Abstract
Passive immunotherapy with monoclonal antibodies has improved outcome for patients with B-cell malignancies, although many still relapse and little progress has been made with T-cell malignancies. Novel treatment approaches are clearly required in this disease setting. There has been much recent interest in developing therapeutic approaches to enhance antitumor immune responses using novel immunomodulatory agents in combination with standard of care treatments. Here we report that intravenous administration of the Toll-like receptor 7 (TLR7) agonist, R848 in combination with radiation therapy (RT), leads to the longstanding clearance of tumor in T- and B-cell lymphoma bearing mice. In combination, TLR7/RT therapy leads to the expansion of tumor antigen-specific CD8+ T cells and improved survival. Furthermore, those mice that achieve long-term clearance of tumor after TLR7/RT therapy are protected from subsequent tumor rechallenge by the generation of a tumor-specific memory immune response. Our findings demonstrate the potential for enhancing the efficacy of conventional cytotoxic anticancer therapy through combination with a systemically administered TLR7 agonist to improve antitumor immune responses and provide durable remissions.
Key Points
Systemic administration of a synthetic TLR7 agonist combined with radiation can prime a cytotoxic T-cell response against lymphoma cells.
The addition of a systemic TLR7 agonist to radiotherapy primes a memory immune response that may prevent recurrence of lymphoma.
Introduction
Passive immunotherapy with monoclonal antibodies have improved the outcome for patients in a wide range of B-cell malignancies.1 Despite this success, little or no progress has been made with T-cell lymphoma; furthermore, many patients with B-cell lymphoma continue to relapse and develop progressive disease after treatment.2 Clearly, additional therapeutic approaches are urgently required. Recent approaches exploiting novel active immunotherapies have focused on developing therapeutic vaccines or immunomodulatory antibodies and small molecules that facilitate the generation of durable antitumor immune responses. To date, several immunomodulatory approaches have demonstrated efficacy in preclinical and early phase clinical studies, including anti-CTLA4 and anti-CD40 monoclonal antibody, and small molecule agonists of the Toll-like receptor (TLR) family member TLR9.3-7
TLRs recognize a diverse repertoire of highly evolutionarily conserved pathogen-associated molecular patterns present on foreign pathogens and are constitutively expressed by both professional antigen-presenting cells, such as the dendritic cell (DC) and macrophage, and by effector B, T, and natural killer cell populations.8-10 Signaling through individual TLRs can direct the phenotype of the ensuing immune response through modulation of cytokine production and polarization of CD4+ helper, CD8+ effector, and TReg cell responses.11-13 Agonism of TLR7, which is localized intracellularly to endosomal membranes by viral guanosine and/or uridine-rich single-stranded RNA, or by synthetic agonists, can engender strong TH-1 biased immune responses through the activation of plasmacytoid DC and myeloid DC subsets.14,15 This phenotype of response is optimal for eliciting durable antitumor immune responses as it supports the activation and expansion of CD8+ T cells. Currently, imiquimod (Aldara 5% cream, 3M) is the only TLR7/8 agonist to receive FDA approval and is licensed in oncology for the topical treatment of basal cell carcinoma and other dermatologic malignancies.16 With the exception of therapeutic vaccination approaches, topical administration of TLR agonists is of limited utility in the treatment of the majority of solid tumors and in settings of nondermatologic disseminated disease.
In this study, we have investigated intravenous administration of the TLR-7 agonist R848 in combination with radiation therapy (RT) in syngeneic models of T- and B-cell lymphoma. We hypothesized that the combination of a systemically administered TLR-7 agonist in combination with local RT would prime systemic antitumor immune responses. In this setting, RT has the advantage that most non-Hodgkin lymphomas are radiosensitive and importantly has minimal deleterious systemic effect on immune effector cells in contrast to the immunosuppression seen with chemotherapy. Here we demonstrate that intravenous administration of R848 can enhance the therapeutic efficacy of RT by the generation of durable antitumor CD8+ T cell–dependent immune responses, leading to long-term survival and to the induction of protective immunologic memory. This study provides proof of principle for translation to early phase clinical trial.
Methods
Mice and cell lines
C57Bl/6 and BALB/c mice were obtained from Harlan. All animal experiments were approved by a local ethical committee and performed under a United Kingdom Home Office license. The B-cell lymphoma line A20, the T-cell lymphoma line EL4, and its ovalbumin-expressing derivative EG7 were obtained from ATCC and maintained in RPMI 1640 medium supplemented with 10% FCS, 1% L-glutamine (Invitrogen), and 50μM 2-mercaptoethanol (Sigma-Aldrich). EG7 media was also supplemented with 0.5 mg/mL G418 (Invitrogen). All cell lines were routinely screened for Mycoplasma contamination.
Measurement of systemic immune activation by R848
Tumor-bearing mice received a single intravenous dose of R848 (Alexis Biochemical) at 3 mg/kg, 7 days after tumor implantation. At 2 and 4 hours after administration of R848, mice were killed and spleens harvested for profiling of CD69 expression on T and B cells (CD69, eBioscience; CD4, CD8, and CD19, BD Biosciences PharMingen). DC activation was assessed by expression of CD40, class II MHC, and CD80 (BD Biosciences PharMingen). Plasma was isolated from peripheral blood and assayed for IL-1α, IL-2, IL-5, IL-6, IL-10, IL-12p70, IFN-γ, TNF-α, GM-CSF, IL-4, and IL-17 expression using a multiplex bead-based analyte detection system (Bender Medsystems). Experimental groups contained 5 mice per group.
