Abstract
Increased microvessel density contributes to abnormal BM and spleen microenvironment in myelofibrosis (MF). Taking advantage of the JAK2V617F mutation as a marker of malignancy, in the present study, we investigated whether splenic endothelial cells (ECs) obtained from capillaries by laser microdissection or from fresh spleen tissue by cell culture or cell sorting harbored such mutation in patients bearing the mutation in their granulocytes and undergoing splenectomy for therapeutical reasons. To extend the analysis to the ECs of large vessels, endothelial tissue from the splenic vein was also studied. We found JAK2V617F+ ECs in 12 of 18 patients also bearing the mutation in their granulocytes. In 3 patients, the mutation was found in at least 2 different EC samples obtained by laser microdissection, cell culture, or cell sorting. The mutation was detected in the splenic vein ECs of 1 of 6 patients investigated. In conclusion, we provide evidence that some ECs from the spleen and splenic veins of patients with MF bear the JAK2V617F mutation. We suggest that splenic ECs are involved in the process of malignant transformation in MF.
Key Points
Endothelial cells carrying the JAK2V617F mutation can be detected both in the splenic capillaries and in the splenic vein of patients with myelofibrosis.
The mutated endothelium may be involved in the malignant transformation of myelofibrosis, opening new perspectives in its pathogenesis and treatment.
Introduction
Myelofibrosis (MF) is a neoplasm characterized by hematopoietic stem cell–derived clonal myeloproliferation with cytokine-mediated BM fibrosis and osteosclerosis.1 The clinical picture is dominated by anemia, splenomegaly, and evolution toward blast transformation. BM is characterized by global hyperplasia with clustering and dysplasia of megakaryocytes and by constitutive reticulin or collagen fibrosis.2 The molecular mechanisms responsible for the disease remain largely unknown. An involvement of JAK2 signaling pathway by the gain-of-function mutation V617F in exon 14 has been reported in approximately 60% of patients and additional abnormalities affecting other genes, including MPL, TET-2, ASXL1, and EZH2, have been reported to contribute to modifying the disease phenotype.3
Increased BM and spleen vascularity has been found to represent a hallmark of the biology of MF.4,5 This event has been documented to correlate with advanced-stage disease and poor outcome.6 MF shares the neoangiogenesis mechanism with solid tumors, in which the growth of malignant cells is strictly dependent on the development of an adequate blood supply through sprouting of new microvessels from preexisting capillaries.7
A classic concept in tumor angiogenesis is that tumor blood vessels contain genetically normal and stable endothelial cells (ECs), whereas tumors cells typically display genetic instability and may harbor several genomic abnormalities. Recent studies, however, have challenged this paradigm because evidence is accumulating that in some cancers, ECs are derived from the tumor itself and that the tumor-derived ECs share the same genetic abnormalities with the malignant cells from which they originate. Such evidence has been provided in hematologic neoplasms such as chronic myeloid leukemia,8,9 lymphoma,10 chronic lymphocytic leukemia,11 myelodysplastic syndromes,12 and multiple myeloma13,14 and in solid tumors such as neuroblastoma,15 glioblastoma,16-19 and melanoma.20 Recently, using laser capture microdissection followed by nested PCR or reverse-transcriptase PCR, Sozer et al showed that ECs lining the lumen of terminal hepatic venules from 3 patients with polycythemia vera and concurrent Budd-Chiari syndrome harbored the JAK2V617F mutation.21 These findings hinted at an identical mutation occurring in both hematopoietic cells and ECs and led to the hypothesis that a common progenitor of the 2 lineages, hemangioblasts,22 could represent the target of neoplastic transformation.
In the present study, we have investigated for the first time the JAK2V617F mutation in ECs derived from the spleen and the splenic vein in patients with MF. Genomic anomalies of ECs in patients with MF could be of biologic relevance given the role that ECs play in the stem cell niche in both the BM and the spleen. Moreover, given the high incidence of thrombosis in the splanchnic area in patients with MF, genetic abnormalities of ECs might influence the coagulation mechanism locally.
Methods
Patients
Spleen and peripheral blood samples were collected from 18 patients with MF following a protocol approved by the institutional review boards for human research of the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo Foundation (Pavia, Italy). At enrollment, all patients gave written informed consent to be part of the study in accordance with the Declaration of Helsinki. Eleven patients had primary MF and the remaining patients had secondary MF after either polycythemia vera or essential thrombocythemia. The patients were referred to the Center for the Study of Myelofibrosis of the IRCCS Policlinico San Matteo Foundation and received splenectomy from 2005-2011 either for transfusion-dependent anemia and/or to relieve symptoms of massive splenomegaly.
EC isolation by laser pressure catapulting microdissection from spleen capillaries
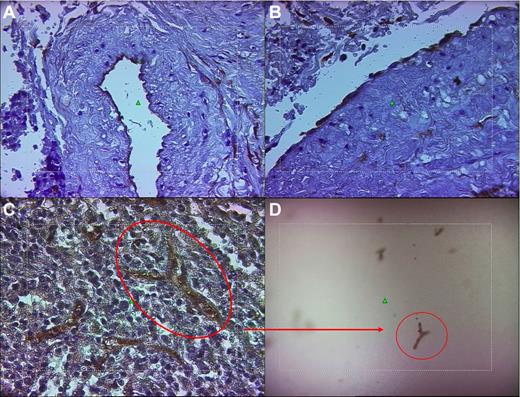
Laser pressure catapulting microdissection (for brevity, laser microdissection [LM]) was performed using a positioning and ablation with laser microbeams (PALM) robot microbeam system (Zeiss) on 4-μm-thick silane- or polyethylene naphtholate-membrane–coated histologic spleen sections. Slides were immunostained with either anti-CD34 (DBS) or anti-CD31 (Dako Cytomation) mAbs, revealed using the streptavidin-biotin-peroxidase–conjugated method (Dako), and finally counterstained with Harris hematoxylin without coverslip. Sections were examined under an inverted microscope (Axiovert 200×, Zeiss) at different magnifications (5×, 10×, 20×, and 40×) to identify CD34+ or CD31+ ECs derived from proliferating splenic capillaries and the splenic vein (Figure 1A-C and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These cells were selected with either the “free drawing” or “predefined geometric elements” options. To allow an accurate and clearcut definition of the targeted cells, laser energy was properly calibrated according to the type of preparation. LM was performed using the objective 40× LD-Arcoplan; both CD34+ and CD31+ microdissected cells were harvested using a catapulting method within the cap of the PCR tube. After a checkpoint function to find the actual recovery of cells within the cap (Figure 1D), cells were digested in appropriate lysis buffer (1 mM EDTA, pH 8.0, 20 mM Tris-HCl, pH 8.0, 0.5% Tween 20, and 400 μg/mL of proteinase K) and processed under the following conditions: 12 hours at 55°C, 10 minutes at 99°C, and 7 minutes at 72°C. DNA samples were then subjected to molecular analysis.
