Key Points
Inhibition of calcineurin-NFAT signaling increases megakaryocyte and platelet counts.
Inhibition of calcineurin-NFAT signaling increases proliferation of megakaryocyte progenitors.
Abstract
The calcium regulated calcineurin-nuclear factor of activated T cells (NFAT) pathway modulates the physiology of numerous cell types, including hematopoietic. Upon activation, calcineurin dephosphorylates NFAT family transcription factors, triggering their nuclear entry and activation or repression of target genes. NFATc1 and c2 isoforms are expressed in megakaryocytes. Moreover, human chromosome 21 (Hsa21) encodes several negative regulators of calcineurin-NFAT, candidates in the pathogenesis of Down syndrome (trisomy 21)-associated transient myeloproliferative disorder and acute megakaryoblastic leukemia. To investigate the role of calcineurin-NFAT in megakaryopoiesis, we examined wild-type mice treated with the calcineurin inhibitor cyclosporin A and transgenic mice expressing a targeted single extra copy of Dscr1, an Hsa21–encoded calcineurin inhibitor. Both murine models exhibited thrombocytosis with increased megakaryocytes and megakaryocyte progenitors. Pharmacological or genetic inhibition of calcineurin in mice caused accumulation of megakaryocytes exhibiting enhanced 5-bromo-2′-deoxyuridine uptake and increased expression of messenger RNAs encoding CDK4 and G1 cyclins, which promote cell division. Additionally, human megakaryocytes with trisomy 21 show increased proliferation and decreased NFAT activation compared with euploid controls. Our data indicate that inhibition of calcineurin-NFAT drives proliferation of megakaryocyte precursors by de-repressing genes that drive cell division, providing insights into mechanisms of normal megakaryopoiesis and megakaryocytic abnormalities that accompany Down syndrome.
Introduction
Recent work implicates a role for the nuclear factor of activated T cells (NFAT) family of transcription factors in normal and pathological megakaryopoiesis. NFAT was originally discovered as a protein that binds the interleukin-2 (IL-2) gene promoter in activated T cells1 and is now known to regulate multiple developmental processes through many target genes.2 Calcineurin, a calcium-activated serine/threonine phosphatase, dephosphorylates NFAT to stimulate its nuclear entry and transcriptional activity. Conversely, phosphorylation inactivates NFAT by promoting its cytoplasmic sequestration. Several NFAT family members, particularly the C1 and C2 isoforms, are upregulated during megakaryopoiesis.3 Overexpression of NFAT or its activation by calcium ionophore induces apoptosis in megakaryocyte-like cell lines and megakaryocytes cultured from human CD34+ cells.4 In idiopathic myelofibrosis, the calcineurin inhibitor FKBP5 is upregulated in hyperplastic megakaryocytes.5 In addition, 2 human chromosome 21 (Hsa21) genes encoding calcineurin-NFAT inhibitors (RCAN1/DSCR1 and DYRK1A) have been implicated in the development of Down syndrome (trisomy 21)-associated transient myeloproliferative disorder and acute megakaryoblastic leukemia.6,7 Together, these data indicate that calcineurin-NFAT inhibits megakaryopoiesis and that attenuation of this pathway may contribute to pathological expansion of megakaryocytes and their precursors.
Presumably, NFAT inhibits megakaryopoiesis by regulating the expression of genes critical for megakaryocyte proliferation, apoptosis, or differentiation. For example, the FASLG gene is induced by NFAT in cultured megakaryocytes and megakaryocytic cell lines.4 FASLG encodes Fas ligand, a member of the tumor necrosis factor superfamily, which engages the Fas receptor to initiate an apoptotic signaling cascade. Transactivation of FASLG by NFAT in activated lymphocytes is critical for elimination of T-cell populations.8 In cultured megakaryocytes, which express Fas receptor, upregulation of FASLG contributes to NFAT-mediated apoptosis.6
Regulation of megakaryopoiesis by calcineurin-NFAT has therapeutic implications, particularly because this signaling pathway is amenable to pharmacologic manipulation. However, most prior studies have defined NFAT effects in cultured megakaryocytes and cell lines. The role of NFAT in megakaryopoiesis in vivo has not been examined closely. Moreover, the genetic pathways through which NFAT regulates megakaryocyte development are not fully defined. Here we show that inhibition of calcineurin-NFAT by systemically administered cyclosporin A (CsA) or by 50% overexpression of DSCR1 via a stably integrated complementary DNA (cDNA) transgene drives megakaryocyte and platelet production in mice. In megakaryocyte precursors, NFAT suppression induces cell-cycle stimulatory genes that are transcriptionally repressed by NFAT in other cell types.9-11 Moreover, human trisomy 21 megakaryocytes exhibit reduced NFAT activity and enhanced expansion in culture. Our findings demonstrate that calcineurin-NFAT negatively regulates megakaryopoiesis in vivo, defines new target genes involved in this process, and supports the hypothesis that suppression of NFAT contributes to Down syndrome–associated hematopoietic abnormalities, including predisposition to megakaryocytic leukemia.
Methods
Animals
C57Bl/6 mice were purchased from Charles River. Dscr1 transgenic mice with a third copy of Dscr1 targeted into the hypoxanthine phospho-ribosyltransferase (Hprt) locus were generated as previously described.12 All male and female mice used in the experiments were 6 to 8 weeks old unless noted otherwise. For CsA drug treatments, wild-type and Dscr1 transgenic (Tg) mice were treated daily (10 mg/kg) via oral gavage with CsA oral solution USP (Watson Pharma, Corona, CA) diluted in peanut oil (Sigma-Aldrich, St. Louis, MO) for 6 to 10 weeks. Age-matched control animals were treated with peanut oil (vehicle) alone. All animal experiments were performed according to protocols approved by the University of Pennsylvania institutional animal care and use committee.
Cell culture
Liquid human megakaryocyte cultures were performed with fetal liver mononuclear cells in serum free medium (StemSpan SFEM, Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 100 ng/mL TPO (R&D Systems, Minneapolis, MN), 40 μg/mL low-density lipoprotein (Calbiochem, San Diego, CA), and 1% Pen/Strep (Gibco Invitrogen, Carlsbad, CA).
To analyze megakaryocyte colony-forming units (CFU), 7,500 harvested murine bone marrow cells/mL were seeded into Megacult-C medium (Stem Cell Technologies) that included 50 ng/mL TPO, 20 ng/mL IL-6, 50 ng/mL IL-11, and 10 ng/mL IL-3. After 7 to 9 days of incubation, cultures were dehydrated, fixed, and stained according to manufacturer instructions.
