Key Points
Serum levels of galectin-1 are significantly higher in patients with cHL than in normal controls.
Galectin-1 serum levels are associated with clinical parameters of tumor burden in patients with cHL.
Abstract
Galectin-1 (Gal1) is a member of a highly conserved family of carbohydrate-binding proteins. It modulates innate and adaptive immune responses and fosters tumor-immune escape. Hodgkin lymphoma (HL) Reed-Sternberg cells overexpress and secrete Gal1, which selectively kills T helper (Th)1 and Th17 cells and cytotoxic T cells and promotes the immunosuppressive Th2/regulatory T-cell–predominant HL microenvironment. We developed a sandwich enzyme-linked immunosorbent assay and assessed serum Gal1 levels in 293 newly diagnosed, previously untreated patients with classical HL (cHL) enrolled in 3 risk-adapted clinical trials. Serum Gal1 levels were significantly higher in patients with cHL than in normal controls (P < .0001). Gal1 serum levels also increased with Ann Arbor stage (P = .012), areas of nodal involvement (P < .0001), and the International Prognostic Score (2-7, P = .019). We conclude that Gal1 serum levels are significantly associated with tumor burden and related clinical features in newly diagnosed cHL patients.
Introduction
Galectin-1 (Gal1), a member of a highly conserved family of carbohydrate-binding proteins, modulates immune responses and fosters tumor-immune escape through specific recognition of N-acetyllactosamine (Gal-β1-4-NAcGlc) residues on the branches of N- or O-linked glycans.1-4 Although Gal1 induces apoptosis of T helper (Th)1 and Th17 cells and cytotoxic T cells, Th2 cells resist Gal1-induced cell death through differential sialylation of cell surface glycoproteins.5 Gal1 also promotes the expansion of regulatory T (Treg) cells,1,5 and Gal1-glycan interactions augment hypoxia-driven tumor angiogenesis.6
Primary classical Hodgkin lymphomas (cHLs) include small numbers of malignant Hodgkin Reed-Sternberg (HRS) cells within a Th2/Treg-skewed inflammatory infiltrate.1,7 In previous studies, we found that HRS cells overexpress Gal1, which selectively kills Th1 and cytotoxic T cells and promotes the immunosuppressive Th2/Treg-predominant Hodgkin lymphoma (HL) microenvironment.1 In cHLs, which exhibit constitutive activation of the activator protein 1 (AP-1) signaling pathway, Gal1 overexpression is driven in large part by an AP-1–dependent enhancer.1,8 In recent immunohistochemical analyses of primary cHLs, increased Gal1 expression was associated with poorer event-free survival.9
Given the broadly immunosuppressive activities of Gal1, we reasoned that this soluble lectin might be a potent marker of disease activity and a novel therapeutic target in cHLs. We previously developed a panel of Gal1 monoclonal antibodies (mAbs) and demonstrated the utility of Gal1 as a diagnostic marker of AP-1–dependent lymphoid malignancies.8,10 A potent neutralizing Gal1 mAb also protected Th1 and cytotoxic T cells from Gal1-induced apoptosis,10 abrogated Gal1-associated tumor angiogenesis,6 and limited the growth of Gal1+ tumors in vivo.6
We have now used a newly developed sandwich enzyme-linked immunosorbent assay (ELISA) to assess the utility of Gal1 as a serum marker of disease burden in cHLs.
Study design
Patients and samples
For this retrospective study, we used frozen serum samples and clinical data from 293 newly diagnosed, previously untreated patients with cHL who were enrolled on Institutional Review Board–approved, risk-adapted German Hodgkin Study Group (GHSG) multicenter clinical trials: HD13 (ISRCTN63474366) for early-stage low-risk disease (clinical stage [CS] IA-IIB with no risk factors, 80 patients)11,12 ; HD14 (ISRCTN04761296) for early-stage disease with risk factors (CS I-IIA with large mediastinal mass, extranodal disease, elevated erythrocyte sedimentation rate (ESR) or >3 nodal areas and CS IIB with elevated ESR or >3 nodal areas, 89 patients)11,13 ; and HD18 (NCT00515554) for bulky localized or advanced-stage disease (CS IIB with bulky mediastinal involvement and/or extranodal involvement and CS III or IV, 124 patients).11,14 In addition, serum samples from 15 healthy normal donors were prepared by centrifuging clotted peripheral blood at 2500 rpm for 20 minutes. This study was conducted in accordance with the Declaration of Helsinki.