Tumor therapy
Mice were inoculated subcutaneously with 3 × 106 EG7, 2 × 105 EL4, or 5 × 106 A20 cells. Irradiations were performed 7 days after inoculation (when tumors were ∼ 100 mm3) using a Pantak HF-320 320-kV x-ray unit (Gulmay Medical). The machine was operated at 300 kV, 9.2 mA, with filtration fitted in the x-ray beam to give a radiation quality of 2.3-mm Cu half-value layer. Mice were positioned at a distance of 350 mm from the x-ray focus, where the dose rate was 0.80 Gy/min. On day 7 after tumor inoculation, R848 was administered intravenously at a dose of 3 mg/kg in a dose volume of 50 μL/10 g in PBS and repeated weekly for up to 5 weeks. For B-cell depletion, mice received 250 μg anti-CD20 monoclonal antibody (IgG2a clone 18B12, a gift from Robert Dunn, Biogen-Idec) as outlined in supplemental Figure 3A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For CD8 depletion experiments, mice were treated with a depleting antibody, YTS169; a gift from M. Glennie, Southampton University, as outlined in supplemental Figure 3B. For tumor rechallenge experiments, long-term surviving mice were implanted contralaterally with either EG7 or EL4 a minimum of 60 days after previous tumor implantation. Additional control mice were also implanted to confirm tumor growth. Experimental groups contained at least 5 mice per group and are representative at least 2 independent experiments.
Measurement of IFN-γ production by CD8+ T cells isolated from long-term surviving mice
For in vitro stimulation, 3.5 × 106 splenocytes from either long-term surviving mice or control mice were cultured for 5 days in RPMI 1640 supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% l-glutamine, 50μM 2-mercaptoethanol, and 10 IU/mL human recombinant IL-2 in the presence of either 1 × 106 EG7 cells irradiated with 25 Gy or 1 μmol/mL SIINFEKL peptide (Anaspec) in 12-well or 24-well plates, respectively. Experimental groups contained at least 3 mice and are representative of 2 independent experiments. After 5 days in culture, cells were restimulated at a 1:1 ratio with 25 Gy irradiated EG7s for 16 hours in the presence of 3 μg/mL brefeldin A (BD Biosciences PharMingen) and 100 IU/mL human recombinant IL-2 (Chiron). For FACS analysis, cells were washed and incubated with rat anti-CD16/32 (eBioscience) to block nonspecific binding and then stained with a FITC-conjugated anti-CD8α monoclonal antibody. Cells were then fixed/permeabilized and stained for expression of IFN-γ using an allophycocyanin conjugated monoclonal antibody (BD Biosciences PharMingen).
Results
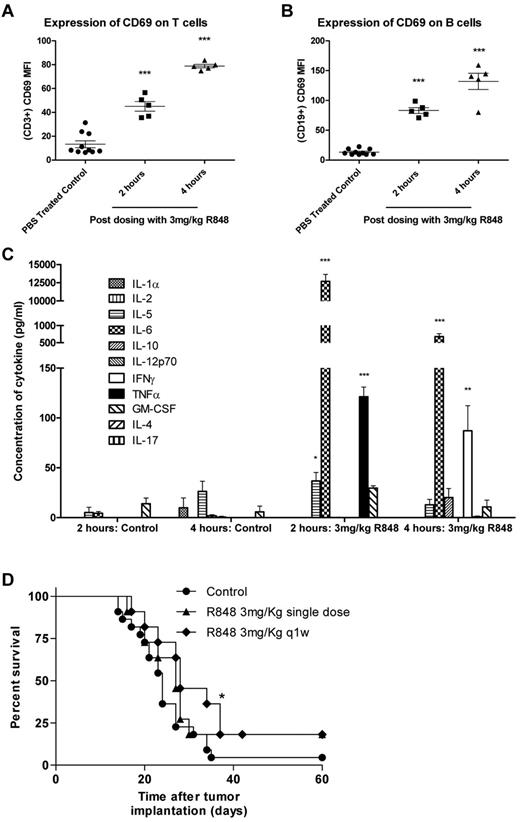
Systemic administration of R848 leads to activation of T and B lymphocytes, induction of cytokine expression, and increased long-term survival in EG7 tumor-bearing mice
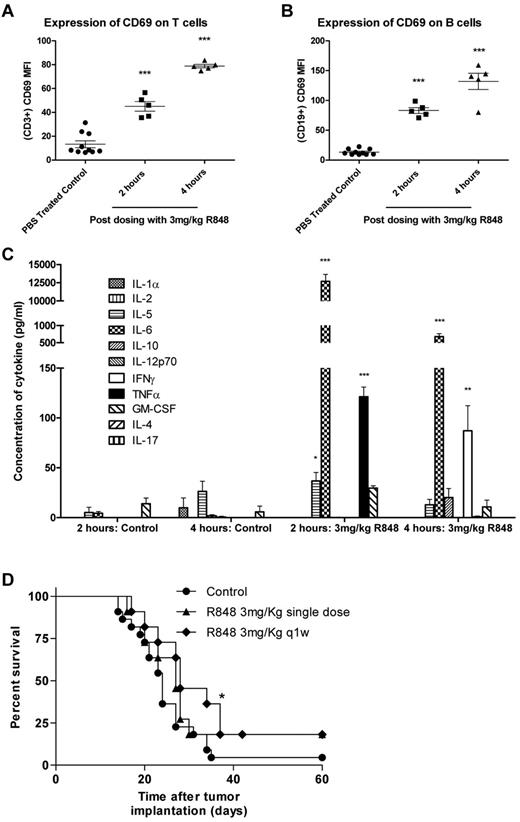
In previously published studies, R848 has been administered locally either by subcutaneous/intratumoral injection or by transdermal absorption. To determine whether intravenous dosing of R848 had the capacity to induce systemic immune activation in tumor-bearing mice, spleens and plasma were harvested 2 and 4 hours after a single intravenous treatment. R848 was well tolerated when dosed systemically at 3 mg/kg and induced the expression of the early activation marker CD69 on T lymphocytes (Figure 1A-B) at both 2 and 4 hours after dosing compared with vehicle-treated controls (P < .001, 2-tailed Student t test). The systemic induction of cytokines in the serum of treated and control mice were also measured by multiplex bead array (Figure 1C). We found that, 2 hours after intravenous administration of R848, plasma concentrations of IL-5 increased by 7-fold, IL-6 by ∼ 3000-fold, and levels of TNF-α, undetectable in untreated mice, also increased significantly (P < .001, 2-tailed Student t test). By 4 hours, levels of IL-6 had dropped by ∼ 10-fold but remained 360-fold higher than in time-matched vehicle-treated mice. In addition, the expression of TNF-α had dropped down to baseline levels, and expression of the TH1 cytokine IFN-γ was found to be significantly elevated (P < .01, 2-tailed Student t test).
