Abstract
T-cell leukemia/lymphoma 1 (TCL1) is an oncogene overexpressed in T-cell prolymphocytic leukemia and in B-cell malignancies including B-cell chronic lymphocytic leukemia and lymphomas. To date, only a limited number of Tcl1-interacting proteins that regulate its oncogenic function have been identified. Prior studies used a proteomic approach to identify a novel interaction between Tcl1 with Ataxia Telangiectasia Mutated. The association of Tcl1 and Ataxia Telangiectasia Mutated leads to activation of the NF-κB pathway. Here, we demonstrate that Tcl1 also interacts with heat shock protein (Hsp) 70. The Tcl1-Hsp70 complex was validated by coimmunoprecipitation experiments. In addition, we report that Hsp70, a protein that plays a critical role in the folding and maturation of several oncogenic proteins, associates with Tcl1 protein and stabilizes its expression. The inhibition of the ATPase activity of Hsp70 results in ubiquitination and proteasome-dependent degradation of Tcl1. The inhibition of Hsp70 significantly reduced the growth of lymphoma xenografts in vivo and down-regulated the expression of Tcl1 protein. Our findings reveal a functional interaction between Tcl1 and Hsp70 and identify Tcl1 as a novel Hsp70 client protein. These findings suggest that inhibition of Hsp70 may represent an alternative effective therapy for chronic lymphocytic leukemia and lymphomas via its ability to inhibit the oncogenic functions of Tcl1.
Key Points
Hsp70 represents an alternative effective therapy for CLL and lymphomas via its ability to inhibit the oncogenic functions of Tcl1.
Heat Shock Protein 70 interacts and regulates Tcl1 expression in leukemia and lymphomas.
Introduction
The T-cell leukemia/lymphoma 1 (TCL1) gene maps to chromosome 14q31.2 and is involved in chromosomal translocations and inversions in T-cell prolymphocytic leukemia. It is also dysregulated in several B-cell malignancies, including B-cell chronic lymphocytic leukemia (B-CLL), some B-cell non-Hodgkin lymphomas, and in germ-cell tumors such as Seminoma and Dysgerminoma.1,2 Normally, Tcl1 protein is expressed in early embryos and fetal tissues, germ T cells, and most B cells.3-5 We showed previously that aggressive human CLLs overexpress Tcl16 and that transgenic mice expressing TCL1 in B-cells developed an aggressive form of CLL.7,8 These previous studies demonstrated that Tcl1 up-regulation is critical for pathogenesis of the aggressive form of human chronic lymphocytic leukemia (CLL). Using a proteomic approach, we also identified Ataxia Telangiectasia Mutated (Atm) as a Tcl1-interacting protein. This interaction is responsible for the activation of NF-κB pathway in hematologic malignancies.9 We proposed the following mechanism: through interacting with Atm, Tcl1 expression causes increased Atm kinase activity, an increase in the phosphorylation of IκBα, and translocation of Rel-A to the nucleus. Tcl1 and Atm have a common target: IκBα, a physiologic NF-κB inhibitor. Indeed, Tcl1 interacts with IκBα,10 whereas Atm phosphorylates it.11,12 As a result of this posttranslational modification, IκBα is subsequently degraded by ubiquitination.13 Because Tcl1 interacts with both IκBα and Atm, coexpression of Tcl1 and Atm resulted in an additive effect on IκBα. As a result of IκBα degradation, active p65 NF-κB active (Rel-A) translocates from the cytosol into the nucleus. Here, Rel-A interacts with the EGR1 promoter sequence and causes an overexpression of Egr1 protein.14
The heat shock protein (Hsp) 70 protein can influence cell survival and the level of Akt.15 Importantly, this protein also plays an essential role in protein folding and stability. Hsp70 is highly expressed in a variety of malignant tumors, and its expression correlates with increased cell proliferation and lympho-node metastases.16-19 In contrast to its low abundance in unstressed normal cells, the inducible Hsp70 protein is present at constitutively elevated levels in human tumors from various anatomic locations. Enhanced Hsp70 expression in tumor cells correlates with resistance to cell death and is associated with poor patient prognosis.17,20-23 Hypoxia, nutrient deprivation, oxidative stress, oncogene activation, and exposure to chemotherapeutic agents lead to alterations in protein structure or processing, as well as up-regulation of Hsp70.
There is a variety of evidence suggesting that Hsp70 is actively secreted, has important extracellular functions, and has prognostic value. First, Hsp70 has been shown to act as a potent danger signal to the immune system.24-27 Second, depletion of Hsp70 induces massive apoptotic death in tumorigenic cells but not in nontumorigenic epithelial cells or in embryonic fibroblast.28,29 These data indicate a cancer-specific cell survival function of this protein is likely. Finally, high levels of circulating Hsp70 in plasma confer a poor prognosis in acute myeloid leukemia and acute lymphocytic leukemia. Circulating Hsp70 therefore could be a valuable prognostic indicator in leukemias.30 These lines of evidence suggest that Hsp70 is required for protecting certain vulnerable proteins whose function is essential and specific for the survival of cancer cells.31 A large number of malignancies are associated with overexpression of Hsps, most notably Hsp27, Hsp70, and Hsp90.30,32 As a result, these proteins represent potential therapeutic targets. Many approaches to targeting Hsps are under investigation, including strategies ranging from design of targeted small molecule drugs and use of peptide-Hsp70 complexes as an immunotherapeutic vaccine approach.33-36 Hsp70 can be targeted by small interfering RNAs (siRNAs), antisense oligonucleotides, or aptamers to down-regulate its expression29,37,38 and by chemical modulators such as myricetin (Myr), an Hsp70 ATPase inhibitor.39,40 We postulated that Tcl1 might interact with proteins involved in critical survival pathways up-regulated in hematologic malignancies. The aim of this work was to identify new partners of Tcl1 and to uncover its role in oncogenesis using a mass spectrometry approach.
