Key Points
PRKCD deficiency causes a novel primary immunodeficiency with B-cell deficiency and severe autoimmunity.
Protein kinase C δ may represent a key factor controlling immune homeostasis and autoimmunity.
Abstract
Primary B-cell disorders comprise a heterogeneous group of inherited immunodeficiencies, often associated with autoimmunity causing significant morbidity. The underlying genetic etiology remains elusive in the majority of patients. In this study, we investigated a patient from a consanguineous family suffering from recurrent infections and severe lupuslike autoimmunity. Immunophenotyping revealed progressive decrease of CD19+ B cells, a defective class switch indicated by low numbers of IgM- and IgG-memory B cells, as well as increased numbers of CD21low B cells. Combined homozygosity mapping and exome sequencing identified a biallelic splice-site mutation in protein C kinaseδ (PRKCD), causing the absence of the corresponding protein product. Consequently, phosphorylation of myristoylated alanine-rich C kinase substrate was decreased, and mRNA levels of nuclear factor interleukin (IL)-6 and IL-6 were increased. Our study uncovers human PRKCD deficiency as a novel cause of common variable immunodeficiency-like B-cell deficiency with severe autoimmunity.
Introduction
Primary B-cell immunodeficiencies (B-PID) constitute a heterogeneous group of immunodeficiencies characterized by defective production of antigen-specific antibodies and predisposition to recurrent and severe infections.1 A high proportion of patients display autoimmune features.2
Fine-tuned B-cell receptor (BCR) signaling is crucial for controlling immune homeostasis, as aberrant BCR signaling predisposes patients to autoimmunity.3
In the last decade, several Mendelian defects causing B-PID have been identified.3,4 Nonetheless, the molecular etiology of these disorders remains elusive in the majority of patients. The advent of high-throughput genomic technologies will be instrumental in defining the spectrum of molecular aberrations underlying primary B-cell deficiencies.
Here we investigated the molecular cause of a common variable immunodeficiency (CVID)-like B-PID with progressive B-cell lymphopenia, an immunoglobulin class switch defect, aberrant immunoglobulin levels, and severe autoimmunity. Combined homozygosity mapping and exome sequencing identified a biallelic mutation in protein C kinaseδ (PRKCD) encoding protein kinase C δ as the molecular cause of this novel PID.
Methods
A detailed description of all experimental methods can be found in the supplemental Methods on the Blood website.
Subjects
This study has been approved by the ethics committee of the Medical University of Vienna, Austria. Biological material was obtained on informed consent in accordance with the Declaration of Helsinki. The patient was followed up and treated at the Klinikum Wels-Grieskirchen, St. Anna Kinderspital Vienna, and the Department of Pediatrics and Adolescent Medicine of the Medical University, Vienna, Austria.
Flow cytometry–based immunophenotyping
Flow cytometry analysis of peripheral blood mononuclear cells was performed on a Beckton-Dickinson LSR Fortessa or FACS Calibur.
Genetic analysis
Sanger sequencing was performed according to standard methods; single nucleotide polymorphism–based homozygosity mapping and exome sequencing were performed as described previously with minor modifications.5
Immunoblot analysis
Immunoblot analyses were performed with the following antibodies: anti-human PRKCD (Cell Signaling, Frankfurt am Main, Germany), anti-phospho (clone D13E4) and total myristoylated alanine-rich C kinase substrate (MARCKS) (clone D88D11; both from Cell Signaling), and anti-GAPDH (clone 6C5; Santa Cruz Biotechnology, Heidelberg, Germany).
Quantitative polymerase chain reaction analysis
mRNA levels of interleukin (IL)-6 and nuclear factor (NF)-IL6 in Epstein-Barr virus–transformed B cells from the patient and his father, upon stimulation with phorbol myristate acetate, were measured by quantitative polymerase chain reaction analysis.
T-cell Vβ spectratyping
T-cell receptor Vβ spectratyping was performed according to Pannetier et al6 with minor modifications.