Representative example of the LM technique for the isolation of mature ECs from the spleen. (A-B) The photomicrographs showing the ECs of the luminal surface of the splenic vein of patients 6 and 9, respectively, after immunohistochemical staining with anti-CD34 mAb and counterstaining with Harris hematoxylin. (C-D) Capillary of the splenic pulp immunostained with anti-CD34 mAb (as in panels A and B) before (C) and after (D) being laser microdissected and catapulted into the cap of a PCR tube. Original magnification for panels A, B, and C was 40× and for panel D, 10× using LD-Arcoplan objectives. The microdissection laser system is equipped on an inverted microscope (Axiovert 200; Zeiss) linked to a Sony DXC-309F 3CCD color video camera.
Representative example of the LM technique for the isolation of mature ECs from the spleen. (A-B) The photomicrographs showing the ECs of the luminal surface of the splenic vein of patients 6 and 9, respectively, after immunohistochemical staining with anti-CD34 mAb and counterstaining with Harris hematoxylin. (C-D) Capillary of the splenic pulp immunostained with anti-CD34 mAb (as in panels A and B) before (C) and after (D) being laser microdissected and catapulted into the cap of a PCR tube. Original magnification for panels A, B, and C was 40× and for panel D, 10× using LD-Arcoplan objectives. The microdissection laser system is equipped on an inverted microscope (Axiovert 200; Zeiss) linked to a Sony DXC-309F 3CCD color video camera.
Spleen ECFC and EC cultures
Spleen tissue specimens of 2-3 cm were obtained immediately after surgery, washed extensively in PBS, further cut into smaller pieces, and reduced to a single-cell suspension using a gentle MACS dissociator (Miltenyi Biotec). Mononuclear cells (MNCs) were obtained by density gradient centrifugation. Endothelial colony-forming cells (ECFCs) were cultured according to Ingram et al23 by plating at least 3 × 107 MNCs onto collagen-coated 30-mm Petri dishes (BD Biosciences) in the presence of the EGM-2 MV medium (Lonza) at 37°C and 5% CO2. Nonadherent cells were discarded after 2 days and thereafter medium was changed every 2-3 days. ECFCs were observed after 12-15 days of culture and identified both on the basis of their typical morphology (fast growing colonies with a cobblestone appearance) and on the expression of a peculiar immunophenotype (CD105+CD144+CD146+CD31+VEGFR-2+CD45−CD14− cells), as described previously.23 For culture of mature ECs, a maximum of 1 × 106 MNCs were plated onto 30-mm Petri dishes with the same medium used for ECFCs. At the end of the culture period, the cells were recovered by trypsinization and used for the evaluation of JAK2V617F mutational status by PCR; for quantification of JAK2V617F, CD14, and CD45 expression by RT-PCR; for DNA content assessment; and for estimation of hematopoietic cell contamination by immunophenotype analysis. This was performed by cytospinning 3-5 × 103 cells onto glass slides and staining by indirect immunolabeling using secondary Abs conjugated with Alexa Fluor 488 (green) or Alexa Fluor 594 (red) fluorochromes (Molecular Probes). As primary Abs, anti-CD144 (Bender Med System), anti-CD31 and anti-VWF (both Dako), and anti-CD45 (BD Biosciences) were used. At least 350 consecutive cells were scored. Micrographs were captured at room temperature using an Olympus IX71 inverted microscope equipped with a CPlan F1 10×/0.30 objective, an Olympia Camedia C-3030 zoom with a 3× optical plus 2.5× digital zoom (Olympus), and Camedia Master Version 2.0 software (Olympus).
Sensitivity to erythropoietin
To determine whether JAK2V617F ECs have an enhanced sensitivity to erythropoietin, splenic ECs (from patient numbers 6, 13, and 17) were incubated with increasing concentrations of erythropoietin (0, 0.1, 1, and 10 IU/mL) for up to 96 hours and the total cell number was assessed after trypsinization. In addition, the effects of the same concentrations on endothelial nitric oxide synthase (eNOS) expression were assessed as described previously.24
Spleen EC purification by cell sorting
Isolation of spleen ECs was also determined by FACS. Splenic MNCs, obtained as described in “Spleen ECFC and EC cultures,” were first enriched by immunomagnetic cell selection using anti-CD34 microbeads (Miltenyi Biotec). CD34+-enriched cells were then stained with PE-conjugated anti-CD146, FITC-conjugated anti-CD31, and APC-Cy7–conjugated anti-CD45 mAbs and sorted on a FACSAria cell sorter (BD Biosciences). Cells belonging to the hematopoietic lineage were excluded according to CD45 surface marker expression. ECs were sorted according to double positivity for the CD146 and CD31 markers (Figure 2).