Western blots
For bone marrow–derived megakaryocytes, femurs were dissected from mice and bone marrow was flushed with saline. Total protein was extracted from bone marrow megakaryocytes or human fetal liver megakaryocytes by cell lysis with RIPA buffer (Sigma-Aldrich). Proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (BioRad, Hercules, CA). Blots were blocked and incubated overnight with primary antibodies according to the manufacturer’s specifications. Antibodies used were directed against Fas ligand, NFATc1, NFATc2, phospho-NFATc2, DSCR1, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) and DSCR1 (Abcam, Cambridge, MA).
Platelet counts
Blood (500 μL per sample) was drawn into a tube containing 50 μL of Aster-Jandl anticoagulant (85 mM sodium citrate, 69 mM citric acid, 111 mM glucose, pH 4.6) from the orbital sinus of each anesthetized mice by using heparinized glass capillary tubes (Fisher Scientific, Hampton, NH). Platelet counts were determined using a HemaVet counter (Triad Associates, Concord, CA).
Immunofluorescence
For bone marrow cytospins, femurs were dissected from mice and bone marrow flushed with saline and placed in a cytospin with 0.2% gelatin-coated slides. Cells were fixed with 4% paraformaldehyde (Fisher Scientific) for 10 minutes at room temperature (RT) followed by blocking in 3% milk in TBS-T for 45 minutes. Anti-mouse NFATc1, NFATc2 (both from Santa Cruz Biotechnology), or isotype-matched control antibody was added and incubated overnight at RT at 1:250 (NFATc1) 1:1000 (NFATc2) dilution factor. Cells were washed with phosphate-buffered saline (PBS)-T before the addition of secondary anti-rabbit-Alexa 594 (1:500; Life Technologies, Grand Island, NY) for 1 hour at RT protected from light. Nuclei were stained with 1% Hoechst dye (Sigma-Aldrich) for 1 minute. Cells were extensively washed with PBS-T, mounted and viewed, and photographed on a fluorescence microscope. Images were taken at RT with a 10× or 20× magnification objective lens and with a digital camera AxioCAM HRc (Zeiss, Thornwood, CT) mounted on Zeiss Imager M1 Axio using Zeiss AxioVision Acquisition software (version 4.5).
Ploidy, TUNEL, and BrdU uptake
All staining procedures included an initial resuspension of bone marrow cells in Dulbecco PBS containing 4% fetal calf serum (HyClone Laboratories, South Logan, UT). Single-cell suspensions were generated by passage through a 100-μM mesh filter (BD Pharmigen, San Jose, CA). Antibodies used: anti–CD41-APC (eBiosciences, San Diego, CA); anti–CD41-FITC (from BD Pharmigen), anti-CD9-PE (Biolegend, San Diego, CA) and propidium iodide (Sigma-Aldrich).
Ploidy analyses were performed as described in McCrann et al.13 Briefly, bone marrow cells were suspended in PBS and fixed in 70% ethanol at −20°C overnight. Cells were then washed and allowed to react with anti-mouse CD41-FITC antibody for 30 minutes at 4°C. Cells were then stained with propidium iodide for 30 minutes in the dark at 37°C, and cellular DNA content was determined by flow cytometry.
To measure apoptosis, in situ terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) labeling was performed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) according to manufacturer's instructions. Briefly, bone marrow cells were harvested from wild-type and Tg mice and 106 cells were immunostained with anti–CD41-APC for 30 minutes at RT, and fixed in 1% paraformaldehyde for 1 hour at 4°C. Cells were permeabilized by 0.2% Triton X-100, followed by incubation in equilibration buffer for 10 minutes. Cells were incubated with TdT reaction mix for 60 minutes, washed, and resuspended in 20 mM EDTA to stop the reaction.
For 5-bromo-2′-deoxyuridine (BrdU)-labeling experiments, BrdU (2 μg/mouse) was injected intraperitoneally followed by isolation of bone marrow cells 8 hours later. A total of 106 cells were immunostained with anti CD41-APC for 20 minutes at 4°C. The BrdU-incorporated cells were stained with FITC-BrdU Flow Kit (BD Pharmingen) according to the manufacturer's instructions. Dead cells were excluded on the basis of forward and side scatter profiles and 7-AAD staining. Flow cytometry was performed on either a FACS LSR or FACS Canto flow cytometers (BD Biosciences) and analyzed with FloJo Software (TreeStar, Ashland, OR).
Flow cytometry for quantification of megakaryocyte precursor populations
Whole bone marrow cells were flushed from femurs, red blood cells lysed, and single-cell suspensions were achieved by filtration through a 70-μm filter. Live/Dead Aqua Fixable Stain (Life Technologies, Carlsbad, CA) was used. Cells were incubated in PBS with 2% bovine serum albumin, 0.1% sodium azide, and these antibodies: PerCP-Cy5.5-anti-mouse CD150, PE-anti-mouse CD9 (both from BioLegend, San Diego, CA), FITC-anti-mouse CD41, APC-Cy7-anti-mouse FcgRII/III (BD Biosciences), APC-anti-mouse CD42, eFlour700-anti-mouse Sca1, PE-Cy7-anti-mouse-ckit, APC-anti-mouse CD42, eFlour450-anti-mouse CD105, and mouse hematopoietic lineage biotin cocktail plus biotinylated anti-mouse CD4, anti-mouse CD5, anti-mouse CD8 (all from eBiosciences), then with AlexaFlour350-anti-biotin (Invitrogen, Carlsbad, CA). A total of 2 × 106 cells per sample were read on a BD LSR II equipped with Diva software and analyzed using FlowJo. Samples were gated for live, single cells and then by appropriate markers to identify each population.
Gene expression analysis by quantitative polymerase chain reaction
Bone marrow cells were isolated and stained with anti-CD41-APC antibody as described previously. Single-cell suspensions were generated by passage through a 70-μM mesh filter. Large and small CD41+ cells were sorted on FACS Diva (BD Biosciences) directly into 1× PBS, pelleted by centrifugation, and lysed in Trizol (Life Technologies). Total cellular RNA was isolated with Trizol and cDNA was prepared by the oligo(dT) method (Life Technologies) from 1 μg total RNA. Polymerase chain reaction was performed using SYBR green dye on an ABI 7900 real-time machine (Life Technologies). Data were analyzed according to the ΔΔCT method, and messenger RNA (mRNA) levels were normalized to those of glyceraldehyde phosphate dehydrogenase. Murine primer sequences are as follows.
Cyclin A2 forward: CAGCCCTGCTCTCGCTGCAT, reverse: AAGAGGAGCAACCCG TCGAGTCT; Cyclin E forward: CAGAGCAGCGAGCAGGAGA, reverse: CAGCTG CTTCCACACCACTG;
Cyclin F forward: ATGGGGAGCGGCGGTGTGAT, reverse: AGGTGGGAGTGGACAGCTCGG;
Cdk4 forward: GAGCGTTGGCTGTATCTTTG; reverse: TCCTTGTGCAGGTAGGAGTG;
Fas Ligand forward: TGGGTAGACAGCAGTGCCAC; reverse: GCCCACAAGATGGACAGGG;
glyceraldehyde phosphate dehydrogenase forward: CCCCTTCATTGACCTCAACTACA; reverse: CGCTCCTGGAGGATGGTGAT.
