Key Points
Independent predictors of stable, undetectable BCR-ABL1 during first-line imatinib therapy were female sex and the BCR-ABL1 value at 3 months.
Time to achieve an MMR influenced time to stable, undetectable BCR-ABL1, suggesting slower dynamics of BCR-ABL1 decline with delayed MMR.
Recent studies have demonstrated that some patients with chronic myeloid leukemia (CML) can maintain remission after discontinuation of imatinib. A prerequisite is stable, undetectable BCR-ABL1. It is not known how many patients achieve this response or the factors associated with its achievement. We examined 423 de novo imatinib-treated patients to determine the cumulative incidence of achieving the discontinuation criteria as defined in the CML8 study (≥2 years of undetectable BCR-ABL1 [Stable MR4.5]), and predictive factors. After 8 years of imatinib, the cumulative incidence of Stable MR4.5 was 36.5%. Therefore, 9% to 15% of first-line imatinib-treated patients would maintain remission after discontinuation. The BCR-ABL1 level at 3 months and factors at diagnosis were examined for association with Stable MR4.5: Sokal risk, age, sex, and assigned imatinib dose. The only independent predictors were female sex (54.4% vs 27.2%; P = .018) and the 3-month BCR-ABL1 (P < .001). The highest cumulative incidence of Stable MR4.5 after 8 years was 78.2% for patients with BCR-ABL1 ≤ 0.10%IS at 3 months (n = 38). Time to major molecular response (MMR) influenced the time to reach Stable MR4.5 (P < .001), suggesting slower dynamics of response with a delayed MMR. The findings justify the focus on rapid reduction of BCR-ABL1 as a strategy to maximize potential suitability for imatinib discontinuation studies. The Iris trial was registered at http://www.clinicaltrials.gov as NCT00006343. The Tops trial was registered at http://www.clinicaltrials.gov as NCT00124748. The TIDEL I trial was registered at www.ANZCTR.org.au as ACTRN12607000614493. The TIDEL II trial was registered at www.ANZCTR.org.au as ACTRN12607000325404.
Introduction
Imatinib therapy has radically changed the prognosis for patients with chronic myeloid leukemia (CML), and most enjoy a normal life expectancy.1 Despite excellent efficacy, treatment-related adverse effects remain problematic for some patients and can reduce quality of life. This is particularly evident for younger patients.2 Those 18 to 39 years old receiving long-term imatinib therapy reported lower physical and emotional functioning compared with matched healthy population control participants, resulting in restrictions in work and other daily activities. Fatigue was the most commonly reported symptom in adults.2
The opportunity to maintain remission after discontinuation of imatinib would be desirable for many patients otherwise facing the prospect of life-long therapy. Imatinib discontinuation studies have demonstrated that approximately 40% of patients can maintain remission at 12 to 36 months after imatinib discontinuation, if the patients are carefully selected.3,,-6 The first prospective studies that demonstrated a sustained response after discontinuation only included patients with stable, undetectable BCR-ABL1 transcripts using strict polymerase chain reaction (PCR) sensitivity criteria.3,,-6 Imatinib discontinuation in patients with persisting detectable BCR-ABL1, regardless of the quality of molecular or cytogenetic response, led to relapse in all patients.7,8 The Stop Imatinib (STIM) trial and the Australasian Leukaemia & Lymphoma Group (ALLG) CML8 discontinuation trial used similar PCR sensitivity criteria for patient selection, and the overall probability of maintaining molecular remission after stopping imatinib was similar: 39% and 42%, respectively.3,,-6 For patients without prior interferon-alpha therapy, the probability of maintaining remission was slightly lower: 34% and 32%, respectively.3,,-6
The proportion of first-line imatinib-treated patients who reach the STIM or CML8 criteria for discontinuation is currently unknown. However, it is known that BCR-ABL1 transcripts remain detectable for many years in most patients treated with imatinib.9 Consequently, identifying the factors associated with stable, undetectable BCR-ABL1 may lead to rationally designed therapeutic strategies aimed at increasing the proportion of patients who are eligible for discontinuation studies. Therefore, we assessed factors at commencement of first-line imatinib therapy and the dynamics of BCR-ABL1 decline during therapy to determine their association with stable, undetectable BCR-ABL1.