Sorting strategy for the isolation of CD34+CD146+CD31+CD45− cells from spleen-derived MNCs. Splenic MNCs were first enriched in CD34+ cells by immunomagnetic selection, followed by staining with anti-CD146, anti-CD31, and anti-CD45 mAbs, and then sorted according to CD34, CD31, and CD146 positive and CD45 negative expression. Contaminating platelets were excluded by size gating.
Sorting strategy for the isolation of CD34+CD146+CD31+CD45− cells from spleen-derived MNCs. Splenic MNCs were first enriched in CD34+ cells by immunomagnetic selection, followed by staining with anti-CD146, anti-CD31, and anti-CD45 mAbs, and then sorted according to CD34, CD31, and CD146 positive and CD45 negative expression. Contaminating platelets were excluded by size gating.
Splenic vein EC separation
Small fragments of splenic vein (0.5-1.5 cm) were obtained from 11 patients at the time of surgery. The vessel specimen was cut longitudinally and opened with the endothelial lumen facing up. The surface was gently washed twice with PBS without Ca and Mg and collagenase (1 mg/mL; Sigma-Aldrich) was carefully dispensed on the endothelial surface. The vein fragment was then incubated for 10-15 minutes at 37°C and 5% CO2, followed by thorough washing of the luminal surface with PBS and EGM-2 medium. Finally, the washing medium, consisting of PBS and EGM-2 medium and containing ECs lining the lumen of the vein, was recovered, pelleted by mild centrifugation, resuspended in EGM-2MV medium, and cultured onto collagen-coated Petri dishes, as described in “Spleen ECFC and EC cultures.”
Array-CGH
Molecular karyotyping of both granulocytes and ECs obtained in culture was performed through array-comparative genomic hybridization (array-CGH) using the Agilent 60K kit (Human Genome CGH Microarray; Agilent Technologies) according to the manufacturer's protocol. The platform used is made up of 63 000 probes distributed along the whole genome with an average spacing of 54.5 kb and a real average resolution of approximately 150 kb. Data analysis was performed using Agilent Genomic Workbench Standard Edition Version 6.5.0.58 software. Oligo positions were in reference to hg19. A female poll reference DNA (Promega) was used in all experiments.
JAK2V617F mutation detection by PCR and quantitative PCR
The mutational status for JAK2 of the spleen ECs obtained by LM, cell culture, or cell sorting was determined using the BsaXI digestion method as described previously25 (a detailed description is available in supplemental Methods). JAK2V617F allele burden in the DNA of granulocytes and in the RNA of ECs obtained in culture was measured by allele-specific quantitative PCR, as described previously.26 The JAK2V617F allele burden was calculated by comparison with a standard curve obtained by mixing DNA from a patient with 100% allele burden and that from a healthy subject in different proportions (2%, 5%, 12.5%, 25%, 50%, 75%, 95%, and 100%). To quantify the number of mutated transcripts, a standard curve was obtained by mixing cDNA from a patient 100% mutated with that from a healthy subject with the same JAK2 expression levels.
RNA isolation and CD45/CD14 detection by RT-PCR
Total RNA was extracted from cultured ECs with the miRNeasy Mini Kit (QIAGEN), purified from contaminating DNA by on-column digestion with the RNase-free DNase Set (QIAGEN), according to the manufacturer's instructions, and quantified with a Nanodrop 1000 spectrophotometer (Thermo Scientific). cDNA synthesis was carried out using the iScript Kit (Bio-Rad). In brief, 150 ng of each total RNA sample was reverse transcribed using a blend of oligo-dT and random primers, diluted with nuclease-free water to 3.75 ng/μL (total RNA equivalent), and stored at −80°C. Primers for CD45 and CD14 for EvaGreen assays were designed using the Beacon Designer Version 7.9 software (Premier Biosoft International; supplemental Table 1). Quantification of transcripts was carried out in a 15-μL reaction mixture containing 1× SsoFast EvaGreen Supermix (Bio-Rad) and 400nM concentrations of each primer. The PCR conditions were 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 5 seconds. Melting curves were generated after amplification in the range of 65-95°C with increments of 0.5°C every 5 seconds. For each experiment, 11.3 ng of cDNA was used and each sample was tested in triplicate. The PCR data were collected using the CFX96 Real-Time System (Bio-Rad) and data were analyzed using qbasePLUS Version 2.11 software.
Analysis of DNA content
Cultured ECs were collected and, after the addition of a commercial solution containing propidium iodide and RNAse (DNA QC Particles; BD Biosciences), incubated for 30 minutes in the dark. At least 1.5 × 104 cells were acquired on a FACSCalibur flow cytometer (BD Biosciences) and the cells' DNA content was analyzed using the ModFit LT Version 3.0.3 software program (Verity Software House).
FISH analysis of ECs in spleen sections
FISH analysis was performed on deparaffinized spleen sections using an anti-JAK2 probe (9p24; Kreatech Diagnostic) in a dual-color (red and green) split assay on metaphase interphase spreads using a procedure described previously.27
Results
Patient population
Fifteen patients were male and 3 were female with a median age of 62 years (range, 51-70), a median disease duration of 65 months (range, 9-210), and a median disease severity score28 of 4 (range, 3-5). Median spleen weight was 3.6 kg (range, 2.2-4.6). Of 18 JAK2V617F-mutated patients, 11 had an allelic burden compatible with a homozygous status in their granulocytes and 7 were heterozygous. The assessment of the mutational status of hematopoiesis was performed at diagnosis and confirmed just before splenectomy (Table 1). At the time of surgery, 5 patients had cytogenetic analysis performed and all displayed a normal karyotype. No patients experienced splanchnic vein thrombosis before splenectomy.