Results and discussion
Clinical and laboratory characterization
The index patient (now 12 years of age) was born to consanguineous parents (first-degree cousins) of Turkish origin (supplemental Figure 1). His father was diagnosed with Behçet’s disease and mild autoimmune thyreoiditis at 40 years of age. The mother is asymptomatic. The patient’s medical history is characterized by multifaceted manifestations of recurrent severe infections and autoimmunity as specified below.
Infections.
From the first year of life onward, the patient experienced repeated episodes of infections, including urinary tract infections, gastroenteritis, upper and lower respiratory tract infections, and otitis media, prompting tonsillectomy and adenoidectomy within the first 4 years of life. Frequency and severity of infections decreased after commencement of immunoglobulin substitution at the age of 4 years.
Autoimmunity and immune dysregulation.
The first manifestation of autoimmunity occurred at 15 months of age, when the patient presented with nephrotic syndrome. Renal biopsy revealed membranous glomerulonephritis (Figure 1A; supplemental Figure 2). Partial remission was achieved with steroid treatment with remaining mildly impaired renal function (low-grade proteinuria, hematuria; supplemental Table 1). By 3 years of age, hepatosplenomegaly (supplemental Figure 3) and generalized lymphadenopathy became apparent, prompting an in-depth diagnostic workup, which revealed low-grade viremia of human herpes virus subtypes 6 and 7. Herpes viremia was transient, whereas lymphadenopathy persisted. Several lymph node biopsies revealed nonspecific reactive follicular hyperplasia (Figure 1B). Bone marrow aspiration did not reveal any signs of malignancy (not shown). In the following years, additional manifestations of autoimmunity including relapsing polychondritis developed. Latent hypothyroidism was detected; organ-specific autoantibodies were absent. At the age of 8 years, aseptic endocarditis and pulmonary embolism were diagnosed, and laboratory investigations suggested the diagnosis of antiphospholipid syndrome (positivity of anti-nuclear antibodies, anti-dsDNA, and anti-cardiolipin IgG antibodies; supplemental Table 2), prompting anticoagulation therapy and low-dose steroid therapy.
Clinical and immunological characterization of the index patient. (A) First renal biopsy was performed at the age of 15 months. Granular deposition of IgG along the periphery of the capillary loops (left) as seen in membranous nephropathy (MGN) was confirmed by transmission electron microscopy (TEM) (right), which showed electron dense deposits between basement membrane and podocytes (P), as well as deposits partially in resolution and incorporated by basement membrane material (arrows), consistent with MGN stage I to III (CL, capillary loop). (B) Histopathological analysis of a lymph node biopsy revealed unspecific, reactive follicular hyperplasia (arrow) but not the characteristic lymph node changes of autoimmune lymphoproliferative syndrome (ALPS) associated with CD95/FAS mutations (ALPS type 0/1a). The left and middle panels show hematoxylin and eosin stains, and the right panel shows anti-CD20 staining. (C) Representative FACS plots illustrating the aberrant B-cell phenotype including B-cell lymphopenia, decreased IgM- and IgG memory B cells, and increased numbers of CD21low B cells. (D) Longitudinal analysis illustrates progressive decrease of CD19+ B cells and (E-G) persistence of the aberrant distribution of B-cell subsets. *First episode of nephrotic syndrome. #Treatment with anti-CD20. The dotted lines indicate the age-related 25th and 75th percentiles of the corresponding cells.23
Clinical and immunological characterization of the index patient. (A) First renal biopsy was performed at the age of 15 months. Granular deposition of IgG along the periphery of the capillary loops (left) as seen in membranous nephropathy (MGN) was confirmed by transmission electron microscopy (TEM) (right), which showed electron dense deposits between basement membrane and podocytes (P), as well as deposits partially in resolution and incorporated by basement membrane material (arrows), consistent with MGN stage I to III (CL, capillary loop). (B) Histopathological analysis of a lymph node biopsy revealed unspecific, reactive follicular hyperplasia (arrow) but not the characteristic lymph node changes of autoimmune lymphoproliferative syndrome (ALPS) associated with CD95/FAS mutations (ALPS type 0/1a). The left and middle panels show hematoxylin and eosin stains, and the right panel shows anti-CD20 staining. (C) Representative FACS plots illustrating the aberrant B-cell phenotype including B-cell lymphopenia, decreased IgM- and IgG memory B cells, and increased numbers of CD21low B cells. (D) Longitudinal analysis illustrates progressive decrease of CD19+ B cells and (E-G) persistence of the aberrant distribution of B-cell subsets. *First episode of nephrotic syndrome. #Treatment with anti-CD20. The dotted lines indicate the age-related 25th and 75th percentiles of the corresponding cells.23
Immunological workup.