Methods
Patients
Between July 2000 and September 2009, a total of 423 patients with newly diagnosed chronic-phase CML were enrolled in 4 consecutive clinical trials of 400, 600, or 800 mg of imatinib daily and were monitored by peripheral blood molecular analysis at our institution. These trials included a subset of patients from the Novartis IRIS trial (n = 29, all patients enrolled in Australia and New Zealand)10 , a subset of patients from the Novartis TOPS trial (n = 186, all patients enrolled in Australia, New Zealand, Singapore, South Africa, and South America),11 and all patients enrolled in the ALLG TIDEL I study (n = 103)12 and Cohort I of the ALLG TIDEL II study (n = 105).13 Imatinib dose was increased for patients enrolled in the TIDEL I and II studies for failure to achieve time-dependent milestone molecular responses (MRs). All trials were conducted according to the Helsinki Declaration and were approved by national/international ethics committees. All participants gave written informed consent.
Molecular results were included until the time that imatinib was ceased for any reason, or last follow-up. The minimal elapsed time since commencement of imatinib for all patients was 33 months, and the median time of imatinib therapy was 50 months (range, 1-144 months).
Molecular analysis
The real-time quantitative PCR method for measurement of BCR-ABL1 transcripts has been described previously.14 The schedule for molecular monitoring was 3- to 6-months. A confirmed major MR (MMR) was defined as a BCR-ABL1 value of ≤0.10%IS (international scale) at 2 consecutive measurements. The time to MMR was the duration between therapy commencement and the month of the first value of ≤0.10%IS of the confirmed MMR.
The PCR sensitivity defining undetectable BCR-ABL1 was at least 4.5 log below the standardized baseline value, which was established in the IRIS trial.9,15 Therefore, all of the samples defined as undetectable in this study had an absence of BCR-ABL1 transcripts in PCR assays involving RNA of very high quality. Two consecutive measurements of undetectable BCR-ABL1 with a PCR sensitivity of 4.5 log were defined as MR4.5. This classification is the same as we previously defined as a complete MR.4 The time to MR4.5 was the month of the first value of the confirmed MR4.5. If BCR-ABL1 remained undetectable for a continuous period of 24 months of treatment with imatinib, the response was termed Stable MR4.5, which was the key inclusion criterion for enrollment in the ALLG CML8 discontinuation study.4 The time to Stable MR4.5 was the duration from imatinib start to the first of 24 months of undetectable BCR-ABL1.
Statistical analysis
Quantitative factors at the time of imatinib commencement (baseline) were categorized into groups. The achievement of a confirmed MR4.5 and Stable MR4.5 during imatinib therapy was calculated using the cumulative incidence function according to recommendations.16,17 Competing risks were any event that led to permanent cessation of imatinib for any reason, other than completion of study protocol. The observation time of patients with no event of interest or a competing event was the date of the last molecular analysis. The cumulative incidence of response was displayed by an increasing step-function. This curve increased each time a new responder was observed up to the best observed response rate by a certain time point. Univariate and multivariate regression analyses were performed using the Fine and Gray model18,19 to examine the association of Stable MR4.5 with baseline factors and the BCR-ABL1 value at 3 months of imatinib. In these competing risk regression models, achievement of the response was the event of interest, and cessation of imatinib for any reason (including death) was the competing risk. The relative risk and its 95% confidence interval (CI) were calculated for terms included in the competing risks regression models, and the Wald test was used to ascertain the significance of these terms. The Akaike Information Criterion was used to select the best fitting model. The cumulative incidence of MR4.5 and Stable MR4.5 were analyzed based on the time to achieve MMR at landmark time points (3, 6, 12, and 18 months). Patients who achieved the end point or ceased imatinib or whose follow-up was at or before the landmark time point were excluded from the landmark analysis. Frequencies were compared using the χ2 test. The cmprsk package, installed in R statistical software, was used to calculate cumulative incidences and fit the competing risk regression models.