We found that Hsp70 interacts with Tcl1 and regulates its expression. These results were novel and complimented prior reports documenting an interaction between Tcl1 with Akt41 and Atm.9 By inhibiting Hsp70, we observed a decrease in the expression of Tcl1. Together, these data suggest that disruption of the interaction between Hsp70 and Tcl1 deserves further investigation as a potential therapeutic approach for cancer.
Methods
DNA constructs
Human TCL1 full-length was cloned into the pCMV5 vector to obtain both pCMV5-TCL1 wild-type (WT) and pCMV5-TCL1 WT hemagglutinin (HA)–tagged vectors. The dual-luciferase reporter assay system and Renilla luciferase reporter vector pRL-TK were purchased from Promega. pNF-kB-Luc and the construct encoding the kinase domain of mitogen-activated protein kinase kinase 1 (MEKK1) under the transcriptional control of the CMV promoter pFC-MEKK were purchased from Stratagene.
Cells, transfections, and antisera
Human embryonic kidney (HEK)–293 cells were grown in DMEM with 10% FBS and 100 μg/L gentamicin at 37°C and 5% CO2. FuGENE 6 transfection reagent and protease inhibitor mixture tablets were purchased from Roche. A549 lung cancer cells and Daudi and Raji lymphoma cells were grown in RPMI 1640 with 10% FBS and 100 μg/L gentamicin at 37°C and 5% CO2. A549 lung cancer cells and Daudi and Raji lymphoma cells were grown in RPMI 1640 with 10% FBS and 100 μg/L gentamicin at 37°C. Daudi and Raji lymphoma cells and CLL samples were transfected with Nucleofector following Amaxa Biosystems guidelines for cells in suspension. Solution L and program A-020 were used for Daudi and Raji cell lines; solution R and program U-016 were used for CLL cell samples.
Immunoblots were developed using Denville HyGlo enhanced chemiluminescence. Antibodies used were as follows: anti-Tcl1 (sc 32331), anti-Hsp70 (sc 32239), anti-Akt (sc 1618), and anti-Hsp90 (sc 7947) from Santa Cruz Biotechnology; and anti-phospho (Ser 473) Akt (9271) and anti–poly(ADP-ribose) polymerase (PARP; 9532) from Cell Signaling Technology.
Immunoprecipitation analysis
Coimmunoprecipitation experiments were performed by incubating 1 mg of total cell lysates (radioimmunoprecipitation assay [RIPA] buffer) with A/G agarose beads (sc 2003; Santa Cruz Biotechnology) with anti-Tcl1a antibody or anti-Hsp70 antibody overnight at 4°C; after washing, beads were boiled in 1 × SDS sample buffer and proteins were separated on 4% to 20% polyacrylamide gels (Bio-Rad Laboratories). Dithiobis [succinimidylpropionate] (DSP) crosslinker was from Pierce Chemical.
Mass spectrometry studies
Protein pellets were solubilized and digested by trypsin. Protein constituents were identified by liquid chromatography tandem mass spectrometry (LC-MS/MS). Inspection of LC-MS/MS data was undertaken to assess exclusive presence of mass peaks belonging to candidate partner proteins in samples from cells transfected with tagged TCL1-HA.9
Ubiquitination experiments
Total protein lysates in RIPA buffer (1 mg) were incubated overnight at 4°C in rotating condition with anti-Tcl1 antibody (sc 32331; Santa Cruz Biotechnology) and AG/agarose beads and then run on 4% to 20% polyacrylamide gels. The nitrocellulose membranes were probed with anti–HA-horseradish peroxidase to detect ubiquitin (Ub)–HA, anti-Tcl1A.
Luciferase assay
HEK-293 cells were transfected with the indicated constructs. Firefly and Renilla luciferase activities were assayed with the dual-luciferase assay system (Promega), and firefly luciferase activity was normalized to Renilla luciferase activity, as suggested by the manufacturer. All experiments were carried out in triplicate and repeated 3 times with consistent results.
TUNEL assay
Daudi and Raji Lymphoma cells were assessed for the induction of single strand breaks (indicative of apoptosis) by the terminal deoxynucleotidyl transferase mediated X-dUTP nick end labeling (TUNEL) assay using the in situ cell death detection kit (Boehringer/Roche), according to the manufacturer's recommendations.
MTS assay
Human Daudi and Raji lymphoma cells were seeded in triplicate at 25 000 cells per well in a 96-well plate. The proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay (Promega), according to the manufacturer's recommendations.
Patients
B-CLL samples were obtained after informed consent in accordance with the Declaration of Helsinki from patients diagnosed with B-CLL from the CLL Research Consortium (La Jolla, CA). Research was performed with the approval of the Institutional Review Board of The Ohio State University (protocol 2005C0014). In brief, peripheral blood was obtained from CLL patients (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and lymphocytes were isolated through Ficoll/Hypaque gradient centrifugation (GE Healthcare). Cryopreserved cells were processed with RIPA buffer for protein extraction.