Systemic activation of the immune system after intravenous administration of R848 in EG7 tumor-bearing mice leads to increased survival. (A-B) Splenocytes were harvested at 2 and 4 hours, and T (A) and B (B) lymphocytes were analyzed for CD69 expression by flow cytometry. (C) Induction of systemic cytokine responses was measured in the plasma using multiplex assays. Experimental groups contained 5 mice. *P < .05 (2-tailed Student t test). **P < .01 (2-tailed Student t test). ***P < .001 (2-tailed Student t test). (D) Survival curve for EG7 tumor-bearing mice dosed intravenously with R848 either once or weekly. Experimental groups contained at least 7 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test).
Systemic activation of the immune system after intravenous administration of R848 in EG7 tumor-bearing mice leads to increased survival. (A-B) Splenocytes were harvested at 2 and 4 hours, and T (A) and B (B) lymphocytes were analyzed for CD69 expression by flow cytometry. (C) Induction of systemic cytokine responses was measured in the plasma using multiplex assays. Experimental groups contained 5 mice. *P < .05 (2-tailed Student t test). **P < .01 (2-tailed Student t test). ***P < .001 (2-tailed Student t test). (D) Survival curve for EG7 tumor-bearing mice dosed intravenously with R848 either once or weekly. Experimental groups contained at least 7 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test).
To determine whether systemic delivery of a TLR7 agonist could lead to therapeutic antitumor responses, mice bearing EG7 tumors were treated with either a single or weekly intravenous dose of R848 as a monotherapy. Our data demonstrated that, although ineffective at controlling tumor growth when administered as a single dose (P > .05, log-rank; Mantel-Cox test), systemic dosing of R848 moderately improves survival over vehicle-treated control mice when dosed weekly (P < .05, log-rank; Mantel-Cox test; Figure 1D). Using MTS and annexin V/propidium iodide assays, we confirmed in vitro that R848 was not directly affecting the proliferation or viability of the tumor cells (supplemental Figure 1A-B), suggesting that the antitumor activity of systemic TLR7 therapy may have been immune mediated. These data demonstrate that intravenous administration of R848 leads to activation of T and B cells commensurate with the origination of a proinflammatory cytokine milieu in tumor-bearing mice and that monotherapy with R848 provides a modest increase in the survival of EG7 tumor-bearing mice.
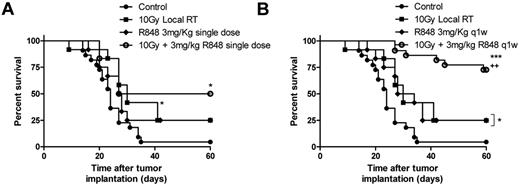
R848 in combination with RT improves survival in a model of T-cell lymphoma
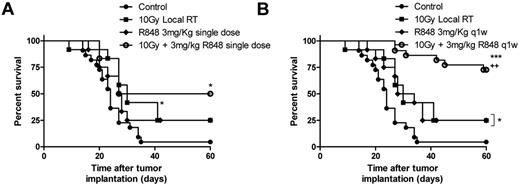
Local RT delivered as a single 10 Gy dose to the tumor improves long-term survival in mice bearing subcutaneously implanted EG7 tumor (Figure 2A; P < .05, log-rank; Mantel-Cox test). The antitumor efficacy was however substantially improved with systemic administration of R848 in combination with RT (Figure 2A-B). R848 when dosed weekly for 5 weeks, but not as a single intravenous dose in combination with RT enhances the efficacy of local RT leading to a substantial increase in the frequency of long-term surviving (LTS) mice (defined as mice surviving > 60 days after therapy) compared with both control mice (75% of mice classified as LTS after treatment with RT and R848 weekly vs 4% of control mice; P < .001, log-rank; Mantel-Cox test) and to mice receiving monotherapy with either 10 Gy RT or R848 dosed weekly (75% of mice classified as LTS after treatment with RT and R848 weekly vs 25% of mice treated with either monotherapy alone; P < .05, log-rank; Mantel-Cox test; Figure 2A-B). These data demonstrate that the therapeutic efficacy of RT and the ability to bring about long-term tumor control can be substantially augmented by combination with a systemically delivered TLR7 agonist.
