Key Points
Endogenous sVEGFR-3 that is expressed by the cornea binds and sequesters VEGF-C and is critical for corneal alymphaticity.
sVEGFR-3 overexpression enhances murine corneal graft transplant survival 5-fold by blocking lymphangiogenesis and hemangiogenesis.
Corneal transparency is a prerequisite for optimal vision and in turn relies on an absence of blood and lymphatic vessels, which is remarkable given the cornea’s proximity to vascularized tissues. Membrane-bound vascular endothelial growth factor receptor 3 (VEGFR-3), with its cognate ligand vascular endothelial growth factor C (VEGF-C), is a major mediator of lymphangiogenesis. Here, we demonstrate that the cornea expresses a novel truncated isoform of this molecule, soluble VEGFR-3 (sVEGFR-3), which is critical for corneal alymphaticity, by sequestering VEGF-C. sVEGFR-3 binds and sequesters VEGF-C, thereby blocking signaling through VEGFR-3 and suppressing lymphangiogenesis induced by VEGF-C. sVEGFR-3 knockdown leads to lymphangiogenesis and hemangiogenesis in the mouse cornea, while overexpression of sVEGFR-3 inhibits lymphangiogenesis and hemangiogenesis in a murine suture injury model. Pax6+/− mice spontaneously develop corneal and lymphatic vessels and are deficient in sVEGFR-3. sVEGFR-3 suppresses hemangiogenesis by blocking VEGF-C–induced phosphorylation of VEGFR-2. Overexpression of sVEGFR-3 leads to a 5-fold increase in corneal transplant survival in mouse models. sVEGFR-3 holds promise as a molecule to control and regress lymphatic-vessel–based dysfunction. Therefore, sVEGFR-3 has the potential to protect the injured cornea from opacification secondary to infection, inflammation, or transplant rejection.
Introduction
The maintenance of corneal transparency is required for optimal vision. Accordingly, the tissue must preserve avascularity and alymphaticity.1 Multiple pathological conditions lead to angiogenesis and lymphangiogenesis in the cornea.2,3 The vascular endothelial growth factor (VEGF) family of molecules dominates the blood4 and lymphatic5 vessel growth-signaling landscape. While the cornea expresses several inhibitors of hemangiogenesis (soluble FLT-1,6 thrombospondins,7 endostatin,8 and pigment-epithelium–derived factor,9 among other factors10 ), few inhibitors of lymphangiogenesis have been reported.11 Blockade of lymphangiogenesis is of particular interest as its excessive expression enables corneal transplant rejection.12
In adults, vascular endothelial growth factor receptor 3 (VEGFR-3) is expressed in the lymphatic endothelium and corneal epithelium.13,14 VEGF-C binds VEGFR-3, promoting lymphangiogenesis15 and contributing to physiological angiogenesis.16 The VEGF-C propeptide undergoes stepwise proteolytic processing to generate ligands with increasing affinity for VEGFR-3, but only the completely processed VEGF-C isoform appears to bind VEGF receptor 2 (VEGFR-2).17 Overexpression of VEGF-C in transgenic mice results in lymphatic endothelial cell hyperplasia and vessel enlargement.18 VEGF-C is chemotactic for inflammatory cells secreting VEGF-A.19 We have shown that soluble VEGF receptor 1 expressed in the cornea sequesters VEGF-A, controlling hemangiogenesis and maintaining corneal avascularity.6 While previous studies have shown that circulating serum forms of soluble VEGFR-3 (sVEGFR-3) can serve as biomarkers in malignancy,20,21 an endogenous tissue-based sVEGFR-3 has not, to our knowledge, been reported or characterized. In this study, we show that sVEGFR-3 is expressed in the cornea and promotes alymphaticity by binding and sequestering VEGF-C, serving as a “sink”; further, it facilitates corneal avascularity by inhibiting the VEGFR-2 phosphorylation effects of VEGF-C.
