Key Points
Administration of AAV8-hAAT-mdtcs vector results in sustained expression of plasma ADAMTS13 activity and antigen.
The AAV8-mediated expression of ADAMTS13 variant is a safe and efficacious approach for treatment of a murine model of TTP.
Severe deficiency of plasma ADAMTS13 activity causes thrombotic thrombocytopenic purpura (TTP), a life-threatening syndrome for which plasma is the only effective therapy currently available. As much as 5% of TTP cases are hereditary, resulting from mutations of the ADAMTS13 gene. Here, we report the efficacy and safety of recombinant adeno-associated virus serotype 8 (AAV8)-mediated expression of a murine ADAMTS13 variant (MDTCS), truncated after the spacer domain, in a murine model of TTP. Administration of AAV8-hAAT-mdtcs at doses greater than 2.6 × 1011 vg/kg body weight resulted in sustained expression of plasma ADAMTS13 activity at therapeutic levels. Expression of the truncated ADAMTS13 variant eliminated circulating ultralarge von Willebrand factor multimers, prevented severe thrombocytopenia, and reduced mortality in Adamts13−/− disease-prone mice triggered by shigatoxin-2. These data support AAV vector-mediated expression of a comparable truncated ADAMTS13 variant as a novel therapeutic approach for hereditary TTP in humans.
Introduction
The metalloproteinase ADAMTS13 cleaves ultralarge von Willebrand factor (UL-VWF) multimers on endothelial cell surfaces, in blood, and in growing thrombi; this process is essential for normal hemostasis.1 Inhibition or deficiency of plasma ADAMTS13 activity causes thrombotic thrombocytopenic purpura (TTP),2 a syndrome characterized by thrombocytopenia and microangiopathic hemolytic anemia.3,4 Some patients have end-organ damage including neurologic signs and symptoms and renal failure.3,4 As much as 5% of cases of TTP are hereditary, caused by mutations in the ADAMTS13 gene5,6 that result in deficiency of plasma ADAMTS13 activity. The current treatment of hereditary TTP is plasma infusion to maintain trough levels of ADAMTS13 activity >5% of normal values.7 However, plasma therapy is inconvenient, can result in complications such as allergic reactions, and carries the potential risk for blood-borne infections.8
Gene transfer may provide a superior alternative to plasma therapy. The therapeutic levels of ADAMTS13 activity have been reported in animal models after gene transfer with lentivirus9 and adenovirus10 vectors, as well as after transplantation of hematopoietic progenitor cells after lentiviral transduction.11 However, approaches based on these vectors are limited by safety concerns. Recent clinical studies using adeno-associated virus (AAV)-based gene delivery vectors have documented safety and long-term expression of therapeutic transgenes for two human genetic diseases.12,13 In this study, we report the safety and efficacy of AAV8-mediated expression of a C-terminal truncated ADAMTS13 variant in a murine model of hereditary TTP, providing proof-of-concept to support further development of an AAV8-based clinical gene therapy approach for hereditary TTP.
Study design
The animal protocol was approved by IACUC of the Children’s Hospital of Philadelphia.
Preparation of AAV8 vectors
Recombinant AAV8 encoding a murine ADAMTS13 variant (aa 1-2055) (AAV8-hAAT-mdtcs) or a β-galactosidase (lacZ) (AAV8-hAAT-lacZ) under a human α-1 antitrypsin promoter (hAAT) was prepared according to the method described previously.14 Purified vector was dialyzed in phosphate-buffered saline (PBS) and then supplemented with Lutrol F68 to a final concentration of 0.001%. The purified vector was quantified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis with Coomassie blue staining, comparing VP1, VP2, and VP3 staining intensity by scanning densitometry with an established AAV reference vector. The titer was further confirmed by UV spectrophotometry.15
Administration of AAV vectors
Adamts13−/− mice (CAST/Ei) aged 3 to 4 weeks were injected via a tail vein with AAV8-hAAT-mdtcs (2.6 × 1010, 2.6 × 1011, and 1.3 × 1012 vg/kg) or with AAV8-hAAT-lacZ (1.3 × 1012 vg/kg) as a control. Five mice in each group were injected. Blood (100 μL) was collected from the retinal orbital sinus perplex and anticoagulated with 0.19% sodium citrate before vector administration and at 2, 4, 6, 8, 12, 16, and 20 weeks after injection. Plasma was prepared after centrifugation for 10 minutes at 10 000 rpm and stored in aliquots at −80°C.