Human DSCR1 forward: AGGCTCCAGCTGCATAAGAC; reverse: AGCCAGGTGTGAGCTTCCTA.
Cyclophilin: forward: GAAGAGTGCGATCAAGAACCCATGAC; reverse: GTCTCTCCTCCTTCTCCTCCTATCTTTACTT.
Results
Attenuation of calcineurin-NFAT increases megakaryocyte and platelet numbers in vivo
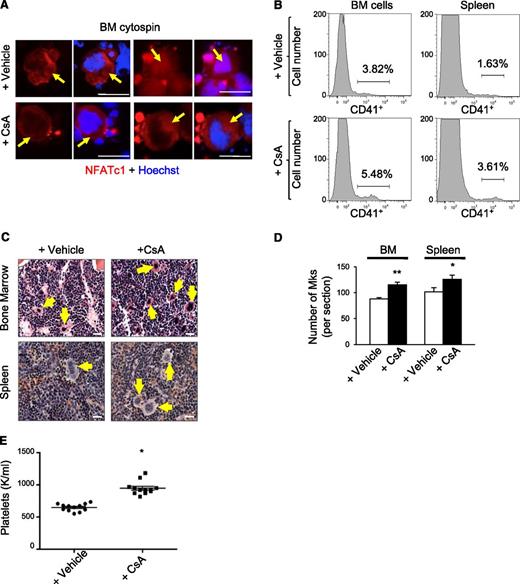
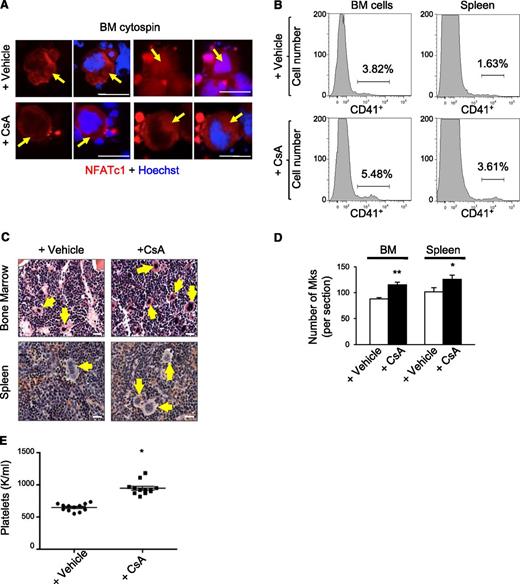
We examined the effects of chronic calcineurin-NFAT inhibition on megakaryopoiesis in vivo by treating wild-type mice daily with the calcineurin inhibitor CsA for 6 weeks. In cycling cells, NFAT shuttles back and forth from the cytoplasm to the nucleus within minutes.14 Accordingly, in both CsA and control (vehicle treated) mice, we detected NFATc1/NFATc2 in the nucleus and cytosol of bone marrow megakaryocytes, which are distinguished by their lobulated polyploid nucleus and large size (Figure 1A and data not shown). However, the proportion of megakaryocytes with nuclear NFATc1 and NFATc2 was reduced more than twofold in CsA-treated mice, consistent with calcineurin inhibition (Figure 1A and supplemental Figure 1A). Flow cytometry analysis of bone marrow and spleen demonstrated two- to threefold increased proportion of cells expressing the megakaryocyte-specific antigen CD41+ in CsA-treated mice compared with controls (Figure 1B and supplemental Figure 1B). In agreement, hematoxylin and eosin staining of femurs demonstrated increased megakaryocytes in CsA-treated mice (Figure 1C-D). CsA-treated mice also exhibited elevated circulating platelet counts as compared with vehicle treated controls (Figure 1E).
CsA stimulates megakaryopoiesis in vivo. (A) Bone marrow cytospins from wild-type mice treated with the calcineurin inhibitor CsA or vehicle for 6 weeks were immunostained for NFATc1 (red) and Dapi (blue) to examine steady-state NFATc1 subcellular localization. Arrows indicate megakaryocytes. Bar represents 50 μM. (B) Representative histogram of CD41-positive bone marrow and spleen megakaryocytes by flow cytometry from wild-type mice treated for 6 weeks with either vehicle alone or CsA. (C) Hematoxylin and eosin images of bone marrow and spleen sections from CsA-treated mice demonstrate increased numbers of megakaryocytes as compared with vehicle-treated mice as evidenced by hematoxylin and eosin. Arrows denote megakaryocytes. Bar represents 25 μM. (D) Quantification of megakaryocytes in bone marrow (BM) and spleen sections counted in 5 low power fields. Values are mean ± S.D. **P < .03; *P < .05. (E) Circulating platelet counts after vehicle or CsA treatment of wild-type mice for 6 weeks; n = 10; *P < .05.
CsA stimulates megakaryopoiesis in vivo. (A) Bone marrow cytospins from wild-type mice treated with the calcineurin inhibitor CsA or vehicle for 6 weeks were immunostained for NFATc1 (red) and Dapi (blue) to examine steady-state NFATc1 subcellular localization. Arrows indicate megakaryocytes. Bar represents 50 μM. (B) Representative histogram of CD41-positive bone marrow and spleen megakaryocytes by flow cytometry from wild-type mice treated for 6 weeks with either vehicle alone or CsA. (C) Hematoxylin and eosin images of bone marrow and spleen sections from CsA-treated mice demonstrate increased numbers of megakaryocytes as compared with vehicle-treated mice as evidenced by hematoxylin and eosin. Arrows denote megakaryocytes. Bar represents 25 μM. (D) Quantification of megakaryocytes in bone marrow (BM) and spleen sections counted in 5 low power fields. Values are mean ± S.D. **P < .03; *P < .05. (E) Circulating platelet counts after vehicle or CsA treatment of wild-type mice for 6 weeks; n = 10; *P < .05.
