotic but also in inflammatory diseases. The strong therapeutic potential of combining the application of the end product of the CD39/CD73 pathway, adenosine, with targeted CD73-based activation has recently been demonstrated in the treatment of experimental rheumatoid arthritis.49 Further studies are warranted to test the potential of targ-CD39 as an anti-inflammatory reagent: in particular because our data suggest that as a result of specific targeting, therapeutic effects can be achieved without systemic side effects.
In conclusion, we showed that delayed targeting of CD39 to a developing thrombus via a scFv, which is specific for activated GPIIb/IIIa and thus activated platelets, is feasible and highly effective at preventing thrombus formation. Based on delayed activation of GPIIb/IIIa on adhering platelets, this approach is tailored to allow a sealing layer of platelets to form before the construct enriches and subsequently prevents pathological thrombus formation and vessel occlusion. The newly designed construct does not cause bleeding time prolongation while being highly effective as an antithrombotic drug. This construct has potential as a novel antithrombotic treatment, promising to break the link between high antithrombotic efficacy and bleeding complications.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Bassler for technical support and Dr Jennifer Rivera for proofreading.
J.D.H. was supported by a scholarship from the University of Melbourne, X.W. was supported by a scholarship from Monash University, and S.K. and A.S. were supported by grants from the German Research Foundation (STR 687/1-1). C.H. and E.L.C. were supported by the National Institutes of Health, C.E.H. was supported by a fellowship from the National Health and Medical Research Council of Australia, and K.P. was supported by a Future Fellowship from the Australian Research Council.
Authorship
Contribution: J.D.H., X.W., and S.K. designed research, performed research, analyzed data, and wrote the paper; C.S. contributed reagents and analytical tools; C.A.H., A.S., E.L.C., and C.E.H. analyzed data and wrote the paper; H.H.N. contributed reagents and analytical tools and wrote the paper; and K.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karlheinz Peter, Baker IDI Heart & Diabetes Institute, PO Box 6492, St Kilda Rd Central, Melbourne, VIC 8008, Australia; e-mail: karlheinz.peter@bakeridi.edu.au.