Reverse transcriptase–polymerase chain reaction
Total RNA was isolated from tissues using Trizol (Invitrogen). The mRNA was converted to cDNA by reverse transcription, followed by polymerase chain reaction amplification of the mdtcs fragment (0.25 kb) using a primer pair (forward: 5′-AGGAATCCTCACTGGTGC-CATCTT-3′ and reverse: 5′-TAGTGAGCACGTAGCGTTC-TGTGT-3′) and the β-actin (0.54 kb) using a primer pair (forward: 5′-TGT TAC CAA CTG GGA CGA CAT CG-3′ and reverse: 5′-TCG GAA CCG CTC GTT GCC-3′).
Plasma proteolytic activity
Plasma ADAMTS13 activity was determined by the cleavage of a murine-specific rFRTES-mVWF73, as previously described.11 Normal plasma pooled from 10 wild-type mice was used for calibration (1 U/mL).
Plasma ADAMTS13 antigen
ADAMTS13 antigen was quantified by a murine-specific enzyme-linked immunosorbent assay (ELISA), as described previously, with modifications.9,16 A rabbit IgG against a murine ADAMTS13-MDTCS fragment (Open Biosystem, Huntsville AL), unlabeled and biotinylated, was used for antigen capture and detection. Purified murine MDTCS fragment was used for calibration.
Plasma VWF multimers
Shigatoxin challenge
Two weeks after AAV vector administration, Adamts13−/− mice (CAST/Ei) were injected via tail vein with 20 pg/g of Shigatoxin-2 (Stx2) (Toxin Technology, Sarasota, FL). The effective dosage was determined by a pilot experiment as the minimal dose of Stx2 necessary to consistently induce thrombocytopenia (>30% drop of platelet count from the baseline) in Adamts13−/− mice (data not shown). Blood (50 µL) was collected through the retinal orbital sinus perplex before and daily for 6 days after Stx2 injection. Complete blood count was performed using a Hemavet M2950HV analyzer (Drew Scientific, Waterbury, CT).
Histology and immunohistochemistry
Plasma alanine aminotransferase levels in mice
Plasma alanine aminotransferase levels were determined by a colorimetric assay (Teco Diagnostics, Anaheim, CA).
Plasma anti-ADAMTS13 antibodies
IgGs against transduced ADAMTS13 variant were determined by ELISA using a purified recombinant mdtcs (2 µg/mL) for capture and a peroxidase-conjugated rabbit anti-mouse IgG (1:5000) (Dako) for detection, respectively.
Results and discussion
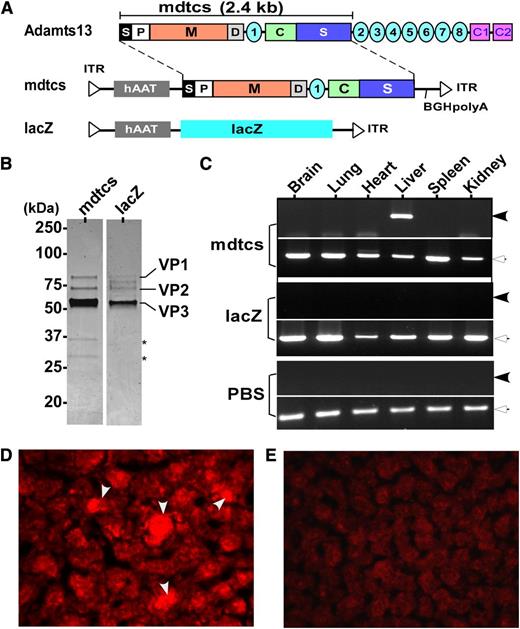
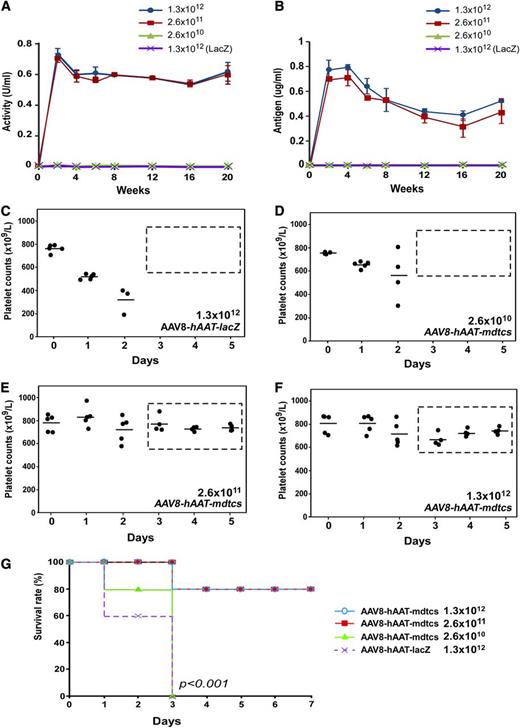
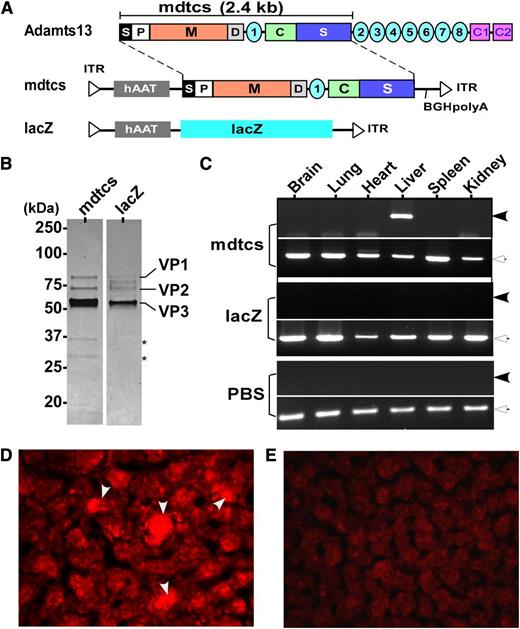
To date, plasma infusion is the only effective prophylaxis or treatment available for hereditary TTP. Here, we report success in correction of a murine model of hereditary TTP by a single intravenous administration of an AAV8 vector encoding a C-terminal truncated murine ADAMTS13 variant (mdtcs) under the control of a liver-specific promoter. AAV8 vectors were produced and highly purified (Figure 1A-B). After intravenous infusion of vector, liver-specific expression of the transgene product was demonstrated by reverse