Animal studies
Mice were maintained and animal experiments were conducted under institutional guidelines established for the Animal Facility at The Ohio State University; Balb/c nu/ν mice were obtained from The Jackson Laboratory. At day 1, tumors were established by injecting Daudi lymphoma cells (200 μL of PBS, 7.5 × 107 cells/mouse) or Raji lymphoma cells (200 μL of PBS, 7.5 × 107 cells/mouse) into the right flanks of female nude mice (6 weeks of age and 25 g of body weight). Tumor size was measured daily until tumors reached 50 mm3 (day 12). Then, Myr (concentration, 20 mg/kg) was injected directly into the tumors and at 12, 15, 18, 21 days. Tumors were measured on the day of the injections and 3 days after the last injection. At that time, the mice were sacrificed and tumors were weighed. Tumor volumes were calculated using the equation V (in mm3) = A × B2/2, where A is the largest diameter and B is the perpendicular diameter. Tumors were excised and small pieces homogenized for few minutes and lysed in RIPA buffer for 30 minutes. Total lysates were analyzed by Western blotting.
Statistics
All graph values represent means ± SEM from 3 independent experiments, with each measured in triplicate. The differences between 2 groups were analyzed with unpaired 2-tailed Student t test and with nonparametric Mann-Whitney test. P < .05 was considered statistically significant and is indicated with asterisks as described in figure legends.
Results
Tcl1 interacts with Hsp70
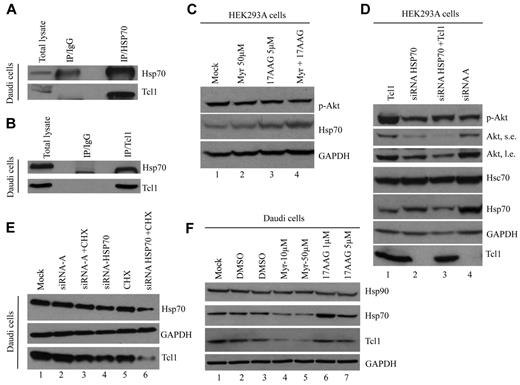
To identify Tcl1-interacting proteins, we used a TCL1 cDNA modified at its 3′ end with a sequence encoding an HA tag (TCL1-HA). The A549 lung cancer cell line was used as recipient and transfected either with WT TCL1 (used as a control) or TCL1-HA and treated with DSP, a crosslinker that fixes protein complexes in vivo. The A549 cell line was used in these initial proof of principle experiments because no CLL cell lines are currently available. Furthermore, these cells can be easily transfected at a very high efficiency, an important requirement for such an experiment. Cells were lysed and Tcl1-HA protein, along with candidate protein partners, was isolated using an HA-conjugated resin. Purified proteins were treated with dithiothreitol to cleave DSP and dissociate complexes and then digested by trypsin; protein constituents were identified by LC-MS/MS.9 From these experiments, a putative interaction between Tcl1 and Hsp70 was observed by mass spectrometry. Hsp70 is an important molecular chaperone that plays a key role in the conformational maturation and stabilization of signaling proteins involved in cell growth and survival. The Tcl1-Hsp70 interaction was observed on immunoprecipitation with either anti-Tcl1 or anti-Hsp70 antibodies and not in lysates immunoprecipitated with immunoglobulin G control (Figure 1A-B).
Hsp70 interacts with Tcl1 and protects it from degradation. (A) Equal amounts of Daudi protein extracts were immunoprecipitated with agarose-conjugated mouse immunoglobulin G (IgG) or anti-Hsp70 antibody and then subjected to SDS-PAGE and to immunoblot using antibodies specific for Tcl1 and Hsp70. (B) In the reverse immunoprecipitation, Daudi cell lysates were immunoprecipitated with anti-Tcl1 antibody. The immunoprecipitates were analyzed by immunoblotting with anti-Hsp70 and anti-Tcl1 antibodies. (C) HEK-293A cells were treated with Myr (50μΜ), 17AAG (5μM), and their combination for 24 hours. Phospho-Akt (Ser 473) and Hsp70 were detected. (D) HEK-293A cells were transfected with siRNA for HSP70 or control siRNA (100nM for 48 hours) and TCL1 plasmid. Indicated proteins were detected by Western blotting. S.e. indicates short exposure; and l.e., long exposure. (E) Daudi lymphoma cells were transfected with siRNA for HSP70 or control siRNA-A, a nontargeting siRNA designed as negative control (100nM/48 hours), and treated with CHX (1.5μM/48 hours) where indicated. Total lysates were subjected to SDS-PAGE and immunoblotted for Hsp70 and Tcl1 antibodies. (F) Daudi lymphoma cells were treated with DMSO (as control), Myr (10 and 50μM), and 17AAG (1 and 5μM) for 24 hours. Total lysates were subjected to SDS-PAGE and immunoblotted for Hsp70, Hsp90, and Tcl1 antibodies.