Gal1 sandwich ELISA
An anti-Gal1 rabbit polyclonal antibody (capture antibody) and a biotin-coupled murine mAb, 8A12 (detection antibody), were generated in our laboratory8,10 and determined to have optimal sensitivity and signal-to-noise ratio. Serum Gal1 levels were assessed according to a standard sandwich ELISA protocol. In brief, 96-well EIA/RIA microplates (Fisher Scientific, Pittsburgh, PA) were precoated with capture antibody at 4 μg/mL (100 μL per well, diluted in 0.05 mol/L carbonate-bicarbonate buffer) at 4°C overnight. After 3 washes with phosphate-buffered saline/0.05% Tween 20 (Sigma-Aldrich, St. Louis, MO), the plate was treated with blocking buffer (1% bovine serum albumin in phosphate-buffered saline/0.05% Tween 20) at room temperature (RT) for 1 hour. Serum-free conditioned media from a Gal1+ HL line, L428, and a serum sample from a healthy normal donor (ND#1) were used as controls in each ELISA plate. All samples were diluted (serum samples, 1:16; L428 serum-free conditioned media, 1:32) and added in duplicates, and then incubated at RT for 2 hours. After the incubation with the detection antibody, biotinylated 8A12 (1:1000), at RT for 2 hours, 100 μL of strepavidin-horseradish peroxidase (1:15 000; Thermo Scientific, Rockford, IL) was added for incubation at RT for 20 minutes. After 5 washes, the reaction was developed by 100 μL of 1-Step Turbo TMB (Thermo Scientific) and stopped by 1 mol/L sulfuric acid. Absorbance at 450 and 570 nm were determined in a SpectraMax M3 Absorbance Microplate Reader (Molecular Devices Inc, Sunnyvale, CA). A standard curve of recombinant His-tagged Gal1 (rGal1) at concentrations of 80 to 0.312 ng/mL with 1:2 serial dilutions (9 points total) was generated and fitted using a 4-parameter nonlinear regression curve for each ELISA. Sample concentrations were calculated by regression analysis using the standard curve.
Statistical analysis
Data analysis was carried out using SAS software version 9.2 (SAS Institute Inc., Cary, NC). A receiver operating characteristic (ROC) curve was plotted to determine the cutoff values for normal vs elevated serum Gal1. Because of skewed distributions, nonparametric analyses of serum Gal1 values were performed using the Wilcoxon 2-sample test or Kruskal-Wallis test for group comparisons. Univariate analyses included Ann Arbor stage (I-IV), number of involved nodal sites (≥3), presence of B symptoms, extranodal disease, and elevated ESR, all of which are well-established parameters of HL tumor burden. Serum Gal1 levels were also analyzed with respect to male sex, age of 45 years or older, stage IV disease, low serum albumin, leukocytosis, and lymphocytopenia, because of the prognostic relevance of these factors in the International Prognostic Score (IPS)15,16 for advanced-stage HL. Nominal P values are presented.
Results and discussion
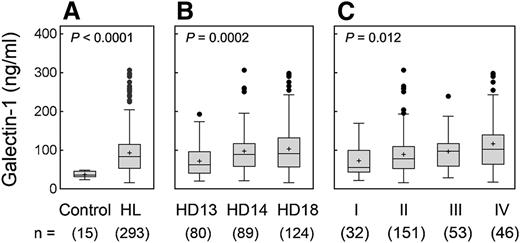
After establishing a sandwich ELISA with purified rGal1 and the newly developed Gal1 antibodies, we assessed the levels of serum Gal1 in 15 healthy, normal donors and 293 newly diagnosed, previously untreated patients with cHL from the GHSG. Serum Gal1 levels were significantly elevated in patients with cHL in comparison with normal controls (P < .0001) (Figure 1A). By plotting a ROC curve, we found that a cutoff value of 49.9 ng/mL distinguished the Gal1 serum levels of patients with cHL from those of normal donors with 100% specificity and 76.5% sensitivity, respectively (data not shown). Patients enrolled on the HD13 trial (for early-stage, low-risk disease), had significantly lower Gal1 levels than patients on HD14 (for early-stage disease with additional risk factors) or HD18 (for bulky localized or advanced-stage disease) (HD13 vs HD14 vs HD18, P = .0002) (Figure 1B and Table 1).