transcriptase–polymerase chain reaction (Figure 1C) and immunohistochemistry (Figure 1D). Control mice that were treated with PBS or AAV8-hAAT-lacZ were negative for ADAMTS13 expression (Figure 1C,E). Plasma ADAMTS13 activity and antigen increased with vector dosage, reaching a comparable plateau of activity (∼0.7 U/mL) (Figure 2A) and antigen level (∼0.8 μg/mL) (Figure 2B) after the medium to high doses. The lack of a dose response at the 2 higher doses suggests possible saturation of vector uptake and/or transgene synthetic capacity by the transduced hepatocytes. The AAV8 doses (>2.6 × 1011 vg/kg) required to achieve therapeutic levels of plasma ADAMTS13 activity and antigen in this study are comparable with those reported for hemophilia B in animal models19,20 and in patients.13
Construction, preparation, and expression of AAV8 vectors. (A) The schematic diagram of AAV constructs. As shown, full-length murine ADAMTS13 protein consists of a signal peptide (S), prodomain (P), a metalloprotease domain (M), a disintegrin domain (D), the first thrombospondin type 1 repeat, a Cys-rich (C), and spacer domain (S) (ie, mdtcs). More distal C-terminus of murine ADAMTS13 contains an additional 7 thrombospondin type 1 repeats (2-8) and CUB domains (for complement C1r/C1s, Uegf, Bmp1). The fragment (∼2.4 kb) encoding amino acid residues 1 to 2055 of murine ADAMTS13, a hAAT promoter, and a bovine growth hormone poly adenylation (BGH-polyA) were cloned into an AAV vector (hAAT-mdtcs). The expression cassette was flanked by 2 inverted terminal repeats (ITR). In addition, a lacZ gene and a hAAT promoter were inserted into the same vector as a control (hAAT-lacZ). (B) The purified recombinant vectors, AAV8-hAAT-mdtcs (lane 1) and AAV8-hAAT-lacZ (lane 2), revealed by Coomassie blue staining. Only 3 viral envelop proteins (VP1, VP2, and VP3) are detected in the final preparations, with the VP3 as the predominant band. Two asterisks indicate 2 minor contaminated proteins or degradation products in lane 1. (C) The amplification of murine ADAMTS13 fragment (∼0.25 kb, closed arrowheads) and β-actin (∼0.5 kb, open arrowheads) mRNA in the brain, lung, heart, liver, spleen, and kidneys in mice treated with AAV8-hAAT-mdtcs (2.6 × 1011 vg/kg) or AAV8-hAAT-lacZ or PBS, as indicated in the figure. The therapeutic transgene product was detected only in the liver of Adamts13−/− mice treated with AAV8-hAAT-mdtcs but not in the controls. (D-E) The positive (arrowheads) and negative staining with anti-murine ADAMTS13 IgG in the hepatocytes 2 weeks after intravenous administration (2.6 × 1011 vg/kg) of AAV8-hAAT-mdtcs and AAV8-hAAT-lacZ, respectively. The staining was performed on the frozen sections after being fixed with ethanol/acetic acid (9/1) for 10 minutes at −20°C. An AlexaFluor568 donkey anti-rabbit IgG (Invitrogen) was used (1:500) for detection (red).