Materials and methods
Animals, antibodies, and recombinant proteins
C57Bl/6J, BALB/c, and Pax6+/− mice (The Jackson Laboratory) were used. Experiments were approved by institutional review boards and conformed to the Association for Research in Vision and Ophthalmology Statement on Animal Research. Antibodies used were VEGF receptor 1 (H-240 and H-225 from Santa Cruz Biotechnology), VEGFR-2 (ab2349, ab9530, ab51873 from Abcam [Cambridge, MA]; PAB12647 from Abnova; and 936-0900 from Invitrogen [Carlsbad, CA]), VEGFR-3 (PA 4835 from Thermo Scientific; H-240, RM0003-5F63, and C-20 from Santa Cruz Biotechnology; and ab72240 from Abcam), and VEGF-C (A-18 and A-16 from Santa Cruz Biotechnology, ab9546 from Abcam, and 2445 from Cell Signaling Technology). Recombinant proteins used were soluble recombinant VEGFR-3 (CRF113B from Cell Sciences), recombinant mouse VEGFR-3/VEGFR-3 Fc chimera (743R3 from R&D Systems), recombinant human VEGF R2/KDR/Flk1Fc chimera (357KD), and VEGF-C recombinant protein (H00007424-P01 from Abnova).
3′ rapid amplification of cDNA ends
A previously described22 method was used to determine the 3′ unique end of sVEGFR-3. Human eyes were obtained from the Utah Lions Eye Bank. Total RNA was extracted from C57Bl/6J mouse and human cornea using the RNeasy Kit (Qiagen, Valencia, CA). A total of 2 μg of RNA was reverse transcribed using complementary DNA (cDNA) cloning primer (QT) CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGCTTTTTTTTTTTTTTTTT. cDNA was amplified using Q0-CCAGTGAGCAGAGTGACG and VEGFR-3 domain 4–specific forward primer ATCAACAAACCTGACACGCTCCTG using a Long Range PCR Kit (Qiagen), with the following polymerase chain reaction (PCR) conditions: 93°C for 3 minutes, 35 cycles of 93°C for 15 seconds, 55°C for 30 seconds, and 68°C for 5 minutes. PCR product was diluted 1:50 and amplified again using QI-GAGGACTCGAGCTCAAGC and VEGFR-3 domain 5–specific primer CCAATCTCTTCGTCGTGCACATCA primers. PCR products were gel excised and the pcr 2.1 TOPO TA cloning kit (Invitrogen) was used to clone the insert. The inserts were sequenced using M13 primers. Once the 3′ end sequence of sVEGFR-3 was determined, the full-length gene was cloned into pCMV Script vector using exon 1 and 3′ end primers.
Corneal suturing and intrastromal injections
The recipient mice were anesthetized by intramuscular injection with ketamine (100 mg/kg body weight) and xylazine (20 mg/kg body weight). To dilate the pupil and anesthetize the cornea, 1% tropicamide ophthalmic solution and 0.5% proparacaine ophthalmic solution were used. Three symmetrical sutures were placed (11-0 nylon, CS160-6; Ethicon). For plasmids, small hairpin RNA (shRNA) and small interfering RNA (siRNA) injections were made under direct microscopic observation, and a nick in the epithelium and anterior stroma of a BALB/c mouse cornea was made in the midperiphery with a 0.5-inch, 30G needle (BD Biosciences, Franklin Lakes, NJ). A 0.5-inch, 33G needle with a 30° bevel on a 10 µL gas-tight syringe (Hamilton, Reno, NV) was introduced into the corneal stroma and advanced 1.5 mm to the corneal center. Two microliters of plasmid solution (1 µg/µL) (pshRNA-sVEGFR-3, pshRNA-CTR, pshRNA-mVEGFR-3, pCMV.sVEGFR-3, and pCMV.CTR) was injected into the stroma to separate corneal lamellae and disperse the plasmid, as performed previously.23 Antibodies (10 µg each anti–VEGF-C antibody and isotype control goat immunoglobulin G [IgG]; Jackson Immunoresearch) were used for injections into mouse cornea as previously reported.24 Eyes were harvested at 3-, 5-, and 10-day intervals for different downstream assays. The efficiency of corneal transfection by naked plasmid exceeded 70%, as gauged by flow cytometry and X-gal staining, as reported previously.6
Immunostaining and imaging
Eyes were harvested 10 days after injections. The corneas were fixed in acetone at room temperature for 20 minutes. After 4 washes in PBST (0.1% Tween20/phosphate-buffered saline), the corneas were incubated in 3% bovine serum albumin/phosphate-buffered saline (BSA/PBS) at 4°C for 3 days. To detect CD-31 and LYVE1, corneas were incubated in 3% BSA/PBS with fluorescein-isothiocyanate–conjugated rat anti–CD-31 antibody (553372, 1:500; BD Biosciences, San Jose, CA) or rabbit anti–LYVE-1 (ab14917, 1:200; Abcam) overnight at 4°C. After 3 washes in PBST, the corneas were incubated in 3% BSA/PBS with Alexa Fluor 546–conjugated goat anti–rabbit IgG (A11071, 1:2000, Invitrogen) for 1 hour at room temperature. After 4 washes in PBST, corneas were mounted on slide glass with Fluoro-gel (Electron Microscopy Sciences, Hatfield, PA). Fluorescence was observed by fluorescence microscope (Carl Zeiss MicroImaging, Thornwood, NY). For area calculation, images were taken in gray mode and calculated by Image J individually. Areas where sutures had fallen out were not included in the final calculations.