R848 enhances the therapeutic efficacy of radiation therapy. Combination of 10 Gy local RT with a single intravenous dose of R848 (A) or weekly dosing of R848 (B). Experimental groups contained at least 7 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test). +P < .05, compared with monotherapy (log-rank; Mantel-Cox test). **P < .01, compared with control mice (log-rank; Mantel-Cox test). ***P < .001, compared with control mice (log-rank; Mantel-Cox test).
R848 enhances the therapeutic efficacy of radiation therapy. Combination of 10 Gy local RT with a single intravenous dose of R848 (A) or weekly dosing of R848 (B). Experimental groups contained at least 7 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test). +P < .05, compared with monotherapy (log-rank; Mantel-Cox test). **P < .01, compared with control mice (log-rank; Mantel-Cox test). ***P < .001, compared with control mice (log-rank; Mantel-Cox test).
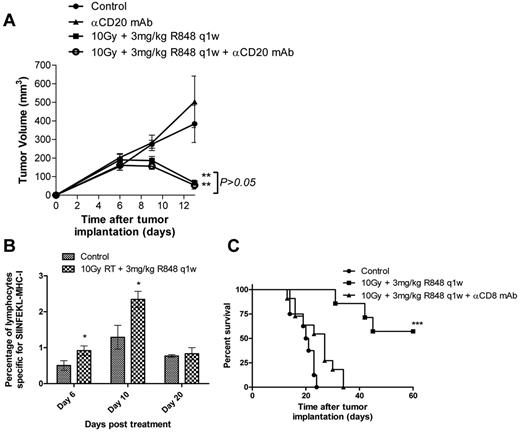
Long-term clearance of tumor with R848 and RT is CD8+ T cell dependent
We next investigated the mechanisms underlying this long-term tumor control observed after combination RT and R848 therapy. Initially, colony-forming assays were used to confirm that R848 was not acting as a radiation sensitizer directly through interaction with the tumor cells. We found that addition of 10μM R848 to EG7 or EL4 cells irradiated at doses of up to 10 Gy in vitro did not effect clonogenic survival compared with irradiation alone (supplemental Figure 2A-B). As the systemic administration of R848 leads to the potent activation of B lymphocytes (Figure 1B) and induction of high levels of IL-6 expression (Figure 1C), we first assessed the potential role for B lymphocytes in mediating the antitumor efficacy observed after combination therapy in the EG7 model (Figure 3A). Depletion of B lymphocytes using an anti-CD20 monoclonal antibody did not impact the therapeutic efficacy of this novel combination (P > .05, Mann-Whitney test). Depletion of B lymphocytes was confirmed by flow cytometry on peripheral blood samples (supplemental Figure 3A).
Therapeutic efficacy of RT and R848 combination is dependent on the activity of CD8+ T-lymphocytes. (A) Depletion of CD20+ B cells does not inhibit the efficacy of 10 Gy local RT and R848 dosed weekly. P > .05 (Mann-Whitney test). **P < .01 (Mann-Whitney test). (B) Serial blood samples were taken 6, 10, and 20 days after the initiation of treatment, and the frequency of SIINFEKL-restricted CD8+ T cells was determined using pentamers by flow cytometry. (C) Tumor-bearing mice received 10 Gy local RT and R848 dosed weekly in addition to a CD8-depleting antibody. Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments. ***P < .001 (log-rank; Mantel-Cox test).
Therapeutic efficacy of RT and R848 combination is dependent on the activity of CD8+ T-lymphocytes. (A) Depletion of CD20+ B cells does not inhibit the efficacy of 10 Gy local RT and R848 dosed weekly. P > .05 (Mann-Whitney test). **P < .01 (Mann-Whitney test). (B) Serial blood samples were taken 6, 10, and 20 days after the initiation of treatment, and the frequency of SIINFEKL-restricted CD8+ T cells was determined using pentamers by flow cytometry. (C) Tumor-bearing mice received 10 Gy local RT and R848 dosed weekly in addition to a CD8-depleting antibody. Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments. ***P < .001 (log-rank; Mantel-Cox test).
Using pentamers specific for the immunodominant class I MHC restricted ovalbumin epitope SIINFEKL, we then determined the impact of combination therapy on the induction of tumor antigen-specific CTLs in mice bearing EG7 tumors (Figure 3B). We observed that the frequency of SIINFEKL-restricted CTLs in the peripheral circulation was significantly higher (∼ 2-fold) 6 and 10 days after treatment with RT and R848 compared with time-matched nontreated control mice (P < .05, 2-tailed Student t test). By day 20, there was no significant difference in the frequency of SIINFEKL-restricted CTLs between mice treated with RT and R848 and nontreated control mice (P > .05, 2-tailed Student t test). Using depleting antibodies, we explored the role of effector T cells in mediating the efficacy of combination RT and R848 therapy in the EG7 model. Whereas the depletion of CD4 T lymphocytes had no effect (data not shown), the depletion of CD8+ T cells completely abrogated the therapeutic efficacy of combination RT and TLR7 agonist therapy (Figure 3C). Depletion of CD8+ T lymphocytes was confirmed by flow cytometry on peripheral blood samples (supplemental Figure 3B).