Construction, preparation, and expression of AAV8 vectors. (A) The schematic diagram of AAV constructs. As shown, full-length murine ADAMTS13 protein consists of a signal peptide (S), prodomain (P), a metalloprotease domain (M), a disintegrin domain (D), the first thrombospondin type 1 repeat, a Cys-rich (C), and spacer domain (S) (ie, mdtcs). More distal C-terminus of murine ADAMTS13 contains an additional 7 thrombospondin type 1 repeats (2-8) and CUB domains (for complement C1r/C1s, Uegf, Bmp1). The fragment (∼2.4 kb) encoding amino acid residues 1 to 2055 of murine ADAMTS13, a hAAT promoter, and a bovine growth hormone poly adenylation (BGH-polyA) were cloned into an AAV vector (hAAT-mdtcs). The expression cassette was flanked by 2 inverted terminal repeats (ITR). In addition, a lacZ gene and a hAAT promoter were inserted into the same vector as a control (hAAT-lacZ). (B) The purified recombinant vectors, AAV8-hAAT-mdtcs (lane 1) and AAV8-hAAT-lacZ (lane 2), revealed by Coomassie blue staining. Only 3 viral envelop proteins (VP1, VP2, and VP3) are detected in the final preparations, with the VP3 as the predominant band. Two asterisks indicate 2 minor contaminated proteins or degradation products in lane 1. (C) The amplification of murine ADAMTS13 fragment (∼0.25 kb, closed arrowheads) and β-actin (∼0.5 kb, open arrowheads) mRNA in the brain, lung, heart, liver, spleen, and kidneys in mice treated with AAV8-hAAT-mdtcs (2.6 × 1011 vg/kg) or AAV8-hAAT-lacZ or PBS, as indicated in the figure. The therapeutic transgene product was detected only in the liver of Adamts13−/− mice treated with AAV8-hAAT-mdtcs but not in the controls. (D-E) The positive (arrowheads) and negative staining with anti-murine ADAMTS13 IgG in the hepatocytes 2 weeks after intravenous administration (2.6 × 1011 vg/kg) of AAV8-hAAT-mdtcs and AAV8-hAAT-lacZ, respectively. The staining was performed on the frozen sections after being fixed with ethanol/acetic acid (9/1) for 10 minutes at −20°C. An AlexaFluor568 donkey anti-rabbit IgG (Invitrogen) was used (1:500) for detection (red).
Plasma levels and therapeutic efficacy of the expressed plasma ADAMTS13 variant in mice. (A-B) The dynamic changes of plasma VWF-cleaving activity (U/mL) and antigen (μg/mL) over time (weeks), as determined by rFRETS-mVWF73 and ELISA, respectively, in mice treated with various doses of AAV8-hAAT-mdtcs. Each time point represents the mean ± SD of 5 individual mice (n = 5). Wild-type murine plasma pooled from 10 mice was used as a standard for the calibration of proteolytic activity (1 U/mL). A purified recombinant mdtcs fragment spiked into the Adamts13−/− murine plasma was used for calibration of plasma antigen levels. (C-F) The daily platelet counts before (day 0) and after the Stx2 challenge in Adamts13−/− mice that were pretreated with a single dose of AAV8-hAAT-lacZ (1.3 × 1012 vg/kg) or various doses of AAV8-hAAT-mdtcs as indicated. (G) The Kaplan-Meier survival rates over 7 days in mice treated with control vector and therapeutic AAV8 vector at various doses and challenged with Stx2. A P value < .001 is considered statistically significant.
