Abstract
Hematopoietic cell transplantation (HCT) and prolonged chemotherapy are standard postremission strategies for adult acute lymphoblastic leukemia in first complete remission, but the optimal strategy remains controversial. There are no randomized trials of allogeneic HCT. In the present study, updated individual patient data were collected and analyzed from studies with information on availability of matched sibling donor (used to mimic randomization) and from randomized trials of autograft versus chemotherapy. Data from 13 studies including 2962 patients, excluding Philadelphia chromosome–positive patients, showed a survival benefit for having a matched sibling donor for patients < 35 years of age (OR = 0.79; 95% CI, 0.70-0.90, P = .0003) but not for those ≥ 35 years of age (OR = 1.01; 95% CI, 0.85-1.19, P = .9; heterogeneity P = .03) because of the higher absolute risk of nonrelapse mortality for older patients. No differences were seen by risk group. There was a trend toward inferior survival for autograft versus chemotherapy (OR = 1.18; 95% CI, 0.99-1.41; P = .06). No beneficial effect of autografting was seen compared with chemotherapy in this analysis. We conclude that matched sibling donor myeloablative HCT improves survival only for younger patients, with an absolute benefit of approximately 10% at 5 years. Improved chemotherapy outcomes and reduced nonrelapse mortality associated with allogeneic HCT may change the relative effects of these treatments in the future.
Key Points
No beneficial effect of autografting was seen in comparison to chemotherapy for adults with acute lymphoblastic leukemia in first remission.
In this individual patient data worldwide meta-analysis, sibling donor myeloablative transplant improved survival for younger patients.
Introduction
Acute lymphoblastic leukemia (ALL) is an aggressive malignancy that constitutes approximately 20% of cases of adult leukemia. With modern chemotherapy protocols, complete remissions are achievable in approximately 80%-95% of adult patients below the age of 55 years, but the majority of patients relapse. Allogeneic hematopoietic cell transplantation (HCT), autologous HCT, and prolonged consolidation and maintenance therapy have been widely used as postremission strategies to decrease the risk of relapse in ALL. However, there is uncertainty about the optimal consolidation strategy for patients in first complete remission (CR1).
Case-control comparisons have been constructed between allogeneic HCT and chemotherapy,1,2 but a major concern with these comparisons is selection bias. There have been no trials in the field of allogeneic blood and marrow transplantation in which patients with an available donor have been randomized between allogeneic HCT and chemotherapy. In the absence of a true randomization, genetic randomization is an established way of comparing allogeneic HCT with chemotherapy or autologous HCT.3 By comparing, among those who had been typed with an intention to transplant if there was a matched related donor, the outcome of those with a donor versus those without one, selection bias can be avoided. Several prospective and retrospective studies have been published on this topic but have produced conflicting data. The guidelines of various major organizations such as the American Society of Blood and Marrow Transplantation,4 the National Marrow Donor Program (http://marrow.org/Physicians/When_to_Transplant/Referral_Guidelines.aspx), and the European Blood and Marrow Transplant Group (http://www.ebmt.org/Contents/Resources/Library/EBMTESHhandbook/Documents/EBMT2008_Cap21.pdf) on the use of allogeneic and autologous HCT in adult patients with ALL in CR1 are not consistent. These discrepancies have led to differing practices for the application of HCT in adult patients with ALL in CR1 as reflected by the ongoing debate on this issue.5,6 The main reasons for these discrepant recommendations may be related to the relatively small sample sizes for the majority of these studies. The results also depend on outcome of the comparative conventional treatment arm and whether the patients with an available donor received allogeneic HCT. Therefore, we undertook a systematic review and meta-analysis of all prospective clinical trials and selected retrospective studies meeting strict criteria comparing the outcomes of allogeneic HCT, autologous HCT, and chemotherapy in adult patients with ALL using an intent-to-treat approach. We used individual patient data (IPD) from the relevant clinical studies, which allowed us to assess the impact of important patient- and disease-related variables.