Results
Stable MR4.5
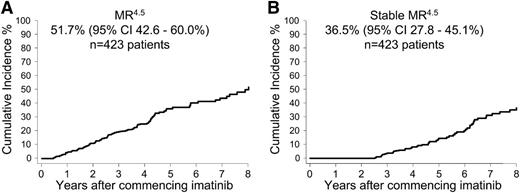
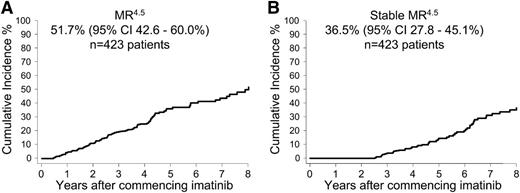
Few patients achieved a confirmed MR4.5 within the first years of imatinib: 19 (4.5%) of 423 patients at 12 months and 49 (12%) of 423 patients at 24 months. The cumulative incidence of MR4.5 after 8 years of imatinib on an intent-to-treat basis was 51.7% (95% CI, 42.6%-60.0%) (Figure 1A). Of the 121 patients with MR4.5, 80 patients had at least a 24-month follow-up with imatinib after achieving MR4.5. Of these 80 patients 51 (64%) had Stable MR4.5 (undetectable BCR-ABL1 for 24 months). The other 29 (36%) of the 80 patients did not maintain a stable response; however, only one of the 29 lost MMR. BCR-ABL1 values fluctuated between positive and negative for 25 of the 29 patients (median 2 positive samples ranging from 0.001% to 0.013%IS), and 3 patients had ongoing positivity.
Overall cumulative incidence of confirmed MR4.5 and Stable MR4.5 after 8 years of imatinib therapy. (A) MR4.5 and (B) Stable MR4.5. Of the patients with at least 24 months of follow-up after MR4.5, 64% had continuous, undetectable BCR-ABL1.
Overall cumulative incidence of confirmed MR4.5 and Stable MR4.5 after 8 years of imatinib therapy. (A) MR4.5 and (B) Stable MR4.5. Of the patients with at least 24 months of follow-up after MR4.5, 64% had continuous, undetectable BCR-ABL1.
The earliest time point that patients reached a Stable MR4.5 was at 30 months of imatinib, which occurred in only 4 (0.9%) of 423 patients. The cumulative incidence of Stable MR4.5 after 8 years of imatinib on an intent-to-treat basis was 36.5% (95% CI, 27.8%-45.1%) (Figure 1B).
Factors for prediction of Stable MR4.5
We investigated the possible associations between the achievement of Stable MR4.5 and a number of baseline variables, including Sokal risk score, age, sex, and assigned imatinib dose (400, 600, or 800 mg daily) (Table 1). In the regression model, patient sex had a statistically significant association with the cumulative incidence of Stable MR4.5 (women vs men, 54.4% vs 27.2% after 8 years; P < .001). There was also a higher cumulative incidence of Stable MR4.5 when patients with a low Sokal risk score were compared with those with a high Sokal risk (45.3% vs 21.5% after 8 years; P = .029). There was no difference in the frequency of female sex in the Sokal risk groups or within assigned imatinib dose cohorts (data not shown; each P > .4). Age did not differ by sex; median age of both men and women was 48 years.
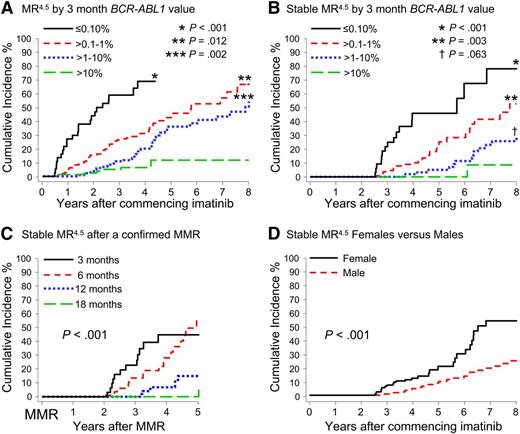
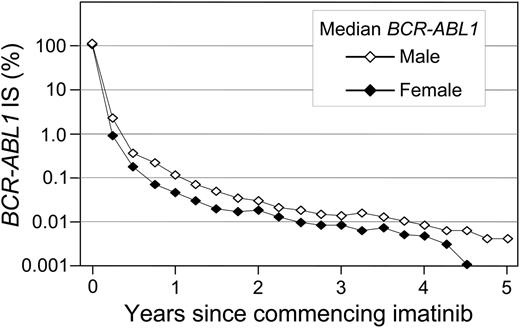
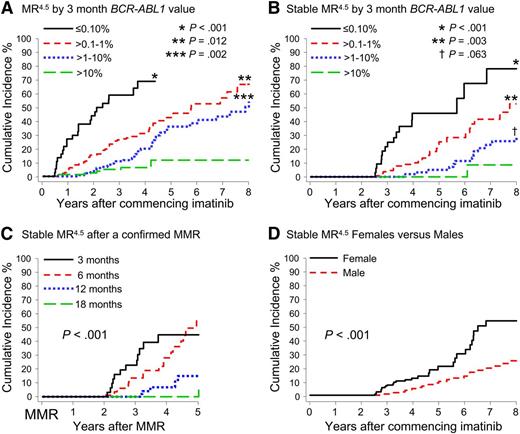