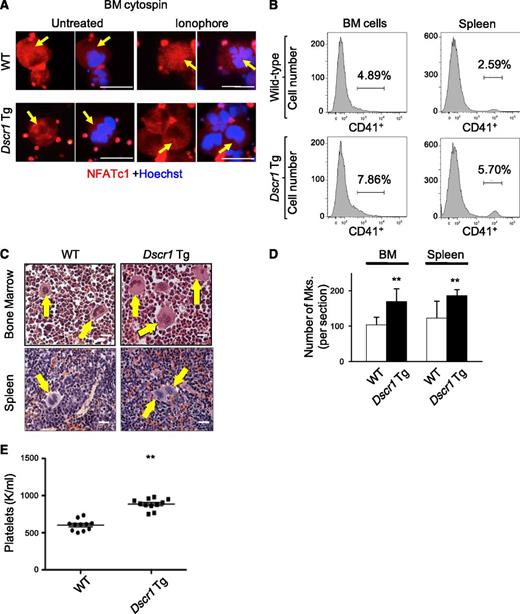
To validate these results in a genetic model of calcineurin suppression, we examined megakaryopoiesis in Tg mice containing a single extra copy of Dscr1 cDNA inserted into the Hprt locus. These animals exhibit a ∼1.5-fold increase in expression of the endogenous calcineurin inhibitor, DSCR1.12 Bone marrow megakaryocytes from control and Dscr1 Tg mice demonstrated NFATc1 and NFATc2 expression in the cytoplasm (Figure 2A and data not shown). However, upon treatment of cells with calcium ionophore to activate calcineurin, NFATc1 relocalized from the cytoplasm to the nucleus in control megakaryocytes but not in Dscr1 Tg ones (Figure 2A and supplemental Figure 1A). Thus, a single extra copy of Dscr1 is sufficient to suppress calcineurin activation preventing NFAT nuclear import. Dscr1 Tg animals exhibited increased megakaryocytes in hematopoietic tissues revealed by flow cytometry analysis for CD41 (Figure 2B and supplemental Figure 1C) and histological examination (Figure 2C-D). In addition, platelet counts were elevated in Dscr1 Tg mice compared with control littermates (Figure 2E). Thus, inhibition of calcineurin-NFAT by systemic CsA treatment or by 50% increased DSCR1 induces megakaryopoiesis and thrombopoiesis.
Increased expression of DSCR1 attenuates calcineurin signaling and leads to an increase in megakaryocytes in vivo. (A) Bone marrow (BM) cytospins from wild-type (WT) and Dscr1 Tg mice were treated with and without ionophore and immunostained with anti-NFATc1 (red) and Hoechst (blue). Arrows indicate megakaryocytes. Bar represents 50 μM. (B) Quantification of CD41-positive BM and spleen megakaryocytes either from WT littermate controls or Dscr1 Tg mice by flow cytometry. (C) Hematoxylin and eosin images of BM and spleen sections from Dscr1 Tg mice demonstrate increased numbers of megakaryocytes. Arrows denote megakaryocytes. Bar represents 25 μM. (D) Quantification of megakaryocytes counted in 5 low power fields is shown on the right. Values are mean ± S.D. **P < .03; *P < .05. (E) Circulating platelet counts in Dscr1 Tg mice and WT littermate controls (n = 11).
Increased expression of DSCR1 attenuates calcineurin signaling and leads to an increase in megakaryocytes in vivo. (A) Bone marrow (BM) cytospins from wild-type (WT) and Dscr1 Tg mice were treated with and without ionophore and immunostained with anti-NFATc1 (red) and Hoechst (blue). Arrows indicate megakaryocytes. Bar represents 50 μM. (B) Quantification of CD41-positive BM and spleen megakaryocytes either from WT littermate controls or Dscr1 Tg mice by flow cytometry. (C) Hematoxylin and eosin images of BM and spleen sections from Dscr1 Tg mice demonstrate increased numbers of megakaryocytes. Arrows denote megakaryocytes. Bar represents 25 μM. (D) Quantification of megakaryocytes counted in 5 low power fields is shown on the right. Values are mean ± S.D. **P < .03; *P < .05. (E) Circulating platelet counts in Dscr1 Tg mice and WT littermate controls (n = 11).
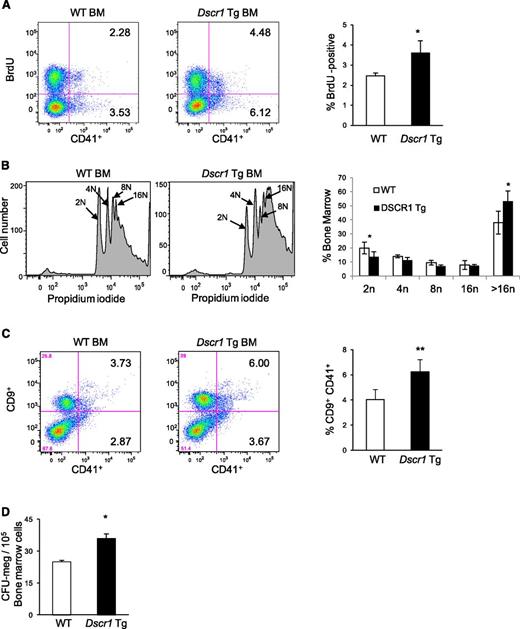
Increased proliferation of megakaryocyte precursors in Dscr1 Tg mice
Expansion of megakaryocytes occurs through increased proliferation, decreased apoptosis, or both. In vivo BrdU uptake demonstrated that DNA replication was increased by about 40% in CD41+ bone marrow megakaryocytes from Dscr1 Tg mice compared with littermate controls (Figure 3A). This could represent either increased cellular proliferation or increased endomitosis, which occurs during megakaryocyte maturation. To distinguish between these possibilities, we examined bone marrow megakaryocyte ploidy by staining the cells for DNA. In Dscr1 Tg mice 53.33% ± 7.3% CD41+ megakaryocytes contained >16N DNA content as compared with 38.1% ± 8.1% in wild-type mice (Figure 3B). A comparable increase in megakaryocytes with >16N DNA content was also observed in megakaryocytes isolated from the spleen of Dscr1 Tg mice (supplemental Figure 2). Thus, there is an increase in mature megakaryocytes in Dscr1 Tg mice. We also examined megakaryocytes for expression of CD9, a surface marker expressed on both early and late megakaryocytes (Figure 3C).15,16 The CD9+CD41+ population was increased in Dscr1 Tg mice. Moreover, bone marrow from Dscr1 Tg mice contained increased CFU-megakaryocytes compared with controls (Figure 3D). Collectively, these findings indicate that calcineurin-NFAT suppression by modest overexpression of DSCR1 causes increased proliferation and accumulation of megakaryocytes in the bone marrow and spleen.
Increased proliferation of megakaryocyte progenitors in Dscr1 transgenic mice. (A) Increased BrdU uptake by CD41+ bone marrow (BM) megakaryocytes from Dscr1 Tg mice as compared with wild-type (WT) littermate controls. Quantification of BrdU-positive CD41+ cells (right); *P < .03 (n = 4). (B) Cellular DNA content was examined in CD41-positive BM-derived megakaryocytes from WT and Dscr1 Tg mice by flow cytometry. WT CD41+ 2N = 36.3% ± 3.4; Dscr1 Tg CD41+ 2N = 49.7% ± 4.7; *P < .03 (n = 7). (C) Increased numbers of megakaryocyte progenitors in the BM of Dscr1 transgenic mice as indicated by flow cytometry of CD9+CD41+ double-positive cells in the BM. Quantification of CD9+CD41+ cells (right); **P < .03 (n = 4). (D) Methylcellulose colony assays of BM cells from WT and Dscr1 Tg mice (n = 8). The numbers of CFU-megakaryocyte colonies are counted after staining with acetylcholinesterase. Results are shown as mean ± SD. *P = .001.