JAK2V617F mutation in ECs from spleen vessels
| Patient no. . | JAK2V617F allele burden of PMNs . | JAK2V617F spleen . | JAK2V617F splenic vein . | ||||
|---|---|---|---|---|---|---|---|
| LM . | ECs . | ECFCs . | Sorting . | ||||
| CD34+ ECs . | CD31+ ECs . | ||||||
| 1 | 48% | 1Het/2WT/4NA | 2Het/1WT/ | ND | ND | ND | ND |
| 2 | 39% | 3Het/1WT | 3 Het | ND | ND | ND | ND |
| 3 | 49% | 1Het/1NA | 1 Het/2WT | NG | WT | ND | ND |
| 4 | 52% | 2Het | 2 Het/1NA | ND | ND | ND | ND |
| 5 | 69% | 1Hom/1Het2/WT | 3 Het | ND | ND | ND | ND |
| 6 | 81% | 2Hom/3Het | 1Hom/2Het | Hom | WT | Hom | Hom* |
| 7 | 100% | ND | ND | NG | WT | ND | ND |
| 8 | 34% | 1Het/1WT | 1Het/2WT | Het | NG | ND | ND |
| 9 | 49% | ND | ND | WT | WT | ND | WT |
| 10 | 60% | ND | ND | NG | WT | ND | WT |
| 11 | 96% | ND | ND | Het† | NG | ND | WT |
| 12 | 93% | ND | ND | WT | NG | ND | WT |
| 13 | 78% | ND | ND | WT | NG | ND | NG |
| 14 | 15% | ND | ND | Het | WT | ND | NG |
| 15 | 65% | ND | ND | Het | WT | ND | NG |
| 16 | 59% | 2Hom/1Het/2WT | 1Hom/1Het/1WT | WT | WT | Het | WT |
| 17 | 65% | ND | ND | Hom | NG | ND | NG |
| 18 | 45% | ND | ND | NG | NG | ND | NG |
| Patient no. . | JAK2V617F allele burden of PMNs . | JAK2V617F spleen . | JAK2V617F splenic vein . | ||||
|---|---|---|---|---|---|---|---|
| LM . | ECs . | ECFCs . | Sorting . | ||||
| CD34+ ECs . | CD31+ ECs . | ||||||
| 1 | 48% | 1Het/2WT/4NA | 2Het/1WT/ | ND | ND | ND | ND |
| 2 | 39% | 3Het/1WT | 3 Het | ND | ND | ND | ND |
| 3 | 49% | 1Het/1NA | 1 Het/2WT | NG | WT | ND | ND |
| 4 | 52% | 2Het | 2 Het/1NA | ND | ND | ND | ND |
| 5 | 69% | 1Hom/1Het2/WT | 3 Het | ND | ND | ND | ND |
| 6 | 81% | 2Hom/3Het | 1Hom/2Het | Hom | WT | Hom | Hom* |
| 7 | 100% | ND | ND | NG | WT | ND | ND |
| 8 | 34% | 1Het/1WT | 1Het/2WT | Het | NG | ND | ND |
| 9 | 49% | ND | ND | WT | WT | ND | WT |
| 10 | 60% | ND | ND | NG | WT | ND | WT |
| 11 | 96% | ND | ND | Het† | NG | ND | WT |
| 12 | 93% | ND | ND | WT | NG | ND | WT |
| 13 | 78% | ND | ND | WT | NG | ND | NG |
| 14 | 15% | ND | ND | Het | WT | ND | NG |
| 15 | 65% | ND | ND | Het | WT | ND | NG |
| 16 | 59% | 2Hom/1Het/2WT | 1Hom/1Het/1WT | WT | WT | Het | WT |
| 17 | 65% | ND | ND | Hom | NG | ND | NG |
| 18 | 45% | ND | ND | NG | NG | ND | NG |
PMNs indicates polymorphonuclear cells; Het, heterozygous; Hom, homozygous; NA, not amplified; WT, wild-type; ND, not done; and NG, no growth. Numbers in the LM column refer to the number of samples (approximately 100 ECs each) that displayed the reported genotype.
Both ECs from LM (CD34+ and CD31+) and from in vitro culture displayed a homozygous genotype for JAK2V617F.
This EC sample was contaminated by 30% CD45+ cells.
LM of spleen capillaries
Spleen ECs from 8 patients were obtained by LM. To minimize the risk of contamination with hematopoietic cells, 2 set of spleen sections were used for each patient; one was stained with an anti-CD34 mAb (Figure 1) and the other was stained with an anti-CD31 mAb (supplemental Figure 1). In either case, at least 50 capillaries per patient were microdissected by UV laser and catapulted within the cap of a PCR tube (Figure 1), digested, processed, and subjected to molecular analysis. The results of JAK2 mutational analysis are reported in Table 1 and a representative example of the PCR results is shown in Figure 3. For staining with anti-CD34, of a total of 32 samples analyzed (with a median content of 100 ECs per sample), 18 showed the presence of the JAK2V617F mutation, whereas in 8 samples, only wild-type alleles were detected. In 6 samples, the PCR reaction failed. For CD31, of 24 samples analyzed, 17 samples contained the mutation, 6 did not, and 1 sample was not amplified in the PCR reaction. All patients had at least 1 sample of ECs positive for the JAK2 mutation, and 6 of 8 patients had at least 1 wild-type sample, suggesting a different contribution of the mutated cells to the vessel wall. A homozygous mutation was found in 7 samples of ECs in 3 of 4 patients with a homozygous mutation in their granulocytes.
JAK2V617F status of LM captured or cultured ECs from spleen or splenic veins. Shown are patient number 5 (lanes 3-6), patient number 6 (lanes 7-11 and 13), and patient number 9 (lane 12). Lane 1, JAK2V617F+ control; lane 2, JAK2 wild-type positive control; and lane 14, negative control. MW indicates molecular weight. Expected sizes for amplified fragments after digestion with BsaIX are 141, 30, and 12 bp for the wild-type allele, whereas the V617F allele remains undigested (183 bp). Both the 30- and 12-bp fragments derived from digestion of the wild-type alleles ran outside the gel. Heterozygous samples show both 183-bp (mutated) and 141-bp (wild-type) fragments.