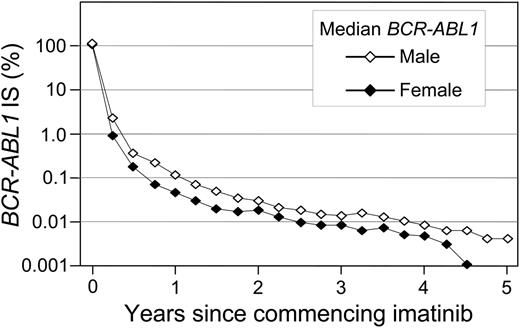
A significant difference in the cumulative incidence of Stable MR4.5 was observed according to the BCR-ABL1 value at 3 months of imatinib (P < .001) (Table 1 and Figure 2B). Patients with BCR-ABL1 ≤ 0.10%IS at 3 months had a significantly higher cumulative incidence of Stable MR4.5 after 8 years compared with patients with BCR-ABL1 > 0.10% to 1%IS (78.2% vs 52.7%; P < .001). Patients with BCR-ABL1 > 0.10% to 1%IS at 3 months had a significantly higher cumulative incidence of Stable MR4.5 than those with BCR-ABL1 > 1% to 10%IS (52.7% vs 29.0%; P = .003). Among the patients with BCR-ABL1 > 10% at 3 months, only 2 reached Stable MR4.5. There was an apparent difference between BCR-ABL1 > 1% to 10%IS and BCR-ABL1 > 10%IS at 3 months (29.0% vs 8.6%; P = .063). Consistent with our finding of a higher cumulative incidence of Stable MR4.5 in women, a higher frequency of BCR-ABL1 ≤ 0.10%IS at 3 months was observed in women compared with men (13.1% vs 6.3%; P = .03). Furthermore, median BCR-ABL1 values during 5 years of imatinib therapy were consistently lower in women compared with men (Figure 3).
Cumulative incidence of MR4.5 and Stable MR4.5 after 8 years of imatinib therapy according to various factors. (A-B) Patients were grouped according to the BCR-ABL1 value at 3 months. The asterisks indicate the P values for the difference from the next BCR-ABL1 group. (C) Cumulative incidence of Stable MR4.5 at 5 years after achieving a confirmed MMR, for the patients who achieved MMR by 18 months (P < .001) (MMR by 6 months vs MMR by 12 months; P = .002). (D) Women had a significantly higher cumulative incidence of Stable MR4.5 than men.
Cumulative incidence of MR4.5 and Stable MR4.5 after 8 years of imatinib therapy according to various factors. (A-B) Patients were grouped according to the BCR-ABL1 value at 3 months. The asterisks indicate the P values for the difference from the next BCR-ABL1 group. (C) Cumulative incidence of Stable MR4.5 at 5 years after achieving a confirmed MMR, for the patients who achieved MMR by 18 months (P < .001) (MMR by 6 months vs MMR by 12 months; P = .002). (D) Women had a significantly higher cumulative incidence of Stable MR4.5 than men.
BCR-ABL1 decline at 5 years: women vs men. The median BCR-ABL1 values are plotted.
BCR-ABL1 decline at 5 years: women vs men. The median BCR-ABL1 values are plotted.
All factors were entered into the multivariate regression model. Female sex (P = .018) and the 3-month BCR-ABL1 value (P < .001) added independent prognostic information for Stable MR4.5 (Table 2).