Gal1 serum levels in cHL patients. Gal1 serum levels were assessed with a sandwich ELISA. (A) Gal1 levels in cHL patients and normal healthy donors (93.0 ± 56.5 ng/mL vs 36.9 ± 7.8 ng/mL, P < .0001). (B) Gal1 levels in patients on the risk-adapted clinical trials, HD13 (early-stage low-risk), HD14 (early-stage with risk factors), or HD18 (bulky localized or advanced-stage disease). (C) Gal1 levels in cHL patients with Ann Arbor stage I, II, III, or IV disease. Nominal P values are presented.
Gal1 serum levels in cHL patients. Gal1 serum levels were assessed with a sandwich ELISA. (A) Gal1 levels in cHL patients and normal healthy donors (93.0 ± 56.5 ng/mL vs 36.9 ± 7.8 ng/mL, P < .0001). (B) Gal1 levels in patients on the risk-adapted clinical trials, HD13 (early-stage low-risk), HD14 (early-stage with risk factors), or HD18 (bulky localized or advanced-stage disease). (C) Gal1 levels in cHL patients with Ann Arbor stage I, II, III, or IV disease. Nominal P values are presented.
We next evaluated the potential association of Gal1 serum levels with Ann Arbor stage and B symptoms, 2 major determinants for assigning HL patients to risk-adapted therapy.17 Gal1 levels increased with Ann Arbor stage (I vs II vs III vs IV, P = .012, Figure 1C and Table 1; I-II vs III-IV, P = .006, data not shown) and with B symptoms (P = .047) (Table 1).
We also assessed the potential association of Gal1 levels with the HL IPS using the accepted groupings of IPS 0/1 vs 2-7.15,16 Patients with an IPS of 2-7 had significantly higher Gal1 levels than patients with an IPS of 0 or 1 (P = .019) (Table 1). Increased Gal1 levels were also associated with 2 of 7 individual IPS risk factors15,16 : extranodal involvement (stage IV disease, P = .011) and lymphocyte count <600 per mm3 or <8% of total white cell count (P = .036). Gal1 levels were also significantly elevated in patients with cHL and 2 additional adverse prognostic factors18 : ≥3 involved nodal sites (P < .0001) and elevated ESR (P = .007) (Table 1). Direct analyses of the association between Gal1 serum levels and outcome await completion of the ongoing HD18 clinical trial.14
In conclusion, Gal1 serum levels are elevated and associated with clinical features reflective of increased tumor burden in newly diagnosed cHL patients. Given the demonstrated role of Gal1 in tumor-immune escape, angiogenesis, and metastasis, analyses of circulating Gal1 levels may inform risk-adapted and targeted treatment strategies for patients with cHL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the German Hodgkin Study Group for its participation in this study.
This work was supported by the Leukemia and Lymphoma Society (SCOR #7009-12) (M.A.S.) and (DFG #SFB832, TP19) (E.P.v.S.).
Authorship
Contribution: J.O. designed and performed research, collected, analyzed and interpreted data, and wrote the manuscript; A.P. performed statistical analysis; E.P.v.S. contributed vital reagents and clinical data; K.S.R. contributed vital reagents; S.P. contributed vital reagents and clinical data; G.A.R. analyzed the data; D.N. performed statistical analysis; A.E. contributed vital reagents and clinical data and analyzed and interpreted data; and M.A.S. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: J.O., G.A.R., and M.A.S. have submitted a patent application on galectin-1 monoclonal antibodies. The remaining authors declare no competing financial interests.
Correspondence: Margaret A. Shipp, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: margaret_shipp@dfci.harvard.edu.