Immunoprecipitation and western blotting
After euthanizing the mice, corneas were dissected under a stereomicroscope and placed in 200 µL RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and Halt phosphatase inhibitor (Fisher Scientific, Pittsburgh, PA). After homogenization with a sonic dismembrator (Fisher Scientific), 150-µL samples were subjected to immunoprecipitation for VEGFR-3 using protein-A–coated magnetic beads (Dynabeads; Invitrogen) and rabbit polyclonal antibody VEGFR-3 (H-240 SC-20734; Santa Cruz) following the manufacturer’s instructions. The proteins were eluted with Laemmli buffer for 10 minutes at 70°C and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10%) under native reducing conditions. The protein was transferred to a nitrocellulose or polyvinylidene difluoride membrane and incubated for 1 hour with blocking buffer (3% BSA and 0.05% Tween-20 in Tris-buffered saline [TBS]) and then incubated with appropriate antibody (1:200-1000) in blocking buffer for 1 hour at room temperature. The membrane was washed once in TBST (0.05% Tween-20/TBS) and twice in TBS. Finally, the membrane was incubated with the appropriate secondary horseradish peroxidase (HRP)-linked antibody in blocking buffer for 30 minutes at room temperature. After washing once with TBST and thrice with TBS, the bands were illuminated by an ECL-PLUS western blot detection kit (Amersham Biosciences, Pittsburgh, PA) and detected by FOTO/Analyst Electronic Imaging System (Fotodyne, Hartland, WI).
VEGFR-2 immunoprecipitation was performed using VEGFR-2 antibody–conjugated Sepharose beads (#5168; Cell Signaling Technology) from 200 μL human umbilical vein endothelial cell (HUVEC) cell lysate at 1 mg/mL following the manufacturer’s protocol. After 7% sodium dodecyl sulfate polyacrylamide gel electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane. After blocking for 1 hour with 5% BSA/TBST, the membrane was stained with VEGFR-3 antibody (1:200, sc-20734; Santa Cruz Biotechnology) or VEGFR-2 antibody (1:1000, #2479; Cell Signaling Technology) in 5% BSA/TBST overnight at 4°C. After washing, the membrane was incubated with the appropriate HRP-conjugated secondary antibody. The signals were developed with ECL reagent (RPN2232, GE Healthcare) and captured by an electronic imaging system (FOTO/Analyst; Fotodyne).
To test VEGF-A–induced VEGFR-2 phosphorylation, HUVEC were stimulated with 20 ng/mL VEGF-A (catalog number 100-20; PeproTech, Rocky Hill, NJ) or 20 ng/mL VEGF-A + 500 ng/mL recombinant sVEGFR-3 (CRF113-005; Cell Science, MA) for 10 minutes after 24 hours of serum starvation. To test VEGF-C–induced VEGFR-2 phosphorylation, HUVEC were stimulated with 20 ng/mL, 100 ng/mL, or 500 ng/mL VEGF-C (2179-VC-025, R&D Systems) and 500 ng/mL VEGF-C + 1750 ng/mL recombinant sVEGFR-3 for 5 minutes after 24 hours of serum starvation. For western blot, the same VEGFR-2 antibody described above and phospho–VEGFR-2 antibody (1:1000, #3770; Cell Signaling Technology) were used.