Landmark analysis of Stable MR4.5 according to the time to MMR
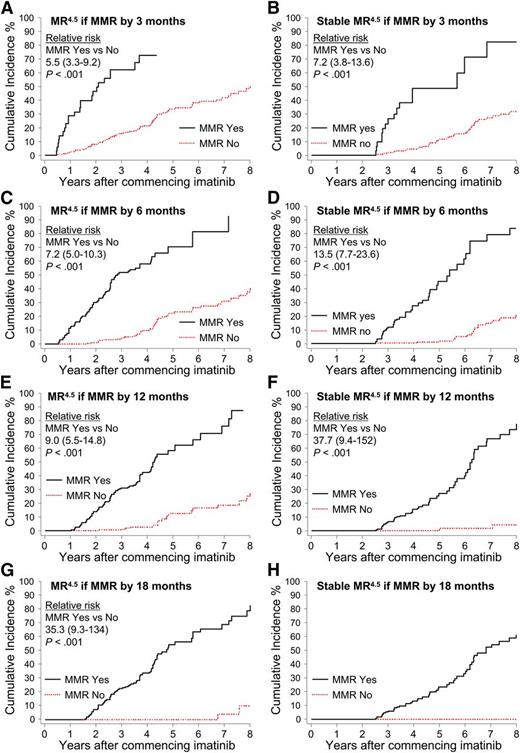
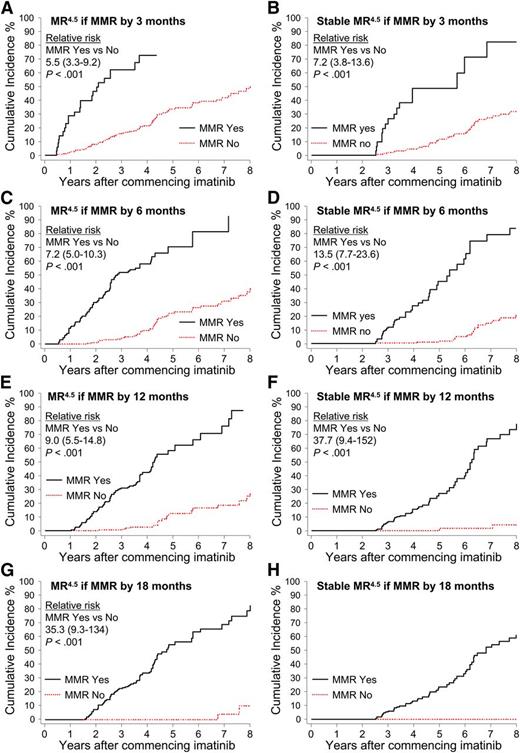
Patients were divided into groups according to the time taken to achieve a confirmed MMR up to 18 months after imatinib initiation: by 3 months, more than 3 to 6 months (6 months), more than 6 to 12 months (12 months), and more than 12 to 18 months (18 months). Landmark analyses demonstrated that patients with MMR by these time points had a significantly higher cumulative incidence of MR4.5 and Stable MR4.5 compared with patients without MMR at these time points (Figure 4). None of the 52 patients who achieved MMR after 18 months are known to have achieved Stable MR4.5 (Figure 4H), and only 2 had a confirmed MR4.5. Both of these patients achieved MMR at 21 months and reached MR4.5 at 60 and 69 months after first achieving MMR. For the 52 patients, the median time to MMR was 27 months (range, 21-87 months), and the median follow up after achieving MMR was 26 months (range, 0-88 months). It is possible that some of these patients will achieve Stable MR4.5 with longer follow-up.
Landmark analyses of MR4.5 according to the time to MMR. (A-H) Patients were divided into groups according to the time to achieve MMR. The relative risk for graph H could not be determined, as no patients in the “No-MMR” group experienced the event.
Landmark analyses of MR4.5 according to the time to MMR. (A-H) Patients were divided into groups according to the time to achieve MMR. The relative risk for graph H could not be determined, as no patients in the “No-MMR” group experienced the event.
Patients who achieved MMR at a later time point subsequently achieved Stable MR4.5 more slowly, if at all. An analysis was performed for the MMR groups to examine the time to achieve Stable MR4.5 after having achieved MMR. Delayed achievement of MMR was associated with slower dynamics of BCR-ABL1 decline (P < .001) (Figure 2C).
Discussion
The STIM and CML8 imatinib discontinuation studies commenced enrollment in 2007 and have established that approximately 40% of patients with stable, undetectable BCR-ABL1 for at least 2 years during imatinib therapy using similar, strict PCR sensitivity criteria can maintain long-term remission after discontinuation of imatinib.3,5,6 The number of first-line imatinib-treated patients who qualify for imatinib discontinuation using the established criteria is unknown but is predicted to be low because BCR-ABL1 usually remains detectable for many years.9 The aim of this study was, first, to determine the proportion of imatinib-treated patients reaching the eligibility criteria for discontinuation, which was stable, undetectable BCR-ABL1 for 24 months. This information is vital not only to our patients but also to enhancement of planning for future clinical studies. Second, we aimed to explore the factors associated with reaching this response such that future interventions may be targeted at strategies to expand the pool of eligible patients for a discontinuation trial. Whether this will lead to higher rates of remission than in the STIM and CML8 trials is unknown but will be addressed in future trials that will investigate discontinuation after alternative kinase inhibitor therapy, less stringent PCR criteria, and enrollment of patients with shorter duration of stable response.