Hsp70 interacts with Tcl1 and protects it from degradation. (A) Equal amounts of Daudi protein extracts were immunoprecipitated with agarose-conjugated mouse immunoglobulin G (IgG) or anti-Hsp70 antibody and then subjected to SDS-PAGE and to immunoblot using antibodies specific for Tcl1 and Hsp70. (B) In the reverse immunoprecipitation, Daudi cell lysates were immunoprecipitated with anti-Tcl1 antibody. The immunoprecipitates were analyzed by immunoblotting with anti-Hsp70 and anti-Tcl1 antibodies. (C) HEK-293A cells were treated with Myr (50μΜ), 17AAG (5μM), and their combination for 24 hours. Phospho-Akt (Ser 473) and Hsp70 were detected. (D) HEK-293A cells were transfected with siRNA for HSP70 or control siRNA (100nM for 48 hours) and TCL1 plasmid. Indicated proteins were detected by Western blotting. S.e. indicates short exposure; and l.e., long exposure. (E) Daudi lymphoma cells were transfected with siRNA for HSP70 or control siRNA-A, a nontargeting siRNA designed as negative control (100nM/48 hours), and treated with CHX (1.5μM/48 hours) where indicated. Total lysates were subjected to SDS-PAGE and immunoblotted for Hsp70 and Tcl1 antibodies. (F) Daudi lymphoma cells were treated with DMSO (as control), Myr (10 and 50μM), and 17AAG (1 and 5μM) for 24 hours. Total lysates were subjected to SDS-PAGE and immunoblotted for Hsp70, Hsp90, and Tcl1 antibodies.
In Figure 1C, the treatment of HEK-293A cells with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17AAG) resulted in Hsp70 overexpression (lanes 3 and 4); this effect is thought to occur via a compensatory mechanism because of drug-mediated down-regulation of Hsp90.42 To determine whether Hsp70 activity could regulate phospho-Akt and Tcll1 expressions, we analyzed HEK-293A cells (Figure 1D) and Daudi lymphoma cells (Figure 1E) after treatment with siRNA for Hsp70. Because Hsp7043 and Tcl141 have a common role in the up-regulation of PI3K/AKT pathway activity, we demonstrated that the down-regulation of Hsp70 (Figure 1D lane 3) reduced the level of phospho-Akt expression compared with controls (Figure 1D lane 1). In addition, Hsp70 is crucial for regulating Tcl1 expression. In fact, treatment of Daudi lymphoma cells with cycloheximide (CHX) and siRNA for HSP70 reduced of Tcl1 (Figure 1E lane 6) compared with controls (Figure 1E lanes 3-5). Treatment of Daudi lymphoma cells with Myr also showed a tremendous reduction of endogenous Tcl1 and Hsp70 proteins (Figure 1F lanes 4 and 5).
Inhibition of Hsp70 affects Tcl1 and phospho-Akt expression
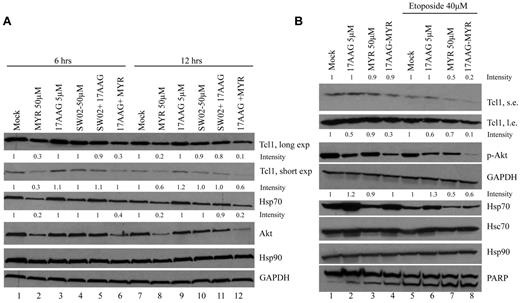
Hsp70 regulates the stability and functions of multiple oncogenic client proteins. Because we demonstrated an association between Tcl1 and Hsp70, we analyzed whether the chaperone activity of Hsp70 also was essential for Tcl1 protein stability. To this purpose, we treated Daudi cells with the Hsp70 inhibitor Myr, Hsp70 activator SW02, or the Hsp90 inhibitor 17AAG. The reduction of Tcl1 protein levels observed (Figure 2A lanes 2, 6, 8, and 12) suggested that the molecular-chaperoning activity of Hsp70 plays a crucial role in Tcl1 stability in Daudi lymphoma cells. At the 12-hour time point, combined treatment with 17AAG and Myr led to an almost complete loss of Akt expression.
Inhibition of Hsp70 affects Tcl1 expression. (A) Daudi lymphoma cells were treated with the Hsp70 inhibitor Myr, 17AAG, Hsp70 activator SWO2, and combinations as indicated for 6 and 12 hours. Cells were lysed and protein extracts were subjected to SDS-PAGE and analyzed by Western blotting with indicated antibodies. The numbers above the blots indicate the intensity of the band expressed as ratio gene product (TCL1, HSP70, or AKT1)/GAPDH and normalized to Mock 6 hours or Mock 12 hours. (B) Daudi lymphoma cells were treated with 17AAG (5μM), Myr (50μM), and their combination for 24 hours, with or without Etoposide (40μM) for 12 hours. Cells lysates were analyzed by Western blotting with indicated antibodies. The numbers above the blots indicate the intensity of the band expressed as ratio gene product (p-AKT1, HSP70)/GAPDH and normalized to Mock or Mock-Etoposide.
Inhibition of Hsp70 affects Tcl1 expression. (A) Daudi lymphoma cells were treated with the Hsp70 inhibitor Myr, 17AAG, Hsp70 activator SWO2, and combinations as indicated for 6 and 12 hours. Cells were lysed and protein extracts were subjected to SDS-PAGE and analyzed by Western blotting with indicated antibodies. The numbers above the blots indicate the intensity of the band expressed as ratio gene product (TCL1, HSP70, or AKT1)/GAPDH and normalized to Mock 6 hours or Mock 12 hours. (B) Daudi lymphoma cells were treated with 17AAG (5μM), Myr (50μM), and their combination for 24 hours, with or without Etoposide (40μM) for 12 hours. Cells lysates were analyzed by Western blotting with indicated antibodies. The numbers above the blots indicate the intensity of the band expressed as ratio gene product (p-AKT1, HSP70)/GAPDH and normalized to Mock or Mock-Etoposide.