Plasma levels and therapeutic efficacy of the expressed plasma ADAMTS13 variant in mice. (A-B) The dynamic changes of plasma VWF-cleaving activity (U/mL) and antigen (μg/mL) over time (weeks), as determined by rFRETS-mVWF73 and ELISA, respectively, in mice treated with various doses of AAV8-hAAT-mdtcs. Each time point represents the mean ± SD of 5 individual mice (n = 5). Wild-type murine plasma pooled from 10 mice was used as a standard for the calibration of proteolytic activity (1 U/mL). A purified recombinant mdtcs fragment spiked into the Adamts13−/− murine plasma was used for calibration of plasma antigen levels. (C-F) The daily platelet counts before (day 0) and after the Stx2 challenge in Adamts13−/− mice that were pretreated with a single dose of AAV8-hAAT-lacZ (1.3 × 1012 vg/kg) or various doses of AAV8-hAAT-mdtcs as indicated. (G) The Kaplan-Meier survival rates over 7 days in mice treated with control vector and therapeutic AAV8 vector at various doses and challenged with Stx2. A P value < .001 is considered statistically significant.
The efficacy of the ADAMTS13 variant expressed in this animal model was evaluated by the reduction in the size of plasma VWF multimers and protection against shigatoxin-induced “TTP-like” syndrome. Previous studies have shown that Adamts13−/− mice are prothrombotic with increased formation of “string-like” UL-VWF polymers on the endothelial cell surface after stimulation21,22 and increased ratios of high-molecular-weight to low-molecular-weight VWF multimers in circulating blood,9,11,23 which contribute to the accelerated rate of thrombus formation after oxidative injury.16,23,24 An infusion or expression of human (or murine) full-length ADAMTS13 or the C-terminal truncated variants via a lentiviral vector or transplantation of reconstituted hematopoietic progenitor cells in these mice can correct prothrombotic phenotypes.9,11,16,21,22 In this study, we demonstrate that AAV8-mediated expression of a murine MDTCS fragment at vector doses ≥2.6 × 1011 vg/kg is sufficient to markedly reduce circulating UL-VWF or large VWF, thereby reducing ratios of high to low molecular weight multimers in plasma (supplemental Figure 1). When challenged with Stx2, known to trigger “TTP-like” syndrome in Adamts13−/− CAST/Ei mice,17 a significant drop (40%-60%) in platelet counts was observed after 24 to 48 hours (Figure 2C). This severe thrombocytopenia was not detected in Adamts13−/− mice that received AAV8-hAAT-mdtcs at doses of 2.6 × 1011 vg/kg (Figure 2D) or 1.3 × 1012 vg/kg (Figure 2E) 2 weeks before Stx2 challenge. All mice (5/5) in the Adamts13−/− cohort without vector treatment died within 3 days, but only 1 mouse (1/5) within each of the two vector-treated cohorts died (mortality rate, 20%) (P < .001) (Figure 2G).
Blood smears and immunohistochemistry revealed the presence of red blood cell fragmentation and VWF-enriched microthrombi in the heart, kidneys, brain, and pancreas in Stx2-challenged Adamts13−/− mice pretreated with PBS or AAV8-LacZ control vector, but these pathological changes were not observed in mice pretreated with AAV8-hAAT-mdtcs or wild-type mice (data not shown). These findings are consistent with the induction of a “TTP-like” syndrome in Adamts13−/− mice not receiving AAV8-hAAT-mdtcs that was prevented in mice that received therapeutic doses of the vector. Neither elevation in plasma alanine aminotransferase (supplemental Figure 2) nor antibodies against the murine ADAMTS13 variant (supplemental Figure 3) were detected, even at 10-fold higher vector doses, supporting that expression in liver and secretion of the truncated metalloprotease is safe.
Together, these results demonstrate that AAV8-mediated liver expression of an ADAMTS13 variant is safe and efficacious in correcting prothrombotic and TTP phenotypes in mice. Our findings provide proof-of-concept supporting the development of an AAV-based gene transfer approach for treatment of hereditary TTP in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr David Ginsburg at the University of Michigan, Ann Arbor, for providing us with the Adamts13−/− mice.
This study was supported by grants from the American Heart Association–National Established Investigator Award (AHA-EIA 0940100N) and the National Institutes of Health (HL-074124, project 3) (X.L.Z.).
Authorship
Contribution: S.-Y.J., J.X., J.B., J.F.W., and X.L.Z. designed the research, performed the experiments, analyzed the data, and wrote the manuscript; and S.Z. performed experiments and revised manuscript. All authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.-Y.J. is the Department of Hematology, Affiliated Hospital of Yanbian University, Yanji, China. The current affiliation for J.X. is the Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, The Children’s Hospital of Philadelphia, 34th Street and Civic Center Blvd, 816G Abramson Research Center, Philadelphia, PA 19104; e-mail: zheng@email.chop.edu.