The cumulative incidence of Stable MR4.5 in our analysis of 423 first-line imatinib-treated patients was 36.5% after 8 years of imatinib. Having first achieved a confirmed MR4.5, approximately 36% of patients did not sustain undetectable BCR-ABL1 for the subsequent 24 months, and most of these manifest intermittently positive values. We did not identify any factors associated with the stability of undetectable BCR-ABL1 after its first achievement during the subsequent 24 months (data not shown). For those with Stable MR4.5, factors at commencement of imatinib therapy were investigated. Univariate analysis showed a significantly higher cumulative incidence of Stable MR4.5 for women vs men and for patients with a low vs high Sokal risk score. The BCR-ABL1 value at 3 months of imatinib also significantly predicted Stable MR4.5. BCR-ABL1 ≤ 0.10%IS (MMR) at 3 months was associated with the highest rate of Stable MR4.5 (78.2% after 8 years); however, less than 10% of patients achieved this response. The level of BCR-ABL1 reduction in the first months of tyrosine kinase inhibitor therapy has demonstrated importance in subsequent MR, complete cytogenetic response, event-free survival, progression-free survival, and overall survival for patients treated with imatinib, nilotinib, and dasatinib.10,20,,,,-25 Our analysis has demonstrated that the level of BCR-ABL1 reduction at 3 months is also important for the achievement of Stable MR4.5 and potential recruitment to a discontinuation study.
By multivariate analysis, the BCR-ABL1 value at 3 months and female sex added independent predictive information for attainment of Stable MR4.5. Furthermore, women achieved greater reduction of BCR-ABL1 than men during the first few years of imatinib therapy. It is not clear whether this is related to differences in dose interruptions or drug adherence, which are known to influence the MR,26 or whether this is attributed to biological differences, such as pharmacokinetics or immune response. In one study, men had higher rates of nonadherence to imatinib.27 However, imatinib treatment interruptions were twice as likely to occur in women compared with men in another study.28 Pharmacokinetic differences might be relevant: a correlation between imatinib trough plasma concentration and response has been described, and concentrations were up to 30% higher in women compared with men.29,30 This finding may be related to differences in body weight, which mean that women on average receive a higher milligram per kilogram dose. An immune response during the initial stages of imatinib treatment is hypothesized to be a determinant of the dynamics of BCR-ABL1 decline.31,32 Whether sex-specific immune responses play a role in the rate of decline is unknown. Further studies are warranted to investigate the difference that we observed. However, the positive effect of female sex on various cancer outcomes, including chronic lymphocytic leukemia, lung cancer, retinoblastoma, and glioblastoma, is well described, although the underlying biological mechanism has not yet been fully explored.33,,,-37 Improved event-free or progression-free survival for women with CML has not been reported. However, Marin et al reported significantly higher rates of MMR and complete MR in women after 8 years in a study of 282 patients initially treated with 400 mg of imatinib,22 which is consistent with our findings.
We found that a delay in the time to reach MMR led to a subsequent delay in the time to achieve a Stable MR4.5 (Figure 2C), suggesting differences in the dynamics of BCR-ABL1 decline. This difference did not appear to be related to a difference in the BCR-ABL1 value at the time of reaching MMR (within 1.8-fold for each time to MMR group, data not shown). Interestingly, only 2 of 52 patients with an MMR that was achieved after 18 months of imatinib (MMR at 21 months for both patients) have so far reached MR4.5. Patients with an MMR at later time points may still achieve Stable MR4.5 with longer follow-up. Mathematical modeling studies in CML have consistently described a rapid initial decline of BCR-ABL1 during the first few months of imatinib in most patients, followed by a more gradual decline that may continue for many years, which corresponds to the long-term BCR-ABL1 dynamics.38,,-41 The STIM study did not find that a delay in achieving undetectable BCR-ABL1 led to a significantly greater risk for molecular recurrence after discontinuation of imatinib.3 However, other small studies of imatinib discontinuation in up to 19 first-line imatinib-treated patients found that molecular recurrence was associated with such a delay.42,43 Importantly, the strongest predictor of molecular recurrence in all treatment discontinuation studies was a high Sokal risk score.3,5,6,42 Continuous, long-term molecular monitoring during kinase inhibitor therapy will be essential to define the MR kinetics and their association with relapse after discontinuation.