Increased proliferation of megakaryocyte progenitors in Dscr1 transgenic mice. (A) Increased BrdU uptake by CD41+ bone marrow (BM) megakaryocytes from Dscr1 Tg mice as compared with wild-type (WT) littermate controls. Quantification of BrdU-positive CD41+ cells (right); *P < .03 (n = 4). (B) Cellular DNA content was examined in CD41-positive BM-derived megakaryocytes from WT and Dscr1 Tg mice by flow cytometry. WT CD41+ 2N = 36.3% ± 3.4; Dscr1 Tg CD41+ 2N = 49.7% ± 4.7; *P < .03 (n = 7). (C) Increased numbers of megakaryocyte progenitors in the BM of Dscr1 transgenic mice as indicated by flow cytometry of CD9+CD41+ double-positive cells in the BM. Quantification of CD9+CD41+ cells (right); **P < .03 (n = 4). (D) Methylcellulose colony assays of BM cells from WT and Dscr1 Tg mice (n = 8). The numbers of CFU-megakaryocyte colonies are counted after staining with acetylcholinesterase. Results are shown as mean ± SD. *P = .001.
Increases in megakaryocyte progenitor populations in Dscr1 Tg mice
Several markers have been used to describe different megakaryocyte progenitor populations by flow cytometry with cell surface markers that include CD150, CD105, CD9, and CD41.17,18 The bipotential megakaryocyte/erythroid progenitor, called MEP (megakaryocyte/erythroid progenitor), bipotential erythroid/megakaryocyte progenitor, or pre-megakaryocyte/erythrocyte in various studies, contain the majority of cells that later produce platelets in vivo, and have the highest capacity to form megakaryocyte colonies in vitro.17,18 This population is Lin-C-kit+Sca1−FcγRII/III− and CD150+, and is further identified in studies by Pronk et al as CD105− or by Ng et al as CD105loCD9lo.17,18 We used both of these gating strategies (supplemental Figure 3) to identify the MEP population in the bone marrow of wild-type and Dscr1 Tg mice and found an increase in bipotential MEPs in Dscr1 Tg mice (Figure 4A). Because this population contributes the largest percentage of platelets after transplant,18 this finding is in agreement with our finding of increased platelet counts.
Increased megakaryocyte precursors and increased expression of cell cycle–related genes in megakaryocytes from Dscr1 transgenic mice. (A) Percent of bone marrow cells from wild-type (WT) or Dscr1 Tg mice that are the PreMegE (pre-megakaryocyte/erythrocyte) population identified by either CD150+ and CD105− after gating for Lin-C-kit+Sca1− (left) or by CD105loCD9lo expression (right). (B) Quantitative polymerase chain reaction analysis of the indicated cell-cycle genes in sorted populations of small (P1) (left) and large (P2) (right) CD41+ cells; **P < .03; (n = 3) (gated on the live population by excluding 7AAD positive cells).
Increased megakaryocyte precursors and increased expression of cell cycle–related genes in megakaryocytes from Dscr1 transgenic mice. (A) Percent of bone marrow cells from wild-type (WT) or Dscr1 Tg mice that are the PreMegE (pre-megakaryocyte/erythrocyte) population identified by either CD150+ and CD105− after gating for Lin-C-kit+Sca1− (left) or by CD105loCD9lo expression (right). (B) Quantitative polymerase chain reaction analysis of the indicated cell-cycle genes in sorted populations of small (P1) (left) and large (P2) (right) CD41+ cells; **P < .03; (n = 3) (gated on the live population by excluding 7AAD positive cells).
We next examined committed megakaryocyte progenitors by expression of CD41 after gating for Lin−C-kit+Sca1−FcγRII/III-CD150+. This population, which represents an intermediate between the bipotential precursor and a fully mature, platelet producing megakaryocyte, was higher in wild-type mice than in DSCR1 Tg mice (supplemental Figure 4A), perhaps suggesting more rapid turnover of these cells. Next we analyzed CD9, CD41, and CD42 expression in bone marrow and spleen megakaryocytes from Dscr1 Tg mice and littermate controls with a general trend of an increase in all megakaryocyte populations observed in Dscr1 Tg mice (supplemental Figure 4B). Previous studies have shown that CD41+ expression identifies the most broad megakaryocyte population, whereas CD9+CD41+ will identify the majority of early and late megakaryocytes and CD41+CD42+ will identify the most mature megakaryocytes.19,20
Taken together, our finding of increased bipotential precursors, decreased committed megakaryocyte progenitors, and an increase in mature CD41+CD42+ megakaryocytes is consistent with a model in which inhibition of calcineurin-NFAT leads to rapid cycling through the committed megakaryocyte progenitor phase. To test this, we examined expression of cell-cycle regulatory proteins, previously identified to be NFAT targets11,18 in megakaryocyte progenitors from wild-type or Dscr1 Tg mice. Megakaryocyte size increases during cellular maturation, reaching up to 100 μm in diameter in mature polyploid cells.17 Sorting bone marrow megakaryocytes according to size, as reflected by forward scatter (FSC) (supplemental Figure 4C), we examined the expression of cell-cycle genes in both “small” (less mature) and “large” (more mature) megakaryocytes.
Cell-cycle progression genes are upregulated in megakaryocyte progenitors in Dscr1 Tg mice
In lymphocytes, NFAT family members directly repress transcription of genes that drive the cell cycle.10,11,18 In particular, NFATc2 has been shown to repress both cyclin-dependent kinase 4 (Cdk4) and cyclin A2 (Ccna2).9,21 Consequently, NFATc2-deficient mice demonstrate lymphocyte hyperproliferation and increased cyclin expression.21 We examined the expression of Cdk4, Ccna2, and other cell-cycle regulatory mRNAs in fluorescence-activated cell sorter–purified FSC low (less mature) and FSC high (more mature) CD41+ bone marrow megakaryocytes isolated from Dscr Tg mice and littermate controls (Figure 4B). The mRNA expression of Cdk4, Ccna2, and cyclin F (Ccnf) were all significantly upregulated in FSC low CD41+ bone marrow megakaryocytes from Dscr1 mice, whereas cyclin E (Ccne1) levels were unchanged (Figure 4B). In contrast, only Ccna2 mRNA was modestly increased in large CD41+ megakaryocytes from Dscr1 vs wild-type mice (Figure 4B). These data indicate that NFAT represses a subset of cell cycle–promoting genes during the early stages of megakaryopoiesis.