RT is one of several effective anticancer treatments that result in cellular stress and the expression of a plethora of damage-associated molecular patterns (DAMPs). Those DAMPs recently identified include calreticulin, High Mobility Group Box 1 (HMGB1), and the extracellular release of ATP, which can lead to the activation of antigen-presenting cells, such as the DCs and engender tumor antigen-specific T-cell responses.17-19 We therefore measured the release of the critical DAMPs that are known to be associated with immunogenic cell death and DC activation. The treatment of EG7 and EL4 cells with 10 Gy RT in vitro led to the release of the obligate immunogenic DAMP, HMGB1, but not to the expression of ecto-calreticulin (data not shown). To examine how the synthetic TLR7 agonist R848 may enhance the antitumor CTL response, we next evaluated DC activation in response to coculture with irradiated tumor cells in the presence and absence of agonist. Although the irradiated tumor cells were efficiently phagocytosed by bone marrow–derived DCs after coculture in vitro (supplemental Figure 4A), they were not able to induce DC maturation; and no change was observed in the activation markers CD80 or CD86 (P > .05, 2-tailed Student t test; supplemental Figure 4B). The addition of 1 μg/mL R848 to the coculture medium led to potent activation of DCs, as evidenced by increased expression of CD80 and CD86 while maintaining a high degree of phagocytosis of the irradiated tumor cells (P < .05, 2-tailed Student t test; supplemental Figure 4A-B). Taken together, these data demonstrate that the efficacy of combination therapy with RT and R848 is dependent on the activity of CD8+ T cells and that, in the absence of TLR7 agonism, the outcome of DC interaction with irradiated tumor cells may not be sufficient to drive both their maturation and subsequent ability to prime therapeutic antitumor CTL responses.
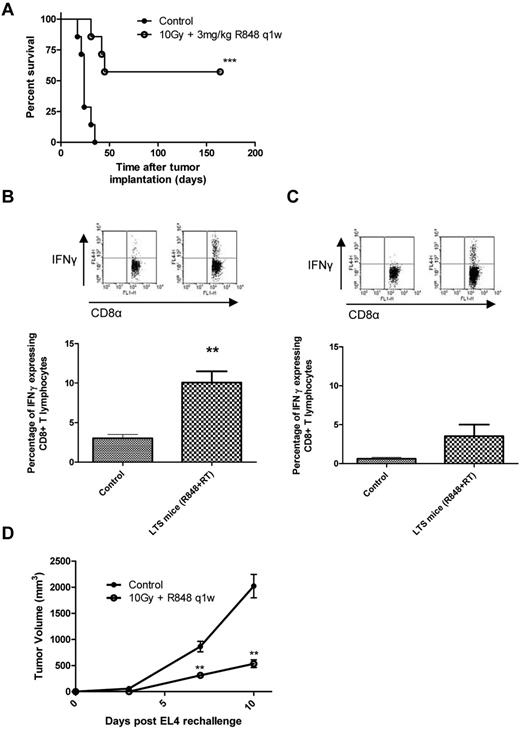
Treatment with R848 and radiotherapy generates long-lived memory T cells specific for multiple tumor-associated antigens
We next investigated whether immunologic memory was engendered in mice treated with 10 Gy and R848 dosed weekly by harvesting splenocytes from LTS mice that had > 150 days of disease-free survival (Figure 4A) and then assessing the capacity of CD8+ CTL to produce IFN-γ after coculture with irradiated EG7 cells (Figure 4B). We found that splenocytes from LTS mice had a significantly greater frequency of IFN-γ–producing CD8+ T lymphocytes after coculture than that of tumor-naive controls (10.06% ± 1.44% vs 3.02% ± 0.48%, respectively; P < .01, 2-tailed Student t test). To determine whether these memory immune responses were SIINFEKL-restricted, splenocytes were also cocultured with SIINFEKL peptide for 5 days and then restimulated with irradiated EG7 cells for 16 hours (Figure 4C). Although there was an increase in the frequency of SIINFEKL-restricted memory IFN-γ+ CD8+ lymphocytes in mice treated with combination TLR7 and RT (3.54% ± 1.47%, combination; vs 0.62% ± 0.2%, control), this was not significant (P = .12, 2-tailed Student t test). These data suggest that the immune response generated after combination RT and R848 therapy is not restricted to the usually immunodominant OVA epitope SIINFEKL and may be specific for multiple tumor-associated antigens. To test this hypothesis, LTS mice originally treated with RT in combination with R848, dosed weekly for 5 weeks, were rechallenged subcutaneously with either EG7 cells or the parental cell line EL4 (which lack expression of OVA). We found that all of the LTS mice were able to completely reject the implanted EG7 cells after contralateral rechallenge (data not shown). When LTS mice originally treated with combination RT and R848 were instead rechallenged with the parental cell line EL4, tumor growth was found to be significantly retarded (P < .01, Mann-Whitney test) compared with the naive control mice (Figure 4D). These data demonstrate that, in addition to OVA-restricted T-cell responses, memory T cells specific for an as yet undefined shared EL4/EG7 tumor-associated antigen(s) were also induced after TLR7 and RT.