Our findings are linked to the molecular remission rates in the CML8 imatinib discontinuation study,4,6 because the PCR discontinuation criteria used in that study were the same as defined by Stable MR4.5. Remarkably, the relapse-free survival for the CML8 and STIM discontinuation studies are very similar.3,,-6 This finding may be related to similar PCR sensitivity criteria for eligibility, which was 4.5 log for CML8 (representative of undetectable BCR-ABL1 values of ≤0.0032IS) and 5 log for STIM (representative of undetectable BCR-ABL1 values of ≤0.001IS). However, there is no universally accepted definition of undetectable BCR-ABL1, which leads to differences in the interpretation of deep MRs. Cross et al have recently highlighted this problem and outlined the requirement for robust, standardized definitions of deep MRs.44 A framework for the advancement of standardization of MR was proposed, which should be widely adopted.
First-line nilotinib or dasatinib leads to earlier achievement of MMR,45,46 which may lead to higher recruitment to discontinuation studies because we found that earlier achievement of MMR was associated with a higher cumulative incidence of Stable MR4.5. However, the cohort of patients achieving early MMR while receiving imatinib may be a biologically favorable group. Many questions remain to be answered regarding the rates of response after discontinuation of first-line nilotinib or dasatinib, the patterns of molecular recurrence, and the factors associated with molecular recurrence. Trials of nilotinib or dasatinib discontinuation have been initiated, and some of these questions may be addressed in the future.47
In conclusion, we have determined the cumulative incidence of Stable MR4.5 after 8 years of first-line imatinib therapy and the factors associated with its achievement, which were female sex and the BCR-ABL1 value at 3 months. On the basis of known remission rates after discontinuation of imatinib in the STIM and CML8 studies for patients without prior interferon therapy (∼33%),3,,-6 we estimate that after 8 years of imatinib therapy ∼9% to 15% of imatinib-treated patients in our cohort would be able to achieve stable molecular remissions after discontinuation of therapy. A longer time to achieve MMR led to a delay in reaching a Stable MR4.5. The findings justify the focus on early achievement of MMR and rapid reduction of BCR-ABL1 as a strategy to expand the proportion of patients potentially eligible for cessation of therapy with the expectation of maintaining molecular remission.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many patients, clinicians, and study coordinators who contributed samples and follow-up data to this study; the staff of the Leukaemia Unit, Adelaide, South Australia; and the Australasian Leukaemia and Lymphoma Group and Novartis for their support, including research support to S.B. and T.P.H.
Authorship
Contribution: S.B. designed and performed the research, analyzed data, and wrote the manuscript; D.T.Y., D.M.R., and T.P.H. contributed to the experimental design and contributed significantly to preparation of the manuscript; J.A.P., C.R.F., H.K.A., A.L.Y., J.G., B.A.J., S.P., and B.S. performed research and contributed to preparation of the manuscript; M.H. and J.F.S. contributed to the experimental design and preparation of the manuscript; N.E.B. and J.R. analyzed data and contributed to preparation of the manuscript.
Conflict-of-interest disclosure: S.B. and T.P.H are advisory board members and receive research funding and honoraria from Novartis Pharmaceuticals, Bristol-Myers Squibb, and Ariad Pharmaceuticals. D.T.Y. receives research funding from Novartis Pharmaceuticals and Bristol-Myers Squibb. D.M.R. is an advisory board member and receives research funding from Novartis Pharmaceuticals and honoraria from Novartis Pharmaceuticals and Bristol-Myers Squibb. M.H. and J.F.S are advisory board members and have received honoraria from Novartis Pharmaceuticals and Bristol-Myers Squibb. J.R. is a former Novartis AG employee and holds Novartis AG stock. The remaining authors declare no competing financial interests.
Correspondence: Susan Branford, Department of Genetics and Molecular Pathology, SA Pathology, PO Box 14, Rundle Mall, Adelaide, South Australia, 5000, Australia; e-mail: susan.branford@health.sa.gov.au.