To determine whether etoposide would enhance the effects of Myr and 17AAG on lymphoma cell death, we tested the effects of these drugs in combination on Daudi lymphoma cells. In Figure 2B, the effects of Myr and 17AAG on Akt loss and Tcl1 loss and their combination are demonstrated with and without etoposide. Consistent with prior published studies, the expression of Hsp70 was increased in response to 17AAG treatment,42 and phospho-Akt and Tcl1 levels were decreased after inhibition of the Hsp70 ATPase activity by Myr (Figure 2B lanes 3-4 vs 1-2; lanes 7-8 vs 5-6).
Hsp70 inhibition induces proteasome-dependent degradation of Tcl1
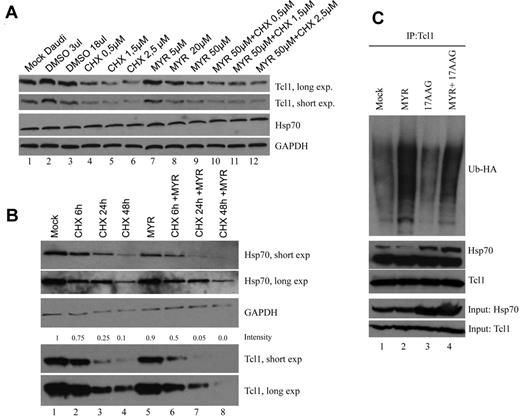
The mechanisms by which Hsp70 loss leads to reduced levels of Tcl1 protein were next investigated. For these studies, CHX was used as an inhibitor of protein synthesis, to evaluate the effect of Myr on Hsp70 expression (Figure 3A).
Myr induces Tcl1 ubiquitination and degradation. (A) Daudi lymphoma cells were treated with CHX, Myr, and their combination for 24 hours. Cells lysates were analyzed by Western blotting with indicated antibodies. (B) Daudi lymphoma cells were treated with CHX, Myr, and their combination for 6, 24, and 48 hours in a time course experiment. The numbers above the blots indicate the intensity of the band expressed as ratio gene product TCL1/GAPDH and normalized to Mock. (C) HEK-293A cells were transfected with TCL1 and UB-HA plasmids. Forty-eight hours later, cells were treated with 17AAG, Myr, and their combination for additional 18 hours and MG132 for 3 hours; Total lysates were immunoprecipitated with anti-Tcl1. The immunoprecipitates were analyzed by immunoblotting with anti-HA and anti-Tcl1.
Myr induces Tcl1 ubiquitination and degradation. (A) Daudi lymphoma cells were treated with CHX, Myr, and their combination for 24 hours. Cells lysates were analyzed by Western blotting with indicated antibodies. (B) Daudi lymphoma cells were treated with CHX, Myr, and their combination for 6, 24, and 48 hours in a time course experiment. The numbers above the blots indicate the intensity of the band expressed as ratio gene product TCL1/GAPDH and normalized to Mock. (C) HEK-293A cells were transfected with TCL1 and UB-HA plasmids. Forty-eight hours later, cells were treated with 17AAG, Myr, and their combination for additional 18 hours and MG132 for 3 hours; Total lysates were immunoprecipitated with anti-Tcl1. The immunoprecipitates were analyzed by immunoblotting with anti-HA and anti-Tcl1.
In a time course experiment, treatment with Myr and CHX in combination (Figure 3B lanes 7 and 8) resulted in an additive reduction of Tcl1 and Hsp70 compared with CHX treatment alone (Figure 3B lanes 3 and 4). The treatment for 48 hours with CHX also affected the expression of Gapdh, but the effect of CHX and Myr in combination on Tcl1 expression is clear.
Akt, a major survival kinase, is subject to regulation by Hsp70 and Tcl1.41 When the ATPase activity of Hsp70 is increased or decreased by certain compounds (SW02 and Myr), Akt levels also were increased or decreased.43 To determine whether Tcl1 expression is controlled by Hsp70, we carried out an ubiquitination assay. We examined whether proteasomal degradation is a mechanism by which the loss of Tcl1 protein occurs after treatment with the Hsp70 ATPase inhibitor Myr. HEK-293 cells were transfected with TCL1 and Ub-HA expression vectors (Figure 3C). Forty-eight hours after transfection, cells were treated with MG132, a proteasome inhibitor, at 10μM for an additional 3 hours and then lysed. Proteins were immunoprecipitated with anti-Tcl1 followed by immunoblotting with anti-HA. Immunoprecipitation of Tcl1 followed by Western blot analysis with an anti-HA (to detect Ub-HA–tagged protein) detected significantly higher levels of ubiquitinated Tcl1 in the presence of Myr and not in presence of and 17AAG (Figure 3C). These data demonstrate that Myr promoted ubiquitination followed by proteasome-dependent degradation of Tcl1 protein. Thus, Tcl1 is an Hsp70 client protein.