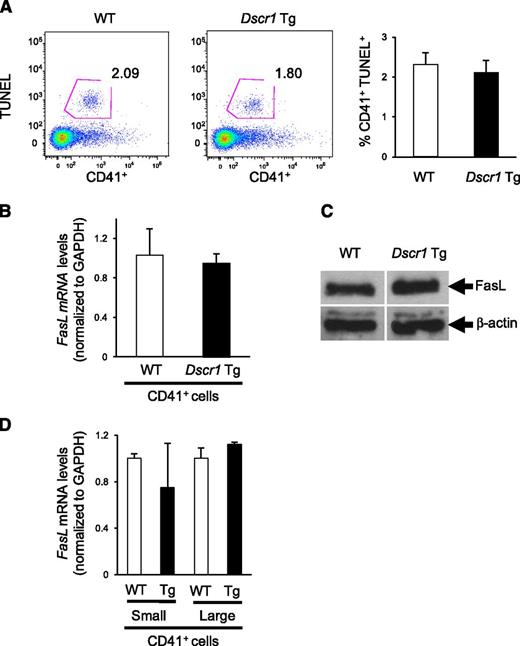
Suppression of calcineurin signaling does not affect megakaryocyte apoptosis or Fas ligand expression
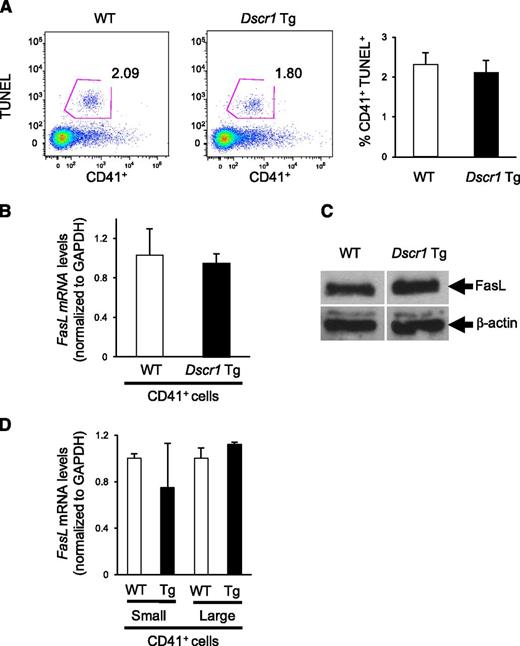
Because previous studies identified Fas ligand (FASLG) as a calcineurin-NFAT target in human megakaryocytes,3,4 we examined both megakaryocyte apoptosis as well as the expression of Fas ligand in bone marrow derived CD41+ cells from Dscr1 Tg and control mice. There was no difference in TUNEL reactivity of CD41+ cells isolated from either Dscr1 Tg or control mice (Figure 5A), indicating similar levels of apoptosis. Correspondingly, upon examination of Fas ligand expression in these cells, we found that both Fas ligand (Faslg) mRNA levels (Figure 5B) and protein expression (Figure 5C) were similar in CD41+ bone marrow megakaryocytes from Dscr1 Tg and control mice. To ensure that NFAT-mediated Faslg regulation was not unique to early megakaryopoiesis, as observed for cell-cycle regulatory genes (Figure 4B), we sorted CD41+ bone marrow cells from Dscr1 Tg and wild-type mice by size (FSC) and quantified Faslg mRNA expression. There was no significant difference in the levels of Faslg mRNA in either small or large CD41+ megakaryocytes or in Dscr1 Tg megakaryocytes as compared with littermate controls (Figure 5D). Finally, we examined circulating thrombopoietin (TPO) levels in CsA-treated mice or Dscr1 Tg mice because TPO is a critical regulator of megakaryopoiesis. We found no differences in TPO expression between wild-type, CsA-treated mice, or Dscr1 Tg mice (supplemental Figure 5), suggesting that inhibition of calcineurin-NFAT signaling had no effect on the production of the megakaryocyte cytokine, TPO.
Megakaryocyte apoptosis and Fas ligand expression is unaffected in Dscr1 transgenic mice. (A) Flow cytometry of TUNEL+ CD41+ bone marrow–derived megakaryocytes from wild-type (WT) and Dscr1 Tg mice. Quantification of CD41+TUNEL+ cells (right). (B) Quantitative polymerase chain reaction analysis of Fas ligand mRNA in sorted CD41+ bone marrow cells (n = 3). (C) Western blot analysis of Fas ligand expression in sorted CD41+ bone marrow cells demonstrate no difference in WT vs Dscr1 Tg megakaryocytes. β-actin was probed as a loading control. (D) Quantitative polymerase chain reaction analysis of Fas ligand mRNA in sorted populations of small and large CD41+ bone marrow cells (n = 3).
Megakaryocyte apoptosis and Fas ligand expression is unaffected in Dscr1 transgenic mice. (A) Flow cytometry of TUNEL+ CD41+ bone marrow–derived megakaryocytes from wild-type (WT) and Dscr1 Tg mice. Quantification of CD41+TUNEL+ cells (right). (B) Quantitative polymerase chain reaction analysis of Fas ligand mRNA in sorted CD41+ bone marrow cells (n = 3). (C) Western blot analysis of Fas ligand expression in sorted CD41+ bone marrow cells demonstrate no difference in WT vs Dscr1 Tg megakaryocytes. β-actin was probed as a loading control. (D) Quantitative polymerase chain reaction analysis of Fas ligand mRNA in sorted populations of small and large CD41+ bone marrow cells (n = 3).
Human trisomy 21 fetal liver–derived megakaryocytes show impaired calcineurin-NFAT signaling
We previously reported that human trisomy 21 (T21) fetal liver megakaryocytes proliferate excessively.22 We confirmed this finding on additional samples of T21 fetal liver (Figure 6A) and showed that DSCR1 mRNA is upregulated in human T21 megakaryocytes compared with control euploid cells (Figure 6B). Specifically, DSCR1 mRNA levels in T21 fetal liver megakaryocytes was increased greater than twofold (P = .035) (Figure 6B). Western blotting showed that T21 fetal liver megakaryocytes express modestly elevated levels of DSCR1 protein and exhibit suppression of NFATc2, as evidenced by its increased phosphorylation (Figure 6C) and reduced nuclear localization, after calcium ionophore treatment (Figure 6D). Thus, increased proliferation of megakaryocytes in human trisomy 21 is associated with elevated levels of DSCR1 and suppression of NFAT.
Calcineurin-NFAT activation is suppressed in human trisomy 21 fetal liver megakaryocytes. (A) Megakaryocyte growth in liquid culture shown as fold change of CD41+ cells (n = 5 euploid (wild-type [WT]) and 5 trisomy 21 [T21]). Results are shown as mean ± SEM. (B) Quantitative polymerase chain reaction analysis of DSCR1 mRNA in euploid (WT) and T21 fetal liver megakaryocytes relative to cyclophilin; P = .035 (n = 5 WT and 5 T21). (C) Western blot indicates increased expression of DSCR1 and phosphorylated NFATc2 (p-NFATc2) in T21 fetal liver megakaryocytes compared with control. (D) Control and T21 fetal liver megakaryocytes were immunostained for NFATc2 (red) and Hoechst (blue) before and after ionophore treatment. Arrows denote megakaryocytes.