Methods
The use of IPD in this project was approved by the Oxford University ethics committee OXTREC. We searched for all trials in adult ALL that included either a randomization of autologous HCT (autograft) versus chemotherapy or in which the recommendation was to treat patients with particular eligibility criteria with an HLA-matched sibling donor transplantation if a matched donor was available and with chemotherapy and/or autograft if not. Trials were sought using electronic searches (supplemental Appendix 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) of MEDLINE, EMBASE, clinical trial registration databases, and meeting abstracts and hand searching for review articles, meeting abstracts, and reference lists. A protocol was written (available at: http://www.ctsu.ox.ac.uk/research/meta-trials/leukemia-metaanalyses/protocol-2009) and principal investigators from the identified trials were invited to join the collaborative group and to provide data and were consulted to ensure completeness of the trial list.
Details collected for each trial protocol included eligibility for HCT, source of stem cells (BM/peripheral blood), conditioning regimen (chemotherapy/total body irradiation + chemotherapy), GVHD prophylaxis (allogeneic HCT only), number of courses of chemotherapy pre-HCT, autologous stem cell source and whether maintenance treatment was given after HCT, plus a summary of chemotherapy in the non-HCT arm.
Data were collected for each individual patient on initial characteristics, donor availability or treatment allocated, outcome and date and type of HCT received (supplemental Table 1). In particular, for the allogeneic HCT trials, the availability of a matched sibling donor was requested with the following categories: not tissue typed, no siblings, siblings but no donor, matched sibling donor, and no donor but unknown whether there was a sibling. Data were checked for internal consistency, for balance of randomization over time, and for compatibility with available published results. The numbers of patients receiving the treatment allocated were compared between groups.
Comparison of autograft with chemotherapy used only the patients randomized between these treatments. For the evaluation of allogeneic HCT with chemotherapy and/or autograft, comparisons were between those with and without a matched sibling donor. Additional sensitivity analyses restricted this to patients known to have siblings. All analyses were by intention to treat.
The primary outcomes were relapse, death without relapse, and death from any cause. Analyses of time to relapse censored at death without relapse. Death without relapse was named as treatment-related mortality (TRM) and censored at relapse. These analyses counted time from date of randomization or date induction treatment started for donor versus no donor comparisons because these were available for all trials.
Analyses used standard methods to obtain overall odds ratios (ORs) from log-rank observed minus expected and variance was calculated for Kaplan-Meier curves for each trial and summed over trials. Descriptive curves were drawn based on the overall event rates and log OR for each year.7 In assessments of allogeneic HCT compared with autograft or chemotherapy (donor versus no donor), the descriptive overall survival curves crossed, with an early hazard from TRM in the donor arm balanced by a later reduction in relapse. Sensitivity analyses used additional statistical tests based on the 5-year probabilities of death and the standard error of the log-rank observed minus expected(s). The log of the ratio of the risks and its standard error, approximated by 1/s, were calculated for each trial and summed to give an overall summary risk ratio and confidence interval (CI).8,9
Subgroup analyses were pre-planned by sex, age, WBC count, immunophenotype, and Philadelphia chromosome status. WBC, immunophenotype, and Philadelphia status were used to define risk groups as very high (Ph+), high (not Ph+ and B-lineage with WBC ≥ 30 or T-lineage and WBC ≥ 100), and standard (remainder). Exploratory analyses looked additionally at results by cytogenetic abnormalities. Heterogeneity between trials and subgroups was tested using χ2 tests and the I2 statistic.9
Results
A total of 20 trials (Table 1) that appeared to be eligible for the donor versus no donor comparison were identified, 8 of which included an autograft versus chemotherapy randomization in the no-donor arm. No additional randomized trials were found. Data were not available for 7 trials (for 2 trials, the investigators considered the treatments used to be of date10,12 and for 5, we were unable to contact the investigators). All trials used traditional adult-intensity chemotherapy regimens.
Autograft versus chemotherapy
Data were available for 5 trials with a randomization between autograft and chemotherapy of the 8 identified (Table 1). Initial characteristics are shown in Table 2. No imbalances between treatments or length of follow-up with the median between 5.6 and 9.4 years were found. Only 32 of the total of 829 patients were known to be Ph+. More than 96% of patients allocated to chemotherapy alone received this treatment, whereas the proportion of patients allocated to autograft who received it in CR1 varied between 59% and 75% (Table 3). The median time to autograft was 2.7 months, with 90% performed within 5 months. Twenty-eight percent of the chemotherapy-treated patients in the autograft arm relapsed within 3 months and 39% within 5 months.