JAK2V617F status of LM captured or cultured ECs from spleen or splenic veins. Shown are patient number 5 (lanes 3-6), patient number 6 (lanes 7-11 and 13), and patient number 9 (lane 12). Lane 1, JAK2V617F+ control; lane 2, JAK2 wild-type positive control; and lane 14, negative control. MW indicates molecular weight. Expected sizes for amplified fragments after digestion with BsaIX are 141, 30, and 12 bp for the wild-type allele, whereas the V617F allele remains undigested (183 bp). Both the 30- and 12-bp fragments derived from digestion of the wild-type alleles ran outside the gel. Heterozygous samples show both 183-bp (mutated) and 141-bp (wild-type) fragments.
Cultured ECs from the spleen
In 14 patients, we investigated freshly isolated cultured ECs obtained from the spleen. Single-cell suspensions from spleen fragments were plated onto collagen-coated Petri dishes in the presence of EGM-2 medium. Mature cells with the morphology of ECs (ie, flat, adherent, spindle-shaped cells) were observed starting from 3-4 days of culture. These cells had a finite proliferative capacity and stopped their growth within 10-14 days, at which time they were trypsinized and divided in 3 aliquots. One aliquot underwent PCR analysis for the detection of JAK2V617F mutation, another was cytospun onto glass slides for EC purity evaluation by immunocytochemistry, and the third aliquot was used for the assessment of CD45 and CD14 RNA by RT-PCR. Mature ECs were obtained from 10 of 14 patients in whom cultures were performed. The V617F mutation was found in the samples of 6 different patients, whereas in 4 patients, only wild-type alleles were detectable. In 4 patients, no EC growth was observed (Table 1). Assessment of CD45+ cell contamination by immunocytochemistry showed the presence of 99.3% ± 0.09% CD144+, 98.8% ± 0.88% CD31+, 97.8% ± 1.01% VWF+, and 0.32% ± 0.07% CD45+ cells in 5 samples (Figure 4 and Table 2), whereas in 1 sample (patient number 11), contamination by 30% of CD45+ hematopoietic cell contamination was demonstrated (Table 2), indicating that the detection of the JAK2V617F mutation could be because of contaminating hematopoietic cells. Concordant results were obtained by RT-PCR, with CD45 and CD14 RNAs detectable at a significant levels only in the sample that was contaminated by CD45+ cells by immunocytochemistry (not shown). In 3 of 5 patients in whom cultured ECs harbored the JAK2V617F mutation (patient numbers 6, 8, and 15), JAK2V617F mRNA levels were quantified by RT-PCR. In all 3 patients, the percentage of mutated transcripts was equal to or greater (patient 6, 80%; patient 8, 83%; and patient 15, 78%) than the percentage of mutated alleles in genomic DNA (patient 6, 81%; patient 8, 34%; and patient 15, 65%). In these same patients, DNA content analysis showed that cells were 100% diploid (Figure 5A). In the other 2 patients (patients 14 and 17), the limited number of cells recovered from the culture precluded analysis of ploidy.
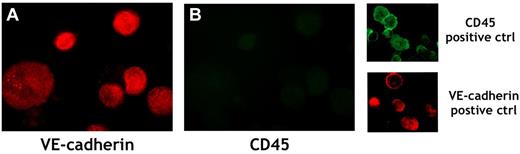
Representative example of immunofluorescent analysis of cultured ECs from splenic MNCs of patient number 6. ECs were detached from the culture dish, cytospun onto a glass slide, and stained with PE-conjugated anti–VE-cadherin mAb (red; A) and with FITC-conjugated anti-CD45 mAb (green; B). MNCs from the peripheral blood of a healthy subject were used as a positive control for CD45 detection, and ECs derived from ECFCs obtained from a healthy subject were used as a positive control for VE-cadherin detection. Original magnification was 250×.
Representative example of immunofluorescent analysis of cultured ECs from splenic MNCs of patient number 6. ECs were detached from the culture dish, cytospun onto a glass slide, and stained with PE-conjugated anti–VE-cadherin mAb (red; A) and with FITC-conjugated anti-CD45 mAb (green; B). MNCs from the peripheral blood of a healthy subject were used as a positive control for CD45 detection, and ECs derived from ECFCs obtained from a healthy subject were used as a positive control for VE-cadherin detection. Original magnification was 250×.
Expression of endothelial (CD144, CD31, and VWF) and hematopoietic (CD45) proteins by ECs isolated in culture from splenic MNCs
| Patient no. . | CD144 . | CD31 . | VWF . | CD45 . |
|---|---|---|---|---|
| 6 | 99.27 | 98.81 | 96.91 | 0.23 |
| 8 | 99.37 | 99.45 | 97.54 | 0.34 |
| 11 | 71.14 | 91.22 | 65.12 | 30.23 |
| 14 | 99.28 | 97.37 | 96.84 | 0.37 |
| 15 | 99.35 | 98.75 | 98.81 | 0.41 |
| 17 | 99.21 | 99.63 | 99.11 | 0.26 |
| ECFCs | 99.63 | 99.08 | 98.96 | 0.12 |
| PB-MNCs | 2.1 | 44.6 | 6.5 | 99.14 |
| Patient no. . | CD144 . | CD31 . | VWF . | CD45 . |
|---|---|---|---|---|
| 6 | 99.27 | 98.81 | 96.91 | 0.23 |
| 8 | 99.37 | 99.45 | 97.54 | 0.34 |
| 11 | 71.14 | 91.22 | 65.12 | 30.23 |
| 14 | 99.28 | 97.37 | 96.84 | 0.37 |
| 15 | 99.35 | 98.75 | 98.81 | 0.41 |
| 17 | 99.21 | 99.63 | 99.11 | 0.26 |
| ECFCs | 99.63 | 99.08 | 98.96 | 0.12 |
| PB-MNCs | 2.1 | 44.6 | 6.5 | 99.14 |
The percentage of positive cells is reported. At least 350 cells were scored per Ab. ECFC-derived cells were used as a positive control for assessment of the expression of EC-specific antigen and peripheral blood–derived MNCs from a healthy donor were used as a positive control for CD45 expression.