Calcineurin-NFAT activation is suppressed in human trisomy 21 fetal liver megakaryocytes. (A) Megakaryocyte growth in liquid culture shown as fold change of CD41+ cells (n = 5 euploid (wild-type [WT]) and 5 trisomy 21 [T21]). Results are shown as mean ± SEM. (B) Quantitative polymerase chain reaction analysis of DSCR1 mRNA in euploid (WT) and T21 fetal liver megakaryocytes relative to cyclophilin; P = .035 (n = 5 WT and 5 T21). (C) Western blot indicates increased expression of DSCR1 and phosphorylated NFATc2 (p-NFATc2) in T21 fetal liver megakaryocytes compared with control. (D) Control and T21 fetal liver megakaryocytes were immunostained for NFATc2 (red) and Hoechst (blue) before and after ionophore treatment. Arrows denote megakaryocytes.
Discussion
The calcineurin-NFAT pathway regulates gene expression in response to changes in intracellular calcium concentration and plays important roles in many tissues, including lymphocytes, neurons, and myocytes.23,24 One study indicated that genetic ablation of the calcineurin B subunit in bone marrow–derived stem cells did not affect the production of nonlymphoid hematopoietic cells.25 However, several more recent lines of evidence suggest roles for calcineurin-NFAT in megakaryopoiesis. First, expression of NFAT isoforms c1, c2, and perhaps c4 increases as CD34+ hematopoietic progenitors differentiate into megakaryocytes.3,7 Second, activation of NFAT inhibits growth of cultured megakaryocytes and megakaryocyte-like cell lines.2 Third, NFAT target genes including RCAN1/DSCR1, CD40LG, and FASLG are induced by NFAT in cultured megakaryocytes and megakaryocytic cell lines.2,6,26 RCAN1/DSCR1 is an Hsa21-encoded calcineurin inhibitor that likely participates in a feedback loop to repress NFAT in calcium-stimulated megakaryocytes during normal megakaryopoiesis as has been shown in other cell types.26-31 Finally, inhibition of NFAT is implicated in the pathogenesis of several disorders of megakaryopoiesis including idiopathic myelofibrosis32 and Down syndrome–associated leukemia.8,9,26 Here we show that inhibition of calcineurin-NFAT by systemic treatment with CsA or Tg overexpression of Dscr1 increases megakaryocyte and platelet numbers in mice. In addition, human trisomy 21 megakaryocytes proliferate excessively in culture and express increased DSCR1 protein associated with decreased NFAT activation. Taken together, these current and previous findings indicate that calcineurin-NFAT inhibits normal and pathological megakaryopoiesis.
Calcineurin-NFAT likely regulates megakaryocyte biology through multiple mechanisms. Our data show that in vivo inhibition of this pathway derepresses Cdk4, Ccna2, and Ccnf in young megakaryocytes and/or megakaryocyte progenitors obtained from bone marrow, thereby enhancing their mitotic rate and expansion. Similarly, calcineurin-NFAT negatively regulates T-cell proliferation via transcriptional repression of an overlapping set of cell-cycle stimulatory genes.9 Recent studies by Arabanian et al showed that in culture-derived human primary megakaryocytes and megakaryocytic cell lines, NFAT induces FASLG expression and stimulates apoptosis through interactions with the Fas death receptor, which is expressed on the same cells.1,2 NFAT is known to negatively regulate lymphocyte numbers by activating FASLG to induce apoptosis.8,33 In agreement with these findings, we found that inhibition of calcineurin-NFAT decreased FASLG expression in cultured megakaryocytes derived from murine fetal liver and in human megakaryocytic cell lines (data not shown). In contrast, our data indicate that chronic inhibition of NFAT by CsA or increased expression of the endogenous calcineurin inhibitor DSCR1 stimulates adult murine megakaryopoiesis in the bone marrow not by repressing FASLG-induced apoptosis, but rather by driving proliferation of megakaryocyte progenitors via enhanced expression of genes that promote cell-cycle progression.
The differences we observed in NFAT activation of Faslg in various megakaryocyte populations may be due in part to the use of cultured fetal liver megakaryocytes and megakaryocyte cell lines as compared with primary endogenous megakaryocytes harvested directly from the bone marrow. It is also possible that NFAT regulates different genes in fetal vs adult megakaryocytes. Previously published studies have demonstrated differences in size, proliferation, and gene expression between fetal liver and adult bone marrow–derived megakaryocyte progenitors that could be due to microenvironmental or cell-intrinsic differences.34-36 NFAT target selection in different cells is undoubtedly modulated by developmental stage, microenvironmental niche, chronicity and amplitude of calcineurin activation, and concomitant influences of other signaling pathways that converge on gene expression. Collective evidence suggests that calcineurin-NFAT signaling affects megakaryocytes through cell intrinsic effects. However, our experiments employing global systemic inhibition of NFAT signaling do not exclude the possibility of indirect effects exerted through alterations in the bone marrow microenvironment. This possibility can be explored in the future through tissue specific gene manipulation and bone marrow transplantation studies.
The calcineurin inhibitor FKBP51 is upregulated in megakaryocytes of patients with idiopathic myelofibrosis and may contribute to the pathophysiology of this disorder.5 Because our data show that attenuation of calcineurin-NFAT signaling stimulates megakaryocyte development in healthy mice, it is plausible to assume that CsA or FK506 could stimulate megakaryocyte and platelet production in patients. However, CsA used clinically for immunosuppression is reported to exert variable effects on platelet counts.37-39 The diverse effects of the underlying disorders of these immunosuppressed patients undoubtedly complicate interpretation of these findings.