Inhibition of Hsp70 down-regulates Tcl1 protein in CLL
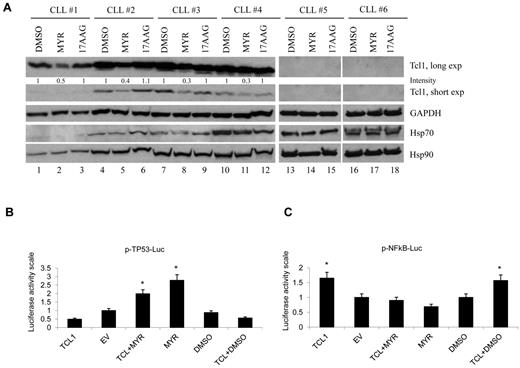
Because Tcl1 is overexpressed in aggressive CLL and most other human B-cell lymphomas, it was of interest to investigate whether our results were relevant to human CLL. Therefore, we studied the effects of HSP70 inhibition in human CLL samples (Figure 4A). Clinical data describing the origin of all CLL patient samples data are summarized in supplemental Table 1. The effects of Myr and 17AAG on Tcl1 expression were examined in CLL patient samples. Tcl1 levels were reduced after treatment with Myr (Figure 4A). These results indicated that Tcl1 stability in patient samples was dependent on Hsp70 activity and that Tcl1 protein was susceptible to degradation after treatment with the Hsp70 inhibitor. As demonstrated previously, Tcl1 expression directly affects the transactivating function of NF-κB.44 We used a commercial system based on the ability of MEKK1 to activate an NF-κB reporter construct pNF-κB-Luc. This construct expresses luciferase under the control of an NF-κB–responsive element. Here, we demonstrated that Myr leads to NF-κB inactivation by disrupting crosstalk between the Hsp70-Tcl1 complex and the NF-κB pathway. Prior reports have described the interaction between TP53 and Hsp70 and the inhibition of TP53 transcriptional activity in the presence of Hsp70.45,46 Consistent with these observations, we demonstrated that Myr increases TP53 promoter activation, blocking Hsp70 activity (Figure 4B-C).
Inhibition of Hsp70 down-regulates Tcl1 protein in CLL. (A) CLL patient samples were treated for 12 hours with mock or Myr (50μΜ) or 17AAG (5μΜ) and then analyzed for Tcl1 and Gapdh by Western blotting. The numbers above the blots indicate the intensity of the band expressed as ratio gene product TCL1/GAPDH and normalized to DMSO. (B) Myr increases TP53 promoter activation, blocking Hsp70 activity. HEK-293A cells were cotransfected for 24 hours with 50 ng of TP53-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 0.75 μg of CMV5-empty vector, or 0.75 μg of CMV5-TCL1 WT were used. Five nanograms of pFC-MEKK was added. Cells were treated with 10μM Myr for 12 hours, where indicated. Data are representative of 3 independent experiments. Data are mean ± SEM of 3 independent experiments, and each is measured in triplicate (*P < .05). (C) Tcl1 activates NF-κB–dependent transcription. HEK-293A cells were cotransfected for 24 hours with 50 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 0.75 μg of CMV5-empty vector, or 0.75 μg of CMV5-TCL1 WT were used. Five nanograms of pFC-MEKK was added. Cells were treated with 10μM Myr for 12 hours, where indicated. Data are representative of 3 independent experiments. Data are mean ± SEM of 3 independent experiments, and each is measured in triplicate (*P < .05).
Inhibition of Hsp70 down-regulates Tcl1 protein in CLL. (A) CLL patient samples were treated for 12 hours with mock or Myr (50μΜ) or 17AAG (5μΜ) and then analyzed for Tcl1 and Gapdh by Western blotting. The numbers above the blots indicate the intensity of the band expressed as ratio gene product TCL1/GAPDH and normalized to DMSO. (B) Myr increases TP53 promoter activation, blocking Hsp70 activity. HEK-293A cells were cotransfected for 24 hours with 50 ng of TP53-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 0.75 μg of CMV5-empty vector, or 0.75 μg of CMV5-TCL1 WT were used. Five nanograms of pFC-MEKK was added. Cells were treated with 10μM Myr for 12 hours, where indicated. Data are representative of 3 independent experiments. Data are mean ± SEM of 3 independent experiments, and each is measured in triplicate (*P < .05). (C) Tcl1 activates NF-κB–dependent transcription. HEK-293A cells were cotransfected for 24 hours with 50 ng of pNF-κB-Luc reporter and 50 ng of pRL-TK Renilla reporter constructs. In addition, 0.75 μg of CMV5-empty vector, or 0.75 μg of CMV5-TCL1 WT were used. Five nanograms of pFC-MEKK was added. Cells were treated with 10μM Myr for 12 hours, where indicated. Data are representative of 3 independent experiments. Data are mean ± SEM of 3 independent experiments, and each is measured in triplicate (*P < .05).