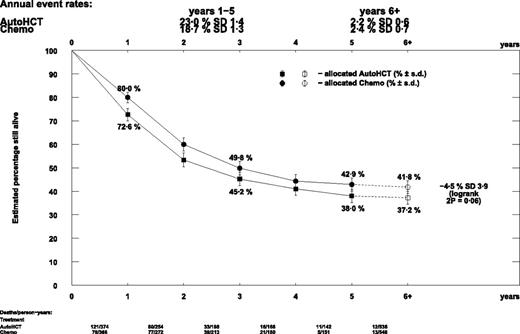
There was no difference in relapse rate seen in patients allocated autograft compared with chemotherapy (OR = 1.12; 95% CI, 0.93-1.34; P = .2). TRM was relatively low at 76 deaths and marginally significantly more with autograft (OR = 1.58; 95% CI, 1.00-2.50; P = .05). This resulted in nonsignificantly poorer survival with planned autograft (OR = 1.18; 95% CI, 0.99-1.41; P = .06; Figure 1). Relapse reduction, and thus survival benefit, occurred chiefly in the first year from randomization with little difference thereafter. Survival at 5 years was 42.9% in the chemotherapy arm and 38.0% in the autograft arm (difference = 4.9%; 95% CI, −2.8%-12.6%; Figure 2). These results were not significantly different in any trial or subgroup.
Effect of autograft versus chemotherapy on overall survival in each trial.
Effect of autograft versus chemotherapy on overall survival in each trial.
Descriptive curve of overall survival by autograft versus chemotherapy.
Descriptive curve of overall survival by autograft versus chemotherapy.
Matched related donor allogeneic HCT versus autograft or chemotherapy
Nineteen trials were found that appeared to compare patients with versus without a matched related donor and to include a recommendation to treat with allogeneic HCT if a sibling donor was available (Table 1). Recommended treatment for the no-donor arm was chemotherapy in 7 trials, autograft in 5 trials, and chemotherapy or autograft in 8 trials. Data were available for 4, 4, and 5 trials (13 in total) in these groups, respectively, with median follow-up from 4-16 years (Table 4). The 5 trials that used both autograft and chemotherapy included a randomization between these, but many patients elected treatment, with 26%-100% of patients in the no-donor arm randomized.
Patient and disease characteristics are shown in Table 4. There were no significant imbalances between comparison arms for these, nor for the number lost to follow-up within 5 years. There were slight differences, in opposite directions, in the lengths of follow-up in the EORTC 06861 and PMH 92 trials (median follow-up: donor = 12.2 years, no donor = 10.5 years, P = .03; donor = 7.6, no donor = 10.3 years, P = .04, respectively). Between 0% and 36% of patients were Ph+. A substantial number of patients in the no-donor arm received an allogeneic HCT in first remission using an unrelated donor, mainly when patients were Ph+ (28% vs 7% for negative or unknown; P < .0001). Because this contaminated the comparison arm, the Ph+ patients were excluded from further analysis.
After exclusion of the Ph+ patients, 25%-49% of patients had a matched sibling donor (Table 5). Only 8 of the 13 trials supplied information on whether the patients in the no-donor arm had a sibling. In these trials, 36%-60% of those with a sibling had a donor.
The proportion of patients in the sibling donor arm in each trial that received a first remission sibling donor HCT was 61%-97% (Table 3). Median time to HCT was 5 months (supplemental Figure 1). In the no-donor arm, 62%-100% of patients received chemotherapy in trials comparing with chemotherapy, 63%-84% received autograft in the autograft comparisons, and in those trials with a randomization between autograft and chemotherapy, 18%-37% received autograft and 60%-79% received chemotherapy. Although the number of patients receiving allogeneic HCT in the no-donor arms was generally below 10%, they were 18% for JALSG-ALL93 and 32% for GRAALL2003.