Detailed laboratory evaluations were first performed after manifestation of glomerulonephritis at 15 months of age and revealed low IgG levels, whereas levels of IgA and IgM were above the normal range (supplemental Figure 4). B-cell studies showed a reduction of CD19+ B cells, decreased relative proportions of non–class-switched (CD19+CD27+IgD+) and class-switched (CD19+CD27+IgD−) memory B cells, and increased numbers of CD21low B cells (Figure 1C; supplemental Table 3). Longitudinal analyses showed progressive decline of total CD19+ B cells (Figure 1D), increased relative proportion of CD21low B cells (Figure 1E), and decreased frequencies of memory B cells (Figure 1F-G). T-cell studies showed mildly decreased T-cell proliferative responses (supplemental Table 2) in the absence of obvious immunophenotypic aberrations (supplemental Table 3) or skewing of the T-cell receptor Vβ repertoire (supplemental Figure 5). Impaired B-cell function was suggested by the absence of isohemagglutinins. Overall, findings were compatible with a CVID-like phenotype, although the formal criteria including decreased levels of at least 2 classes immunoglobulins7 were not fulfilled.
Treatment.
Because of recurrent respiratory tract infections including pneumonia, immunoglobulin G replacement was initiated at 4 years of age, leading to a decrease of infection frequency. At the age of 9 years, anti-CD20 therapy (2 courses of 375 mg/m2 each) was performed to alleviate autoantibody production. Despite transient normalization of the previously increased IgM levels (supplemental Figure 4), autoantibodies persisted. Since the age of 8 years, the patient has been under continuous treatment with mycophenolate-mofetil and low-dose steroids. Other treatment includes enalapril (angiotensin-converting enzyme inhibitor), anticoagulants, thyroid hormone replacement, and immunoglobulin replacement. With this treatment, the boy has a reasonably good quality of life, without the need for hospitalization or intravenous antibiotics during the last 3 years.
Routine genetic investigation.
A genetic workup revealed no mutations in the ICOS, BAFFR, TACI, or FOXP3 genes, respectively. Surface expression of CD40/CD40 ligand was normal (data not shown). A heterozygous variant in CTLA4 was discovered in both the index patient and his father (rs231775). Homozygosity for this variant has been associated with Graves’s disease, rheumatoid arthritis, and systemic lupus erythematous,8 whereas heterozygosity is associated with autoimmune thyreoiditis9 but not systemic lupus erythematous.10 The clinical presentation of this patient with multiple features of immune dysregulation including glomerulonephritis, lymphadenopathy, relapsing polychondritis, and antiphospholipid syndrome in the context of a CVID-like immune phenotype could thus not be reconciled with the heterozygous CTLA4 variant alone. Hence, we initiated further genetic investigations to detect the molecular background of the patient’s disease.