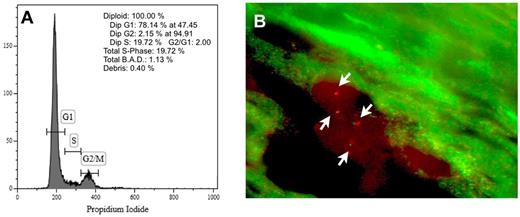
DNA content of cultured splenic ECs and FISH analysis of ECs in spleen sections. (A) Representative example of DNA content analysis of JAK2V617F+ ECs obtained from in vitro culture of splenic mononuclear cells of patient number 6. Cells are 100% diploid. (B) Representative microphotograph of FISH analysis of ECs in a spleen section with a dual-color anti-JAK2 probe. Two cells, each with 2 green and 2 red signals (arrows), are shown.
DNA content of cultured splenic ECs and FISH analysis of ECs in spleen sections. (A) Representative example of DNA content analysis of JAK2V617F+ ECs obtained from in vitro culture of splenic mononuclear cells of patient number 6. Cells are 100% diploid. (B) Representative microphotograph of FISH analysis of ECs in a spleen section with a dual-color anti-JAK2 probe. Two cells, each with 2 green and 2 red signals (arrows), are shown.
ECFCs were obtained from the splenic MNCs of 8 patients, whereas in 6 patients, no ECFC growth was observed. The JAK2V617F mutation was never detected in a total of 23 colonies investigated. In 3 of 5 patients in whom both ECFCs and mature ECs were obtained from the same patients, the latter harbored the mutation whereas the former did not (Table 1).
Sensitivity to erythropoietin
As shown in Table 3, no apparent differences in total cell number was found between cultures without erythropoietin and cultures with low concentrations (0.1 and 1 IU/mL) of erythropoietin. A slight increase was observed when cells were incubated with 10 IU/mL of erythropoietin at later time points (72 and 96 hours of incubation). These results were similar to those obtained when nonmutated ECs were exposed to the same erythropoietin concentrations (not shown). With regard to eNOS expression, whereas a 3-fold increase was observed in the presence of 10 IU of erythropoietin after 12 hours of incubation, no differences with respect to control (no erythropoietin added) were observed at lower concentrations (0.1 and 1 IU) at any time point tested (1, 4, and 12 hours).
Effects of different concentrations of erythropoietin on the in vitro growth of JAK2V617F+ ECs
| Incubation time, h . | Erythropoietin concentration, IU/mL . | |||
|---|---|---|---|---|
| 0 . | 0.1 . | 1 . | 10 . | |
| 0 | 1 × 103 | 1 × 103 | 1 × 103 | 1 × 103 |
| 12 | 0.98 × 103 | 0.99 × 103 | 1.01 × 103 | 0.99 × 103 |
| 24 | 1.04 × 103 | 1.02 × 103 | 1.1 × 103 | 1.06 × 103 |
| 48 | 1.34 × 103 | 1.28 × 103 | 1.31 × 103 | 1.53 × 103 |
| 72 | 1.57 × 103 | 1.62 × 103 | 1.88 × 103 | 2.01 × 103 |
| 96 | 1.91 × 103 | 1.87 × 103 | 2.06 × 103 | 2.49 × 103 |
| Incubation time, h . | Erythropoietin concentration, IU/mL . | |||
|---|---|---|---|---|
| 0 . | 0.1 . | 1 . | 10 . | |
| 0 | 1 × 103 | 1 × 103 | 1 × 103 | 1 × 103 |
| 12 | 0.98 × 103 | 0.99 × 103 | 1.01 × 103 | 0.99 × 103 |
| 24 | 1.04 × 103 | 1.02 × 103 | 1.1 × 103 | 1.06 × 103 |
| 48 | 1.34 × 103 | 1.28 × 103 | 1.31 × 103 | 1.53 × 103 |
| 72 | 1.57 × 103 | 1.62 × 103 | 1.88 × 103 | 2.01 × 103 |
| 96 | 1.91 × 103 | 1.87 × 103 | 2.06 × 103 | 2.49 × 103 |
Results are the mean of the total cell number recovered from the cultures of the mutated ECs of the 2 patients tested (patients 6 and 17) with increasing doses of erythropoietin at different time points (see “Methods” for details).
Array-CGH
In 7 patients (patients 6, 8, 14, 15, 16, and 17), array-CGH was performed to investigate the presence of cytogenetic alterations both in granulocytes and in cultured ECs. Molecular karyotyping showed the absence of chromosomal abnormalities in both types of cells in all patients tested.
FACS-sorted ECs from the spleen
In 2 patients, FACS sorting was performed and CD146+ CD31+CD45− cells were obtained from immunomagnetically selected splenic CD34+ cells (Figure 2). In 2 of 2 samples (obtained from patients homozygous for the mutation in their granulocytes), the mutation was detected by PCR followed by digestion with Bsa XI (Table 1). In one patient, only mutated alleles were detected in the sorted ECs (Figure 6), whereas in the other, both mutated and wild-type alleles were detected.
JAK2V617 mutation in FACS-sorted splenic ECs from patient number 6. Splenic ECs show only mutated alleles, confirming the homozygosity found in the circulating polymorphonuclear cells of this patient. The HEL cell line was used as a positive control for the JAK2V617F mutation; the K562 cell line was used as a positive control for the wild-type JAK2 gene. Amplified fragments, obtained as described in “Methods” and in Baxter et al25 are shown before (−) and after (+) digestion with BsaXI. The expected sizes for amplified fragments after digestion with BsaIX are 241, 189, and 30 bp for the wild-type allele; the V617F allele remained undigested (460 bp). The 30-bp fragment derived from the digestion of the wild-type alleles ran outside the gel.