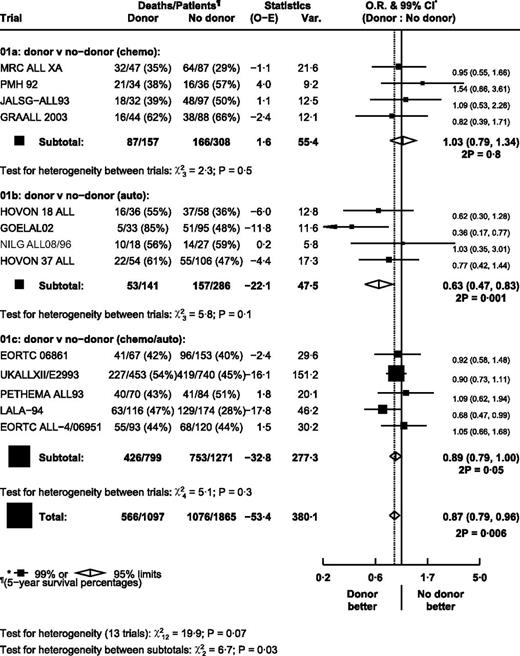
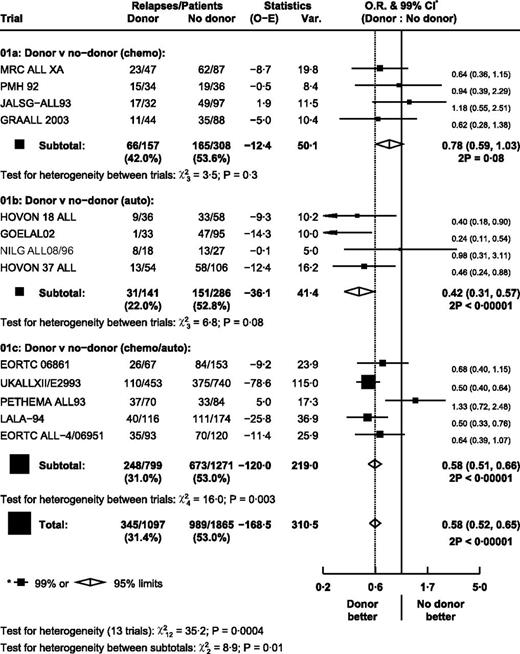
There were significantly fewer relapses in the donor arm (OR = 0.58; 95% CI, 0.52-0.65; P < .000 01; Figure 3). However, there was significant heterogeneity between trials in the autograft/chemotherapy comparison (P = .003), with PETHEMA ALL93 being an outlier. Exclusion of the PETHEMA trial removed the heterogeneity within this subgroup without making any substantial change to the effect estimates (autograft/chemotherapy comparison: OR = 0.54; 95% CI, 0.47-0.62; overall OR = 0.55; 95% CI, 0.49-0.62). There was heterogeneity between subgroups (P = .01), with the largest effect in the autograft comparison and the smallest in the chemotherapy comparison.
Effect of donor versus no donor on time to relapse in each trial. Ph+ patients were excluded.
Effect of donor versus no donor on time to relapse in each trial. Ph+ patients were excluded.
TRM was less frequent than relapse, but was substantially higher in the donor arm (OR = 2.36; 95% CI, 1.94-2.86; P < .00001; Figure 4). PETHEMA ALL93 was the only apparent outlier, but exclusion of this trial made no material difference on the effect estimates (overall OR = 2.49; 95% CI, 2.04-3.04). Results were not significantly different in any subgroup either for relapse or TRM. Overall survival was significantly longer in the donor arm (OR = 0.87; 95% CI, 0.79-0.96; P = .006; Figure 5). Heterogeneity between trials within subgroups was not significant.
Effect of donor versus no donor on TRM in each trial. Ph+ patients were excluded.
Effect of donor versus no donor on TRM in each trial. Ph+ patients were excluded.
Effect of donor versus no donor on overall mortality in each trial. Ph+ patients were excluded.
Effect of donor versus no donor on overall mortality in each trial. Ph+ patients were excluded.