Myr induces reduced proliferation and apoptosis in lymphoma cells in vitro experiments
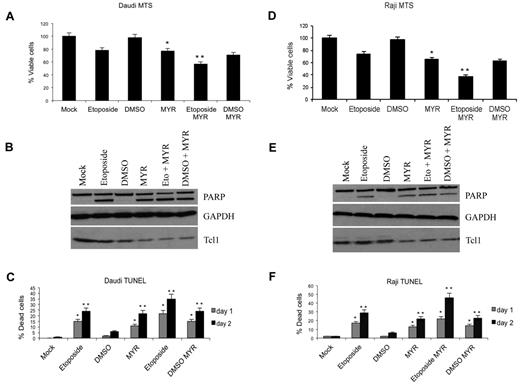
We investigated the effect of Tcl1 depletion by the Hsp70 inhibitor Myr on the proliferation and viability of Daudi and Raji lymphoma cells that express both Tcl1 and Hsp70. Treatment with Myr led to reduced Tcl1 expression and a statistically significant reduction in cell proliferation as determined by MTS assay (*P < .05, **P < .005; Figure 5A,D). Further analysis indicated the reduced cell proliferation was due at least in part to apoptosis, because significant increases in TUNEL positivity also were observed after treatment with Myr (Figure 5C,F). Consistent with these results, immunoblot analysis of lysates from Daudi and Raji lymphoma cells, 24 hours after treatment with Myr, etoposide, or both indicated reduced Tcl1 expression and processing of PARP (Figure 5B,E).
Myr induces apoptosis and reduction of proliferation in lymphoma cells in vitro experiments. (A,D) Daudi and Raji lymphoma cells were treated with etoposide (40μM) for 12 hours and/or Myr (50μM) for 24 hours as indicated and then analyzed by MTS assay. Bar graphs show SD for values from 3 experiments (*P < .05, **P < .005). (C,F) Daudi and Raji lymphoma cells were treated with etoposide (40μM) for 12 hours and/or Myr (50μM) for 24 hours as indicated and then analyzed by TUNEL analysis. Bar graphs show SD for values from 3 experiments (*P < .05, **P < .005). (B,E) Cells lysates from described experiment were immunoblotted with anti-Tcl1, anti-Parp, and anti-Gapdh antibodies.
Myr induces apoptosis and reduction of proliferation in lymphoma cells in vitro experiments. (A,D) Daudi and Raji lymphoma cells were treated with etoposide (40μM) for 12 hours and/or Myr (50μM) for 24 hours as indicated and then analyzed by MTS assay. Bar graphs show SD for values from 3 experiments (*P < .05, **P < .005). (C,F) Daudi and Raji lymphoma cells were treated with etoposide (40μM) for 12 hours and/or Myr (50μM) for 24 hours as indicated and then analyzed by TUNEL analysis. Bar graphs show SD for values from 3 experiments (*P < .05, **P < .005). (B,E) Cells lysates from described experiment were immunoblotted with anti-Tcl1, anti-Parp, and anti-Gapdh antibodies.
Hsp70 ATPase activity inhibition affects proliferation and growth of tumor xenografts
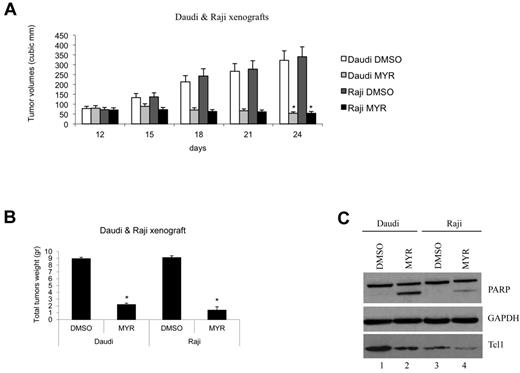
The effects of Myr on Tcl1 expression and lymphoma progression were evaluated in vivo. For these studies, xenograft models of lymphoma with Daudi or Raji cells were used (Figure 6). Mice bearing either Daudi or Raji xenograft tumors treated with Myr showed a 4- to 5-fold reduction in volume compared with control (Figure 6A-B). At the end of the experiment, total proteins extracted from tumors were analyzed by Western blotting for analysis of Tcl1 expression (Figure 6B-C). Consistent with the in vitro results, these in vitro data illustrated the effect of Hsp70 on regulating Tcl1 protein stability.
Hsp70 ATPase activity inhibition affects proliferation and growth of tumor xenografts. (A) Graph representing tumor volumes at indicated days during the experiment for the 4 groups of mice indicated (5 mice/group). Two groups of mice were subcutaneously injected with 6 × 107 Daudi lymphoma cells and another 2 groups with 4 × 107 Raji lymphoma cells (day 1). When tumors reached 50 mm3 (day 12), Myr (20 mg/kg) or DMSO in a volume of 50 μL was injected directly into the tumors at 12, 15, 18, and 21 days. Tumors were measured on the day of the injection and 3 days after the last injection. (*P < .05). (B) Xenograft tumors were weighed. (C) Total lysates from tumors were analyzed by Western blotting and tested with indicated antibodies (*P < .05).
Hsp70 ATPase activity inhibition affects proliferation and growth of tumor xenografts. (A) Graph representing tumor volumes at indicated days during the experiment for the 4 groups of mice indicated (5 mice/group). Two groups of mice were subcutaneously injected with 6 × 107 Daudi lymphoma cells and another 2 groups with 4 × 107 Raji lymphoma cells (day 1). When tumors reached 50 mm3 (day 12), Myr (20 mg/kg) or DMSO in a volume of 50 μL was injected directly into the tumors at 12, 15, 18, and 21 days. Tumors were measured on the day of the injection and 3 days after the last injection. (*P < .05). (B) Xenograft tumors were weighed. (C) Total lysates from tumors were analyzed by Western blotting and tested with indicated antibodies (*P < .05).