Long-term surviving mice treated with radiation and R848 are protected against subsequent tumor rechallenge by the induction of tumor-specific memory CD8+ cells. (A) Survival curve for EG7 tumor-bearing mice after combination therapy with 10 Gy local RT and R848 dosed weekly. ***P < .001 (log-rank; Mantel-Cox test). (B-C) Splenocytes were isolated from treatment naive control mice and from long-term survivors originally treated with local RT and R848 dosed weekly and cocultured with either 25 Gy irradiated EG7 cells (B) or SIINFEKL peptide (C) before being restimulated with fresh 25 Gy irradiated EG7 cells. **P < .01 (2-tailed Student t test). Experimental groups contained at least 3 mice and are representative of 2 independent experiments. (D) At > 100 days after initial tumor inoculation, a cohort of long-term surviving mice were rechallenged contralaterally with EL4 cells. **P < .01 (Mann-Whitney test). Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments.
Long-term surviving mice treated with radiation and R848 are protected against subsequent tumor rechallenge by the induction of tumor-specific memory CD8+ cells. (A) Survival curve for EG7 tumor-bearing mice after combination therapy with 10 Gy local RT and R848 dosed weekly. ***P < .001 (log-rank; Mantel-Cox test). (B-C) Splenocytes were isolated from treatment naive control mice and from long-term survivors originally treated with local RT and R848 dosed weekly and cocultured with either 25 Gy irradiated EG7 cells (B) or SIINFEKL peptide (C) before being restimulated with fresh 25 Gy irradiated EG7 cells. **P < .01 (2-tailed Student t test). Experimental groups contained at least 3 mice and are representative of 2 independent experiments. (D) At > 100 days after initial tumor inoculation, a cohort of long-term surviving mice were rechallenged contralaterally with EL4 cells. **P < .01 (Mann-Whitney test). Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments.
Radiation dose-fractionation further enhances the efficacy of combination RT and R848
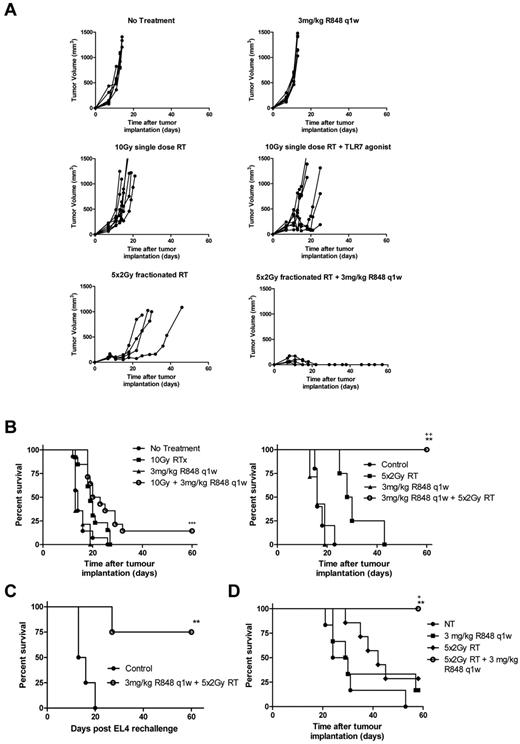
Combination therapy with R848 dosed weekly and local RT delivered as a single 10 Gy fraction decreased tumor growth and increased long-term survival significantly compared with nontreated control mice in both the EG7 (Figure 2B) and EL4 models (Figure 5A-B; P < .01, log-rank; Mantel-Cox test). However, unlike the therapeutic response observed in the EG7 model where 75% of mice were able to completely reject their tumor after treatment with local RT and R848 (dosed weekly), only 15% of EL4 tumor-bearing mice, which lack the expression of OVA, were able to completely reject their tumors. There was a modest increase in survival in EL4 tumor-bearing mice treated with R848 and a single 10 Gy fraction versus monotherapy (P = .058, log-rank; Mantel-Cox test). Based on recent reports describing the greater immunogenicity of fractionated radiotherapy compared with single fraction3 as well as reproducing the clinical delivery of RT more accurately, we combined R848 (dosed weekly) with a fractionated radiation regimen composed of 10 Gy delivered in 5 fractions. Initially, we used colony-forming assays to confirm in vitro that the fractionated RT regimen was not more cytoreductive than a single 10-Gy dose of RT (data not shown). In vivo, we observed significantly increased survival in mice receiving combination therapy with R848 and 10 Gy in 5 fractions compared with both nontreated controls and mice receiving either monotherapy alone in both the EG7 model (data not shown) and EL4 model (Figure 5A-B; P < .01, log-rank; Mantel-Cox test). Long-term surviving mice originally implanted with either EG7 (data not shown) or EL4 (Figure 5C) cells and treated with a fractionated RT regimen in combination with R848 were also able to prime a memory immune response after tumor rechallenge, leading to enhanced long-term survival. To expand our observations, we also evaluated this combination in mice bearing established subcutaneously implanted A20 B-cell lymphoma. We again determined that the combination of fractionated dose RT and weekly intravenous R848 administration significantly improved survival compared with monotherapy with either RT or R848 (100% of mice classified as LTS after treatment with fractionated RT and R848 weekly vs 28.5% of mice that received RT alone vs 16.6% that received R848 alone; (Figure 5D; P < .05, log-rank; Mantel-Cox test). In all 3 lymphoma models, complete tumor rejection was observed in 100% of mice after treatment with a combination of R848 (dosed weekly) and a fractionated RT regimen. These data demonstrate that the therapeutic efficacy of combination RT and TLR7 agonist therapy can be further enhanced by RT dose fractionation.