Mutation identification in the PRKCD gene
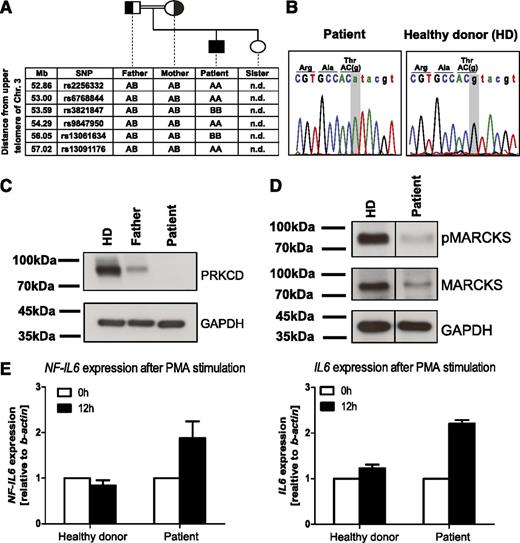
Given the consanguinity in the family, a monogenetic defect with autosomal recessive inheritance was assumed. To uncover the underlying genetic cause, we performed single nucleotide polymorphism array–based homozygosity mapping (Figure 2A; supplemental Table 4) and exome sequencing. Hits from exome sequencing were filtered for homozygous intervals present exclusively in the patient and validated by Sanger sequencing (supplemental Figure 6; supplemental Table 4). Only 2 of these hits showed perfect segregation with the disease: UBXN1 (c. G686A, p. Thr229Met) and PRKCD (c.1352+1G>A) (Figure 2B and supplemental Figure 1, respectively).
Identification of human PRKCD deficiency as a monogenetic B-cell deficiency associated with autoimmunity. (A) Single nucleotide polymorphism array–based homozygosity mapping was performed and revealed several homozygous candidate intervals, including an interval on chromosome 3p21.31. (B) Sanger sequencing validated a splice site mutation in PRKCD, encoding for protein kinase C δ which was homozygous in the patient. (C) Western blot analysis showed absent expression of the corresponding protein product in the patient compared with decreased expression in the heterozygous father and normal expression in a healthy control. (D) Western blot analysis showed defective phosphorylation of MARCKS, a downstream target of PRKCD. (E) Quantitative polymerase chain reaction analysis showed hyperactive NF-IL6 signaling on stimulation using phorbol myristate acetate, as indicated by increased mRNA levels of NF-IL6 and IL6.
Identification of human PRKCD deficiency as a monogenetic B-cell deficiency associated with autoimmunity. (A) Single nucleotide polymorphism array–based homozygosity mapping was performed and revealed several homozygous candidate intervals, including an interval on chromosome 3p21.31. (B) Sanger sequencing validated a splice site mutation in PRKCD, encoding for protein kinase C δ which was homozygous in the patient. (C) Western blot analysis showed absent expression of the corresponding protein product in the patient compared with decreased expression in the heterozygous father and normal expression in a healthy control. (D) Western blot analysis showed defective phosphorylation of MARCKS, a downstream target of PRKCD. (E) Quantitative polymerase chain reaction analysis showed hyperactive NF-IL6 signaling on stimulation using phorbol myristate acetate, as indicated by increased mRNA levels of NF-IL6 and IL6.
While no obvious role for UBXN1 in the patient’s disease pathogenesis could be recognized (Supplemental Materials), PRKCD was considered a plausible candidate, because it has a well-established role in B-cell signaling11,12 and the corresponding Prkcd−/− knockout mouse exhibits various autoimmune manifestations together with generalized lymphadenopathy.13 The murine model also shows splenic lymphocyte hyperproliferation,13 reminiscent of human autoimmune lymphoproliferative syndrome.14 Western blot analysis revealed the absence of PRKCD in the patient, whereas expression was decreased in a heterozygous parent compared with a healthy control (Figure 2C). Lower expression in the heterozygous carrier does not seem to be sufficient to cause disease, because the parents do not present with the characteristic clinical features seen in the patient.