Discussion
Dysregulation of TCL1 in T cells causes T-cell prolymphocytic leukemias3,47 and overexpression of Tcl1 in B cells causes the aggressive form of CLL.7,8 To clarify the function of Tcl1 in leukemia, we studied protein complexes of Tcl1 using a proteomic approach. The A549 lung cancer cell line was used as recipient and transfected either with WT TCL1 (used as a control) or TCL1-HA. The A549 cell line was used in these initial proof of principle experiments because no CLL cell lines are currently available. Furthermore, these cells can be easily transfected at a very high efficiency, an important requirement for such an experiment. To identify the possible interaction between Tcl1 and proteins specifically expressed in B cells, we performed an experiments of mass spectrometry on Raji lymphoma cells expressing endogenous Tcl1 protein, and we confirmed the presence of Hsp70 as putative interactor of Tcl1 (data not shown). We identified a list of putative Tcl1 interactors, published by our group,9 and among the candidates, we focused on Hsp70 because Hsp70 is also overexpressed in many cancers and is involved in the folding of oncoproteins critical for cancer progression.
We investigated and validated this interaction between Tcl1 and Hsp70 using coimmunoprecipitation experiments. Based on these novel data, we propose a mechanism whereby direct interaction with Hsp70 is required for stability of Tcl1. Hsp70 regulates the stability and function of multiple oncogenic client proteins. Indeed, our analysis confirmed that the chaperone activity of Hsp70 also was essential for Tcl1 protein stability. Data from the present study demonstrated that Tcl1 was degraded by ubiquitination during treatment with Myr, a potent and specific inhibitor of Hsp70 ATPase activity. In support of this, treatment of Daudi lymphoma cells with Myr or siRNA for Hsp70 led to a dramatic reduction of both endogenous Tcl1 and Hsp70 proteins.
Hsp7043 and Tcl1, coactivator of Akt,41 have a common role in the up-regulation of PI3K/AKT pathway. The reduction of Tcl1 protein levels observed suggested that the molecular-chaperoning activity of Hsp70 plays a crucial role in Tcl1 stability.
As demonstrated previously, Tcl1 expression directly affects the transactivating function of NF-κB. Here, we demonstrated that Myr affects Hsp70-Tcl1 crosstalk and results in NF-κB inactivation. Prior published studies have described an interaction between TP53 and Hsp70 and the inhibition of TP53 transcriptional activity in the presence of Hsp70.45,46 In the present report, we showed that TP53 transcriptional activation can occur in the presence of Myr, supporting its tumor suppressor activity. Finally, we show that inhibition of Hsp70 reduces the expression of Tcl1 in lymphoma and CLL patient samples. Our results suggest that the down-regulation of Tcl1 protein was a direct effect of Hsp70 inhibition and that Hsp70 is crucial for the stability of Tcl1. Our results provide additional evidence of Tcl1 critical role in lymphomas and aggressive CLL. Myr induces apoptosis and reduces proliferation of lymphoma cells in vitro and in xenograft experiments by targeting Hsp70 and its oncogenic partner Tcl1. Because of its functional role in acute leukemias, Hsp70 represents an important target for antitumor therapy.48 Hsp70 family proteins work with Hsp90 to maintain the stability of their client proteins.49 In turn, the inhibition of Hsp70 by small molecules40 or chemical inhibitors (Myr,43 ; MAL3-101,50 ; 2-phenylthynesulfonamide,23 ; Pifithrin-μ,51 ) was shown to potentiate the apoptotic effects of 17AAG.
MAL3-10150 a recently developed non–ATP-site inhibitor of Hsp70 affected the tumor growth of multiple myeloma cells.51 2-Phenylthynesulfonamide causes disruption of the Hsp70/Hsp90 chaperon system and enhances survival in Eμ-Myc mouse model of lymphomagenesis.50 In addition, Pifithrin-μ was identified as a specific inhibitor of inducible Hsp70 in acute leukemias.48 These Hsp70 inhibitors tested in basic research are candidates for translational trial experimentations as single drugs or in combination with other anticancer drugs. Because Hsp70 inhibitors can kill malignant cells by inhibiting Tcl1 expression and the AKT and NF-κB pathways, our data suggest that the inhibition of Hsp70 and Tcl1 could be further evaluated as a potential new approach for cancer therapy.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kari Green-Church for the mass spectrometry analysis.
The research was supported by National Institutes of health grant P01-CA81534 of the CLL Research Consortium (L.R., T.J.K., C.M.C.).
This work is dedicated to the memory of Pietro Gaudio, Geometra, who died on March 11, 1996.
National Institutes of Health
Authorship
Contribution: E.G., F.P., F.T., and C.M.C. designed the experiments; E.G., F.P., A.N., F.L., M.F., H.-L.S., Y.P., N.Z., M.A.S., and C.L. performed research experiments; A.E., P.G., L.Z.R., and T.J.K. contributed new reagents; E.G., F.P., R.I.A., G.B.L., F.T., and C.M.C. wrote the paper; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo M. Croce, Department of Molecular Virology, Immunology, and Medical Genetics and Comprehensive Cancer Center, The Ohio State University, 1082 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210; e-mail: carlo.croce@osumc.edu.
References
Author notes
E.G. and F.P. contributed equally to this work.