Combination of R848 dosed weekly with an RT regimen composed of 5 fractions of 2 Gy leads to complete eradication of EL4 and A20 tumors. EL4 tumor growth (A) and survival curves (B) after combination therapy with R848 dosed weekly and either a single 10-Gy dose or 5 fractions of 2 Gy local RT. (C) At > 60 days after initial tumor inoculation, a cohort of long-term surviving mice originally treated with 5 fractions of 2 Gy and R848 dosed weekly were rechallenged contralaterally with EL4 cells. (D) Survival curve of mice bearing established A20 tumors after combination therapy with R848 dosed weekly and 5 fractions of 2 Gy local RT. Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test). +P < .05, compared with control mice (log-rank; Mantel-Cox test). **P < .01, compared with monotherapy (log-rank; Mantel-Cox test). ++P < .01, compared with monotherapy (log-rank; Mantel-Cox test). ***P < .001, compared with monotherapy (log-rank; Mantel-Cox test).
Combination of R848 dosed weekly with an RT regimen composed of 5 fractions of 2 Gy leads to complete eradication of EL4 and A20 tumors. EL4 tumor growth (A) and survival curves (B) after combination therapy with R848 dosed weekly and either a single 10-Gy dose or 5 fractions of 2 Gy local RT. (C) At > 60 days after initial tumor inoculation, a cohort of long-term surviving mice originally treated with 5 fractions of 2 Gy and R848 dosed weekly were rechallenged contralaterally with EL4 cells. (D) Survival curve of mice bearing established A20 tumors after combination therapy with R848 dosed weekly and 5 fractions of 2 Gy local RT. Experimental groups contained at least 5 mice and are representative of at least 2 independent experiments. *P < .05, compared with control mice (log-rank; Mantel-Cox test). +P < .05, compared with control mice (log-rank; Mantel-Cox test). **P < .01, compared with monotherapy (log-rank; Mantel-Cox test). ++P < .01, compared with monotherapy (log-rank; Mantel-Cox test). ***P < .001, compared with monotherapy (log-rank; Mantel-Cox test).
Discussion
In this study, we have demonstrated that systemic administration of the imidazoquinolinamine analog Resiquimod (R848) delivered in combination with RT leads to long-term clearance of tumor in models of T- and B-cell lymphoma. R848 is a TLR7-selective agonist that appears to be well tolerated when administered by intravenous injection in the mouse and can potently activate both T and B lymphocytes and induce the expression of the TH-1 cytokines IFN-γ and TNF-α as well as the TH-2 cytokines IL-5 and IL-6. Furthermore, our data demonstrate that weekly systemic administration of R848 in combination with RT leads to the induction of a tumor-specific CD8+ T-cell response. In the EL4, EG7, and A20 models, 100% of mice treated with low-dose fractionated RT (10 Gy in 5 fractions) in combination with R848 dosed weekly were able to completely reject the primary tumor. These long-term surviving mice are protected against subsequent tumor rechallenge by the induction of a tumor-specific memory immune response.
Preclinical studies have demonstrated that subcutaneous vaccination with tumor cells after in vitro irradiation can lead to DC activation in vivo and to the induction of CD8+ T-cell responses that are dependent on the expression and/or release of DAMPs, such as calreticulin and HMGB1 by tumor cells.17,18 Although we also detected the release of the obligate DAMP HMGB1 after irradiation of EG7 and EL4 tumor cells in vitro, coculture of RT-treated tumor cells alone was unable to induce DC activation in our model system. Likewise, we found that in situ vaccination using RT was rarely able to engender an immune response in vivo with the capacity to reject an established tumor (Figure 2A). This latter observation is entirely in keeping with other published studies in a variety of tumor models3,20 and is presumably a consequence of tumor-derived immunosuppression, a characteristic of both clinical and experimental malignancies, which may limit the origination and/or efficacy of a nascent antitumor immune response. Our data suggest that combining an effective anticancer treatment, such as local RT, with immunopotentiating agents, such as TLR agonists, may help overcome this suppressive milieu and elicit tumor-specific immune responses and improved tumor clearance.
Our data support the hypothesis that additional manipulation of the immune system is necessary to elicit durable therapeutic antitumor immune responses after treatment with external beam ionizing radiation. In the present study, weekly systemic dosing of R848 for 5 weeks in combination with RT was associated with greater efficacy compared with combination with a single intravenous dose. This enhancement of the antitumor response after treatment with an extended TLR-dosing schedule was also observed in a study that combined RT with the selective TLR9 agonist CpG oligodeoxynucleotide 1826.21 The precise mechanisms underlying this enhanced antitumor immune response are unknown but may reflect the need for regular priming of the antitumor immune response through repeated exposure to TLR-mediated danger signals. Although further investigation is required, we speculate that this extended dosing schedule may prevent the reestablishment of an immunosuppressive tumor microenvironment and the induction of immunologic anergy after an initial course of RT and TLR7 therapy.