JAK2V617 mutation in FACS-sorted splenic ECs from patient number 6. Splenic ECs show only mutated alleles, confirming the homozygosity found in the circulating polymorphonuclear cells of this patient. The HEL cell line was used as a positive control for the JAK2V617F mutation; the K562 cell line was used as a positive control for the wild-type JAK2 gene. Amplified fragments, obtained as described in “Methods” and in Baxter et al25 are shown before (−) and after (+) digestion with BsaXI. The expected sizes for amplified fragments after digestion with BsaIX are 241, 189, and 30 bp for the wild-type allele; the V617F allele remained undigested (460 bp). The 30-bp fragment derived from the digestion of the wild-type alleles ran outside the gel.
ECs from the splenic vein
In 11 patients, samples of the splenic vein were also obtained after surgery and divided into 2 fragments; one was used immediately for cell culture, as described in “Splenic vein EC separation”; and the other was embedded in paraffin for LM experiments, as described in “EC isolation and laser pressure catapulting microdissection from spleen capillaries.” ECs lining the inner vessel wall were isolated both from the fresh fragment by digestion with collagenase followed by in vitro culture, as described above, and from the paraffin-embedded fragment by LM. Mature ECs were obtained in culture in 6 patients and by LM in 2 other patients. JAK2V617F+ cells were detected in 1 patient both in the culture-derived and in the LM-derived samples (both after staining with anti-CD34 and with anti–CD31-Abs), whereas in the cultures of the remaining 5 patients, only the JAK2 gene in the wild-type status was detected (Table 1). No evidence of CD45+ cells was found in either the cytospins or the RNA of ECs cultured from the JAK2V617F+ sample (not shown).
FISH analysis of ECs in spleen sections
In 4 of 8 patients, FISH analysis with a probe specific for the JAK2 gene was performed (Figure 5). One hundred nuclei were counted per section; 390 nuclei displayed a double signal (indicating 2 copies of the JAK2 gene), 4 nuclei displayed 3 signals, and 3 nuclei displayed 1 signal.
Discussion
In the present study, we provide evidence that ECs from either the spleen or the splenic vein of patients with MF frequently share the same genetic abnormality with the hematopoietic malignant cells: the JAK2V617F mutation. The spleen is a critical organ in the biology of MF featuring neoangiogenetic process that are strictly related to the hematopoietic metaplastic tissue. The vasculature of the spleen is complex, both in term of vessels and ECs. In fact, the arterioles divide into capillaries and sinusoids with different functions, and the EC-leaning capillaries and sinusoids are divided into 2 main pools, either CD34+ and CD8− or CD8+ and CD34−, respectively. We approached the study of spleen ECs using different methods for EC isolation. To avoid contamination of the endothelial splenic samples by hematopoietic cells, we also used all the operationally feasible controls. In particular, we used LM to obtain both CD34+ and CD31+ EC-leaning capillaries and the splenic vein. We also performed cultures of ECs from fragments of fresh tissue to obtain all cells that had the properties necessary to grow in vitro in endothelial medium and showing the morphology and the immunophenotype of both mature endothelium and endothelial progenitor cells. Finally, we also sorted cells with the immunophenotype of ECs from the same splenic fresh tissue. Altogether, 12 of 18 patients investigated for the mutation had documentation of JAK2V617F-mutated ECs in splenic vessels, and 6 of 10 patients for whom ECs were cultured in vitro had documentation of mutated ECs. In addition, in the 12 patients in whom the mutation was found, the mutated ECs were found in a great proportion of vessels. Finally, in contrast to a previous study in which malignant endothelium was obtained in cells derived only from microvessels,21 in the present study, we also found the JAK2 mutation in ECs derived from a large vessel such as the splenic vein. It will be of great interest in future studies to investigate whether mutated ECs could also be isolated from tissues other than the spleen. However, ethical reasons prevent accessibility to extra-hematopoietic and extra-splenic tissues, thus limiting the possibility of searching for JAK2V617F+ ECs in organs not involved in active neoangiogenesis (eg, the spleen of PMF patients) and in large vessels other than the splenic vein. Moreover, the absence of concomitant cytogenetic abnormalities in the hematopoietic cells of patients at the time of splenectomy precluded the possibility of also detecting them in splenic ECs.
An important finding of the present study is the mechanism by which spleen ECs acquire the JAK2V617F mutation. Different hypotheses may be raised. In animal models, the so-called angiogenic monocytes contribute to neovessel formation, acquiring the phenotype of mature ECs at sites of active neoangiogenesis.29 It is possible that monocytes could play a similar role in MF and could therefore be responsible for the detection of the JAK-2 mutation in the splenic endothelium of our samples. However, in humans, it is currently thought that angiogenic monocytes (or CFU-Hill) can favor angiogenesis by secreting proangiogenic factors rather than directly participating in neovessel formation or endothelium turnover.30 An alternative hypothesis is that ECFCs, which circulate at high frequency in the peripheral blood of patients with MF,31 could operate as progenitor cells in the mature endothelium. The existence of a common progenitor to endothelial and hematopoietic cells in which the V617F mutation occurs could explain the involvement of V617F+ ECs in the process of endotheliopoiesis and endothelium renewal. However, we have repeatedly documented that circulating ECFCs lack the JAK2V617F mutation,32 and we further demonstrate herein its absence in the ECFCs resident in the spleen. At variance with our observations, Teofili et al have shown that ECFCs from patients with myeloproliferative disorders can carry the JAK2V617F mutation, rendering it possible that a mutated endothelium can derive from a mutated progenitor cell.33 However, mutated ECFCs were detected only in patients suffering from thrombotic events.33 The patients enrolled in our study never experienced thrombosis and never showed mutated ECFCs. Therefore, it seems unlikely that the mutated ECs that we detected in the spleen of PMF patients could have derived from mutated endothelial progenitor cells.