As for relapse, the difference was greatest in the autograft comparison group (OR = 0.63; 95% CI, 0.47-0.83) and lowest in the chemotherapy comparison, where there was no significant difference (OR = 1.03; 95% CI, 0.79-1.34; heterogeneity between comparison groups P = .03). The number of patients in the no-donor arm who received chemotherapy was generally similar for the trials in the chemotherapy and the chemotherapy/autograft comparisons, approximately 60%-80%. Combining these groups resulted in a nonsignificant favorable effect of having a donor compared with the group in which the majority received chemotherapy (OR = 0.91; 95% CI, 0.82-1.01; P = .09).
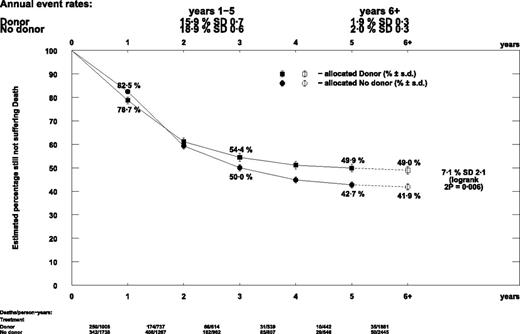
The descriptive curves crossed at just under 2 years from diagnosis (Figure 6), resulting from a detrimental effect in the first year, when most HCTs were performed (OR = 1.27; 95% CI, 1.02-1.59), and a beneficial effect in the second year (OR = 0.74; 95% CI, 0.59-0.92).
Descriptive curve of overall survival by donor versus no donor. Ph+ patients were excluded.
Descriptive curve of overall survival by donor versus no donor. Ph+ patients were excluded.
The only subgroup with evidence of a possible different effect was stratified according to age (P = .03; Figure 7). TRM was higher for those ≥ 35 years of age, with 32% of the donor arm and 14% of the no-donor arm dying without relapse, whereas these figures were 19% and 8% for those < 35 years of age. Therefore, although the effects of having a donor on relapse (beneficial) and TRM (detrimental) rates were similar in the 2 age groups, the higher TRM rate in the older age group resulted in a lack of overall survival benefit for this group (age < 35: OR = 0.79; 95% CI, 0.70-0.90; P = .0003; age > 35: OR = 1.01; 95% CI, 0.85-1.19; P = .9). Survival at 5 years were 55.0% versus 45.1% for patients < 35 years of age, and 39.2% versus 37.2% for age ≥ 35 years (53.7% vs 45.6% and 36.6% vs 35.0% if the autograft comparison group is excluded).
Effect of donor versus no donor on overall mortality in subgroups. Ph+ patients were excluded.
Effect of donor versus no donor on overall mortality in subgroups. Ph+ patients were excluded.
The alternative statistical method comparing the 5-year survival rates gave similar results. Overall survival at 5 years was significantly better in the donor arm (49.9% vs 42.7%, Figure 6; risk ratio [RR] = 0.85; 95% CI, 0.77-0.95; P = .003; supplemental Figure 2). The difference was greatest in the autograft comparison group (RR = 0.61; 95% CI, 0.45-0.81) and least in the chemotherapy comparison, where there was no significant difference (RR = 1.03; 95% CI, 0.79-1.34).
Sensitivity analyses including only patients known to have a sibling resulted in only slight modifications to the ORs, although the uncertainty was increased because of the smaller number of trials and patients (overall survival OR = 0.90; 95% CI, 0.80-1.01).
Discussion
This meta-analysis has shown that standard autograft does not have a beneficial effect compared with chemotherapy for adult patients with ALL in CR1. MSD myeloablative HCT improves survival only for younger patients, with an absolute benefit of around 10% at 5 years.
Recent systematic reviews30-32 have used study-level data, with some additional results supplied by the investigators. Identifying eligible trials for the donor versus no donor comparison is more difficult than for a standard randomized comparison and there are some differences in the trials included (supplemental Table 5). In the Cochrane review,31 it is not clear whether studies on our list were all identified, and if so, at what stage they were rejected. The Ram systematic review30 listed 3 excluded studies and could not obtain data for the main mortality analyses from one. Some trials were included in a “not intention-to-treat” subgroup because the relevant information could not be extracted from publications, a problem avoided by use of the IPD. Despite the differences, it is remarkable and reassuring how similar the overall results are.