Functional consequences of PRKCD deficiency
PRKCD is a member of the protein kinase C family critical for regulation of cell survival, proliferation, and apoptosis.15 In B lymphocytes, PRKCD is involved in BCR-mediated signaling downstream of Bruton's tyrosine kinase and phospholipase Cγ2.11 PRKCD is expected to have an essential function in B-cell tolerance, because the corresponding knockout mouse shows immune-complex glomerulonephritis, splenomegaly, and lymphadenopathy associated with B-cell expansion and defective B-cell tolerance to self-antigen.13 Autoimmunity in Prkcd−/− mice has been linked to defective proapoptotic extracellular signal-regulated kinase signaling during B-cell development.16 Recently, PLCγ2 mutations have been identified in CVID(-like) B-cell deficiency with autoimmunity, highlighting the importance of this pathway for B-cell homeostasis.17,18
To assess functional consequences of PRKCD deficiency, expression of MARCKS, a major PKC target,19 was evaluated. Immunoblot analysis in Epstein-Barr virus–immortalized patient B-cell lines showed reduced total levels of MARCKS, despite contrary literature findings.20 Importantly, MARCKS phosphorylation at Ser167/170, which is critical for translocation of MARCKS from the plasma membrane to the cytoplasm mediating reduction of cell proliferation,21 was abrogated in the patient (Figure 2D). Thus, deficiency of pMARCKS may be related to the lymphoproliferation in the patient.21
On phosphorylation of NF-IL6 at Ser240 by PRKCD, the DNA binding capability of NF-IL6, and consequently IL6 production, is markedly reduced.22 Accordingly, we observed increased mRNA levels of NF-IL6 and IL6 in the PRKCD-deficient patient after phorbol myristate acetate stimulation (Figure 2E), similar to hyperactive NF-IL6 signaling observed in Prkcd−/− mice.13
In summary, we describe PRKCD deficiency as a novel primary CVID-like B-cell deficiency. The index patient of this study exhibited features of immune dysregulation including lymphoproliferation (splenomegaly and lymphadenopathy) and autoimmunity (glomerulonephritis, antiphospholipid syndrome, and relapsing polychondritis) similar to the murine knockout model. However, neither peripheral B-cell lymphopenia nor defective class switch observed in our patient was assessed in the mouse. It cannot be excluded that the known heterozygous variant in CTLA4 in the patient may act as a disease-modifying factor. Future studies will need to comprehensively characterize the clinical and immunological phenotype in a cohort of PRKCD-deficient patients and further dissect the molecular pathophysiology of aberrant PRKCD-signaling in B-cell homeostasis and autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the family for their participation in this study; Georg Ebetsberger-Dachs, Ulrike Habeler, and Christoph Male for clinical care; Karoly Lakatos for providing CT scan pictures; Linda Stöger for help with quantitative polymerase chain reaction analyses; Raphael Ott for technical assistance with library preparation for exome sequencing; Ulrike Koermoeczi and Arno Rottal for expert technical assistance with flow cytometric analyses; and Ciara Cleary for proofreading the manuscript.
This work was supported by an intramural grant from the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences (K.B.) and START Programme of the Austrian Science Fund (FWF) grant Y595B13 (K.B.).
Authorship
Contribution: E.S., E.S.-V., S.K., S.A.B., N.K.P., and W.G. performed all experimental work except serial routine immunological characterization performed by W.F.P. W.E., H.B., A.H., K.A., F.E., M.G.S., W.H., A.P., and E.F.-W. provided clinical care and critically reviewed clinical and immunological patient data. L.M. and R.K. performed histopathological analyses. K.B. conceived this study with help from E.F.-W.; planned, designed, and interpreted experiments; provided laboratory resources; guided E.S., E.S.-V., S.K., S.A.B., N.K.P., and W.G.; and wrote the initial draft of the manuscript with assistance from E.S., E.S.-V., S.K., S.A.B., N.K.P., and E.F.-W. All authors critically reviewed the manuscript and agreed to its publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.G.S. is Division of Pediatric Hematology-Oncology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Graz, Austria.
Correspondence: Kaan Boztug, CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, and Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Lazarettgasse 14, AKH BT 25.3, A-1090 Vienna, Austria; e-mail: kboztug@cemm.oeaw.ac.at; and Elisabeth Förster-Waldl, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail: elisabeth.foerster-waldl@meduniwien.ac.at.
References
Author notes
E.S. and E.S.-V. contributed equally to this study.