Two other mechanisms may potentially lead to the acquisition of external DNA material into ECs of the spleen in patients with MF. Cell fusion could account for JAK2V617F positivity of ECs if a mutated hematopoietic cell has fused with an EC. In addition, phagocytosis of cell-free JAK2V617F+ DNA or of JAK2V617F+ apoptotic bodies by ECs could also account for the detection of the mutation in splenic ECs, because it has been shown previously that cell-free DNA circulates in the peripheral blood of cancer patients.34 Although we cannot rule out these events in a categorical way, our findings of high levels of expression of JAK2V617F in cultured ECs, the absence of aneuploidy and/or tetraploidy in the same cells, and FISH analysis of ECs in spleen sections indicated that no extra DNA was present in the samples that were found to harbor the JAK2V617F mutation. Consistent with this assumption are: (1) the high frequency of mutated ECs detected in our samples, which makes the occurrence of cell fusion unlikely (in fact, we never observed the presence of binucleated cells in ECs cultured in vitro), and (2) the finding of ECs with the JAK2V617F mutation in homozygosity, which makes the occurrence of cell-free DNA phagocytosis very unlikely.
An intriguing hypothesis for explaining the presence of the mutation in splenic ECs and splenic vein is that the MF stem cell (which is a hematopoietic stem cell) actually represents the cell giving rise to mutated endothelia. This event, which has been described in glioblastoma16-19 and in neuroblastoma,15 would assimilate the spleen of patient with MF to a solid tumor and would justify the extension of the phenomenon to the microvessels and to the large vessels of the spleen. It is currently a matter of debate whether differentiation of tumor stem cells into ECs is orchestrated by microenvironmental factors, by specific DNA reprogramming of the neoplastic stem cells, or both.35 The existence of JAK2V617F ECs could have important consequences in the physiopathology of the disease. For example, considering the role that ECs play in the organization and function of the stem cell niche, an altered vascular niche in the spleen could be involved in determining both the increased migratory behavior and the enhanced differentiative predisposition that have been documented recently in CD34+ hematopoietic progenitor cells derived from the spleen of PMF patients compared with their peripheral blood–derived counterparts.36,37
Unexpectedly, no enhanced sensitivity to erythropoietin of JAK2-mutated ECs was observed when proliferation and eNOS synthase expression were assessed after exposure to low concentrations of erythropoietin. However, the effects of erythropoietin on ECs have been reported previously to be subtle and are controversial.38 Therefore, it is possible that a deeper and more extensive investigation (which was outside the scope of this work) will show that the mutation really confers to mutated ECs an increased sensitivity to erythropoietin.
Our work has several potential biases. First, the results obtained by the LM technique suffer from the potential contamination of the individually picked ECs by surrounding nonendothelial (hematopoietic) cells. However, although we cannot formally rule it out, contamination of CD34+ or CD31+ laser-microdissected ECs is unlikely because LM allows accurate single-cell identification and capture. Moreover, should contamination by surrounding cells take place, it would only contaminate the pooled ECs with a minimal number of non-ECs. This would result in negligible quantity of DNA compared with that derived from true ECs. This consideration makes even more unlikely that it was contamination in the EC samples that caused them to be homozygous for the JAK2V617F mutation. The issue of contamination by hematopoietic cells can also be raised for the ECs derived from the in vitro culture. In these experiments, however, we were able to reliably rule out a possible contribution of contaminating hematopoietic cells either by quantitative PCR for the CD45 and CD14 transcripts or by counting CD45+ cells by immunophenotype. In fact, neither CD45/CD14 RNA nor CD45+ cells were observed in ECs cultured from the spleen in 5 of 6 samples investigated. Further evidence that contamination may not account for our results is the patient with JAK2-mutated ECs both in the LM samples and in the in vitro culture at the homozygous state, who had FACS-sorted CD34+CD146+CD31+CD45− ECs without evidence of wild-type alleles in the PCR product.
The results of the present study strongly support the concept that mature ECs carrying the JAK2V617F mutation can be detected both in the splenic capillaries and in a large vessel of the splanchnic area of patients with MF, providing new perspectives in our understanding of the phenotype, evolution, and therapy of this disorder that warrant careful investigations in future studies. Our results also generate several unanswered questions, both at the physiologic and clinical levels. For example, considering the high frequency of splanchnic thrombotic events in MF patients,39 it would be relevant to understand whether the malignant ECs are thrombogenic.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded in part by grants from the Associazione Italiana per la Ricerca sul Cancro (Milan, Italy) Special Program Molecular Clinical Oncology 5 × 1000 to the AGIMM (project number 1005; a detailed description of the project is available at http://www.progettoagimm.it), the Ricerca Corrente IRCCS Policlinico San Matteo Foundation, Pavia, Italy (to G.B.), and the Myeloproliferative Disorder Research Consortium (to G.B.). E.D. is a recipient of a fellowship from the Anatomic Pathology Unit, IRCCS Policlinico San Matteo Foundation (Pavia, Italy).
Authorship
Contribution: V.R. cultured the cells, collected, analyzed, and interpreted the data, and wrote the manuscript; L.V., V.P., G.B., P.C., and A.P. performed the PCR experiments; R.R. and E.D. performed the LM experiments; E.B. and G.F. cultured the cells; L.T. and B.P. performed the FACS sorting; F.N. and O.Z. performed the array-CGH and DNA content analyses; M.M. and R.C. performed the immunocytochemical experiments; M.L., P.G., and G.F. prepared the spleen specimens (ie, splenic tissue processing and paraffin embedding); U.M., M.P., and A.M.V. and designed the experimental plan and revised the manuscript; and G.B. designed the experimental plan, collected, analyzed, and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vittorio Rosti, MD, Unit of Clinical Epidemiology and Center for the Study of Myelofibrosis, IRCCS, Policlinico San Matteo Foundation, Viale Golgi 19, Pavia 27100, Italy; e-mail: v.rosti@smatteo.pv.it.