A recent study from Japan using the databases of the Japan Adult Leukemia Study Group and the Japan Society for Hematopoietic Cell Transplantation performed a decision analysis of allogeneic HCT in adults with Ph− ALL in CR1, and concluded that allogeneic HCT in CR1 was superior in the whole population and in all subgroups.37 Use of IPD allowed us to analyze relapse and TRM separately and to look at the effects in subgroups. Relative effects did not appear to vary across subgroups. However, because TRM was much higher in the ≥ 35 year age group both in the donor and no-donor arms, the balance of benefit and harm differed by age group. Those patients < 35 years of age had improved survival with a donor, whereas there was no evidence of benefit for the older age group. It is clear that toxicity is a major issue for older patients with chemotherapy treatments and HCT. In the case of HCT, nonmyeloablative or reduced intensity conditioning may reduce toxicity, especially in older patients.38 In a recent Center for International Blood and Marrow Transplant Research study, similar outcomes were reported with full-intensity and reduced-intensity conditioning for adult patients with ALL in CR.39
Using patient-level data, we were able to use a consistent definition of high risk across trials, with high risk defined as B-lineage and WBC ≥ 30 × 109/L or T-lineage and WBC ≥ 100 × 109/L because Ph+ patients were excluded. Unlike the age groups, it was not clear that survival benefit differed by risk. TRM was similar across risk groups, and although the relapse rate was higher in the high-risk group, this was true for both the donor and the no-donor arms.
The reduction in relapse seen for those with a sibling donor was greatest in trials using autograft in the no-donor arm and least for those using chemotherapy alone, which is consistent with the suggestion from the randomized comparisons that chemotherapy may be more effective than autograft.
The dilemma of how to best treat an adult patient with ALL in CR1 will continue at present because the risk/benefit ratio associated with allogeneic HCT and chemotherapy will continue to change. Recently, several groups have reported good outcomes of standard-risk adult ALL patients treated with pediatric-inspired chemotherapy protocols.40-42 It should be noted that the trials reported herein used traditional adult-intensity chemotherapy regimens. Moreover, novel treatments such as mAbs have increasingly been used in the chemotherapy protocols and preliminary results of these approaches appear encouraging. In particular, incorporating tyrosine kinase inhibitors for Ph+ ALL may change the HCT approach for these patients. Better supportive care and selection of unrelated donors with the use of high-resolution allele-level HLA typing have improved the safety of HCT.43 Several studies now report outcomes of unrelated donor HCT similar to matched sibling donor in adult patients with ALL.39,44 Moreover, candidacy for allogeneic HCT and chemotherapy alone will be further refined by increasing the monitoring of minimal residual disease in the routine clinical setting. Therefore, it is important to continue to enroll these patients in well-designed clinical trials in the future.
We conclude that a standard autologous HCT has no additional benefit compared with chemotherapy in adult patients with ALL in CR1. Allogeneic myeloablative HCT from a related sibling donor provides a moderate survival benefit, but the absolute long-term survival benefit appears to be confined to younger patients, in whom it is approximately 10%. Continued study of allogeneic HCT versus modern chemotherapy protocols is required to optimize the outcomes of adult patients with ALL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Members of the Acute Leukemia Stem Cell Transplantation Trialists' Collaborative Group secretariat identified trials, obtained datasets, and corresponded with trialists to ensure accurate data (K. Davies, T. Elphinstone, V. Evans, L. Gettins, C. Gregory, S. James, L. MacKinnon, and T. McHugh), and designed and performed analyses (G. Buck, P. Morris, S. Richards, R. Wade, and K. Wheatley).
This work was supported by Cancer Research UK and the Medical Research Council. Funders were not involved in study design, analysis, or reporting.
Authorship
Contribution: V.G., S.R., and J.R. drafted the report and revised it in the light of comments received after circulation to all members of the collaborative group.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of Acute Leukemia Stem Cell Transplantation Trialists' Collaborative Group (ALSCT-TCG) participating institutions, please see supplemental Appendix 1.
Correspondence: Acute Leukemia Stem Cell Transplantation Secretariat, Clinical Trial Service Unit, Richard Doll Building, University of Oxford, Old Road Campus, Roosevelt Drive, Oxford OX3 7LF, United Kingdom; e-mail: alsct.overview@ctsu.ox.ac.uk.