As the systemic administration of R848 leads to the activation of both B and T lymphocytes, we used depleting monoclonal antibodies to CD20, CD4, and CD8 to delineate the role of these important immune effector cells. Importantly, we observed that CD8, but not CD4, T-cell or CD20 B-cell depletion completely abrogated the therapeutic efficacy of the R848/RT combination. Tumor-reactive, SIINFEKL-restricted CTLs could be detected in the circulation for at least 10 days after the initiation of therapy in EG7 tumor-bearing mice. This expansion of tumor-specific CD8+ T cells was found to quickly decline as the level of tumor load decreased. Interestingly, our data revealed that the immune response to irradiated EG7 cells was not restricted to ovalbumin. A large proportion of LTS mice originally implanted with EG7 cells and treated with combination RT and R848 therapy were resistant to rechallenge with EG7 cells. Moreover, a significant growth delay was observed in cohorts of LTS mice (originally bearing EG7 tumor) after rechallenge with parental EL4 cells compared with tumor-naive control mice. Taken together, these data demonstrate the induction of a broad CD8+ T-cell response to multiple tumor cell-associated antigens after combination RT/R848 therapy. The generation of multiple CTL clones specific for diverse TAAs may prove beneficial for therapy. It has previously been shown that exposure of cells to external beam ionizing radiation can enhance MHC class I expression and modulate the peptide repertoire available for presentation.22 Therefore, RT may enhance expression of multiple TAAs and provide novel TAAs, leading to the generation of multiple CTL clones. The danger signals provided by systemic TLR7 therapy may facilitate T cell priming to these antigens.
An important observation made during this study was the demonstration that the combination of R848 and fractionated RT resulted in significantly greater therapeutic efficacy in both the EG7 and EL4 models compared with combination with a single-dose RT, despite the higher radiobiologic effect and expected greater tumor cell lethality of the larger single dose. In the EL4 model, combination of a systemically administered TLR7 agonist with a fractionated course of 10 Gy RT delivered in 5 fractions of 2 Gy led to 100% of mice rejecting their primary tumor (vs 15% when systemic TLR7 therapy was combined with a single dose of 10 Gy). The mechanisms that underlie the differential efficacy observed after combination of TLR7 therapy and either single-dose or fractionated RT are currently unclear and form the basis of ongoing studies. Interestingly, a study that profiled gene expression in breast, prostate, and glioma tumors after both single-dose (10 Gy) and fractionated (5 fractions of 2 Gy) RT revealed significant changes in the tumor microenvironment with several genes, including multiple IFN-related genes uniquely up-regulated after fractionated therapy.23 Given that the induction of IFN is a major downstream effect of TLR7 agonism,24,25 combination with fractionated RT may lead to greater synergy and therapeutic response than with single-dose RT or monotherapy.
Although successful for the treatment of dermatologic malignancy, the therapeutic efficacy of topically administered TLR7 agonists, such as imiquimod (Aldara, Graceway Pharmaceuticals) is generally limited to the treatment site.26 Recently, 2 phase 1/2 clinical trials have evaluated intratumoral delivery of the TLR9 agonist PF-3512676 (Pfizer) in combination with RT in patients with B-cell lymphoma27 and in patients with mycosis fungoides.28 Data from these 2 trials are highly encouraging with evidence of the induction of systemic antitumor immune responses after combination therapy. However, direct intratumoral administration of agents to noncutaneous tumors is often challenging and frequently requires image-guided delivery, such as ultrasound or computed tomography. Systemic delivery of R848 was found to be well tolerated in a phase 2 trial of patients with chronic HCV infection.29 In the treatment of cancer, recent clinical trials of an intravenously administered TLR7 selective agonist (852A, Pfizer) provide the proof of principle that systemic TLR7 therapy can be well tolerated and capable of inducing global immune activation, further strengthening the possibility of translating the findings from this study into early-phase trials of a systemically administered TLR7 agonist in combination with RT.24,25
In conclusion, this study demonstrates, for the first time, that the systemic delivery of a TLR7 agonist can enhance the immune response to radiation-induced tumor cell death in models of T- and B-cell lymphoma. The novel combination of R848 and RT leads to the generation of a tumor-specific CTL response with the capacity to both eradicate primary disease and induce long-term protective immunologic memory. Our data suggest that this therapeutic combination may be a promising approach for the treatment of B- and T-cell malignancies and could be readily translated to early phase clinical trials.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Paterson Institute BRU and Flow Cytometry Core Facilities, Prof Martin Glennie and Alison Tutt for the supply of depleting antibodies, and all members of the Targeted Therapy Group for helpful discussion and critical review of the manuscript.
This work was supported by Leukemia and Lymphoma Research (grant 08075) and Cancer Research UK.
Authorship
Contribution: S.J.D. and J.H. designed the studies; S.J.D. performed research and wrote the manuscript; S.J.D., J.H., M.H.M.M., R.W.W., A.L.A., I.J.S., and T.M.I. analyzed data; and J.H. and T.M.I edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy M. Illidge, Targeted Therapy and Oncology, School of Cancer and Enabling Sciences, University of Manchester, Manchester Academic Health Science Centre, Manchester Cancer Research Centre, Christie NHS Foundation Trust, Wilmslow Road, Manchester, M20 4BX, United Kingdom; e-mail: tmi@man.ac.uk.
References
Author notes
J.H. and T.M.I. contributed equally to this study.