Our data have implications for understanding the pathogenesis of Down syndrome–associated acute megakaryoblastic leukemia. At least 3 genetic aberrations contribute to this malignancy: germline trisomy 21, somatic mutations in the transcription factor gene GATA1, and additional acquired mutations in genes that are largely undefined. Human fetal liver hematopoietic progenitors with trisomy 21 and normal GATA1 alleles contain expanded populations of erythro-megakaryocytic precursors and demonstrate accelerated megakaryopoiesis in vitro.22,40 Thus, trisomy 21 itself drives megakaryopoiesis. However, similar to patients on immunosuppressive therapy, the effects of trisomy 21 on platelet counts in infants with Down syndrome have been variable as well, with some but not all individuals showing thrombocytosis or thrombocytopenia.41-44
Moreover, Hsa21 encodes at least 3 negative regulators of calcineurin-NFAT: DSCR1, DYRK1A, and PCP4. Modest increases in 1 or more of the corresponding proteins conferred by trisomy 21 can produce profound developmental effects by inhibiting NFAT signaling. For example, increased DSCR1 and DYRK1A in Tg mice reduce NFAT activity and recapitulate human Down syndrome cardiac defects.45 Dscr1 Tg mice expressing ∼1.5-fold increased DSCR1 protein expression exhibit endothelial cell defects that restrict angiogenesis, possibly explaining why patients with Down syndrome experience reduced incidence of solid tumors.11,46 Finally, trisomy 21–mediated overexpression of DYRK1A with resultant NFAT activation promotes oncogenesis in a recently described model for murine Down syndrome associated megakaryoblastic leukemia.1 Here we show that decreased NFAT activation caused by ∼1.5-fold increased Dscr1 expression in mice drives megakaryopoiesis and that trisomy 21 human fetal liver–derived megakaryocytes exhibit elevated DSCR1 expression with inactive NFATc2. These findings indicate that increased dosages of 1 or more Hsa21-encoded calcineurin-NFAT inhibitors have major influences on the pathophysiology of Down syndrome. In particular, NFAT inhibition may facilitate early steps in the genesis of Down syndrome–associated acute megakaryoblastic leukemia by expanding an immature megakaryocyte population that is susceptible to further genetic events, including acquisition of mutations in GATA1 and other genes. Determining the role of the calcineurin-NFAT signaling pathway in megakaryopoiesis could identify more effective and less toxic treatments for both Down syndrome–transient myeloproliferative disorder and acute megakaryoblastic leukemia. In addition, improved understanding of calcineurin-NFAT signaling in megakaryopoiesis could illustrate new strategies to raise platelet counts in thrombocytopenia of various etiologies to enhance the production of megakaryocytes and platelets in vitro and to inhibit pathological megakaryopoiesis in conditions such as idiopathic myelofibrosis and essential thrombocytosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Joseph Italiano, members of the S. Ryeom laboratory, and Yu Yao for technical assistance and helpful discussions.
This work was supported by grants from the National Institutes of Health (5T32CA009140-38) (K.S.), (K08 HL093290) (S.T.C.), (RC2 HL101606 and P30DK090969) (M.J.W.), (P01 CA045548 and R01 CA118374) (S.R.); American Society of Hematology (S.T.C.); Leukemia and Lymphoma Foundation (M.J.W.); Garrett B. Smith Foundation (S.R.); TED-driven Foundation (S.R.); and Jerome LeJeune Foundation (S.R.).
Authorship
Contribution: A.Z., S.T.C., and K.S. designed and performed experiments, interpreted results, and wrote the manuscript; A.L., M.P., Y.J.K., and K.-H.B. performed experiments, data analysis, and interpreted results; W.C.A. generated the mouse model and analyzed data; M.J.W. designed experiments, interpreted results, formulated discussions, and assisted with manuscript preparation and editing; and S.R. designed experiments, interpreted results, formulated discussions, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra Ryeom, Department of Cancer Biology, Abramson Family Cancer Research Institute, University of Pennsylvania School of Medicine, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: sryeom@upenn.edu.
References
Author notes
A.Z., S.T.C., and K.S. contributed equally to this work.



![Figure 6. Calcineurin-NFAT activation is suppressed in human trisomy 21 fetal liver megakaryocytes. (A) Megakaryocyte growth in liquid culture shown as fold change of CD41+ cells (n = 5 euploid (wild-type [WT]) and 5 trisomy 21 [T21]). Results are shown as mean ± SEM. (B) Quantitative polymerase chain reaction analysis of DSCR1 mRNA in euploid (WT) and T21 fetal liver megakaryocytes relative to cyclophilin; P = .035 (n = 5 WT and 5 T21). (C) Western blot indicates increased expression of DSCR1 and phosphorylated NFATc2 (p-NFATc2) in T21 fetal liver megakaryocytes compared with control. (D) Control and T21 fetal liver megakaryocytes were immunostained for NFATc2 (red) and Hoechst (blue) before and after ionophore treatment. Arrows denote megakaryocytes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/16/10.1182_blood-2012-04-421172/4/m_3205f6.jpeg?Expires=1769097617&Signature=RcTQXV5wXGZa83Hwn~c84THKt2OCwp6GSzkf7oCqKONbJhDlhZOyScWjQTKsQ8dJdcWNkIjM2UlnEiWZTjzDCHpR8NhRbWY6Cp8c6uE8iFet2D-DPBboWrGjynZdsfxjBR~3pAwSR01~PjEPXPCGVke3lqABRDcgdZj6TWJWCGD7fcYy~JrdUuIeaB0R4fGWKNda0wZSvfgS7OSFekAybkUDrgVWZQLvglMkAYaI0vW86GoOCb8YAqt9nzPytMzWnwOc6tr5jUsfPnYRGnwzXEOPjq605WfMk40JEcw4id0FBFxtSUdadUT8CDXv~cwdX84u2FIYOdjJ4PsDS-4YLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 6. Calcineurin-NFAT activation is suppressed in human trisomy 21 fetal liver megakaryocytes. (A) Megakaryocyte growth in liquid culture shown as fold change of CD41+ cells (n = 5 euploid (wild-type [WT]) and 5 trisomy 21 [T21]). Results are shown as mean ± SEM. (B) Quantitative polymerase chain reaction analysis of DSCR1 mRNA in euploid (WT) and T21 fetal liver megakaryocytes relative to cyclophilin; P = .035 (n = 5 WT and 5 T21). (C) Western blot indicates increased expression of DSCR1 and phosphorylated NFATc2 (p-NFATc2) in T21 fetal liver megakaryocytes compared with control. (D) Control and T21 fetal liver megakaryocytes were immunostained for NFATc2 (red) and Hoechst (blue) before and after ionophore treatment. Arrows denote megakaryocytes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/16/10.1182_blood-2012-04-421172/4/m_3205f6.jpeg?Expires=1769411438&Signature=yuyKPG~kMk52SUE67vG3V7NBycrvc33gLGbcKIewTGXgas6CkrPFhTUifzR3W1-SEnpHnYiChPn-IrpTwrlbFpZCxZWEqQqHX3jY-6hHjHZzvK4DdHnw~S5vjEDu~XXIuR5~ukM3HnAmrnIaW3HotebntCPNw3g33ViPP4qXcnuKM7bmLmg3mILbhRlt-MuxqySMyan3o9PuPs8kEyQrY3U~XbI1Y7xe0kp3u2VLsChan8omO26SMjG-EGCjVoFnvLfrX~wFGYBm-fAt9uuwt0EHzvINd6OmhVqm4hQb8wx8zb75etMAxcgH-h43bpso~0ArpbF~7waFsCLE~Z5F8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)