ELISA
To estimate the presence of the free or bound form of VEGF-C in the cornea and to estimate what percentage of VEGF-C is bound to VEGFR-3 or VEGFR-2, a sandwich enzyme-linked immunosorbent assay (ELISA) was performed from C57Bl/6J corneal lysate in quintuplicate. Anti–VEGF-C antibodies (1 μg/mL, ab9546; Abcam) were coated to 96-well plates overnight. The next day, the plates were washed, corneal lysate (n = 15) and recombinant VEGF-C (H00007424-P01; Abnova) as a standard were added, and plates were incubated for 2 hours at room temperature. Antibodies specific to anti–VEGF-C (sc27128; Santa Cruz), the anti–N-terminal end of VEGFR-3 (sc101563; Santa Cruz Biotechnology), and the anti–N-terminal end of VEGFR-2 (ab51873; Abcam) at a concentration of 2 µg/mL were added and incubated for 2 hours at room temperature with shaking. The wells were washed 5 times with wash buffer. The secondary antibodies donkey anti–goat IgG HRP (1:5000) and goat anti–rat IgG HRP (1:5000) were added, and samples were incubated for another 2 hours at room temperature with shaking. Samples were washed 5 times, 100 µL substrate buffer was added, the samples were incubated for 30 minutes at room temperature, the reaction was stopped, and the absorption was measured with an ELISA reader (Emax; Molecular Devices, Sunnyvale, CA) at 450 nm with λ correction at 570 nm. All measurements were performed in duplicate. Concentrations were calculated from the standard curve and corrected for total protein. To estimate VEGF-C’s relative affinity for VEGFR-3 and VEGFR-2, a competitive sandwich ELISA was performed. Recombinant human VEGF-C (10 µg/mL, H00007424-P01; Abnova) was coated onto 96-well plates overnight, and equal molar amounts of soluble recombinant VEGFR-3 (CRF113B, Cell Sciences) and recombinant human VEGFR2/KDR/Flk1Fc chimera (R&D; 357KD) were added to each well for the competitive binding to VEGF-C. Antibodies to VEGFR-2 (ab51873, Abcam) and VEGFR-3 (SC-20734, Santa Cruz) were added for 2 hours. The wells were washed and HRP-labeled antibodies (antibody to rat IgG, H&L [HRP], ab6734, Abcam, 1:5,000; anti–rabbit IgG, H&L [HRP], NA 934, GE Healthcare) were added for 2 hours. All ELISAs were performed at least twice with a separate set of antibodies to confirm the results.
Corneal transplantation
Penetrating corneal transplantation was performed using previously described methods25 with female mice (8 to 12 weeks old) of the BALB/c strain as graft recipients and mice of the C57BL/6 strain as graft donors (n = 9-12 each group). The donor cornea was marked with a 2-mm trephine and the recipient cornea was marked with a 1.50-mm trephine. The donor graft was sutured into the recipient bed using 6 to 8 10-0 nylon interrupted sutures. Sutures remained in the recipient’s eye for 1 week after transplantation. Subconjunctival injection (15 μl, 1 μg/μl) of plasmids expressing sVEGFR3 and empty pCMV was performed on the day of keratoplasty and postoperatively at 1, 2, 3, and 4 weeks using a gas-tight syringe (Hamilton). We monitored for graft rejection by a standardized opacity grading (0-5) system using previously described methods.25,,-28 Opacity grades 3 and above were considered a graft rejection.
Results
Cloning and characterization of sVEGFR-3
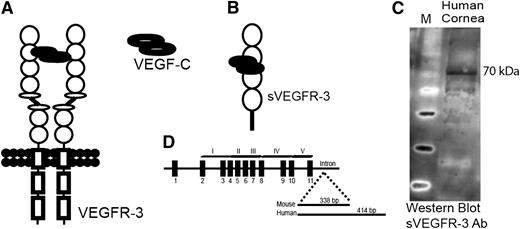
During the course of our studies, we observed a smaller than expected band in western blot using an antibody specific to the N terminus of VEGFR-3 (supplemental Figure 1D). We hypothesized this to be a truncated form of VEGFR-3. 3′ rapid amplification of cDNA ends revealed a transcript of VEGFR-3 messenger RNA (mRNA) with exons 1 to 11 and retention of part of intron 11 (Figure 1D) lacking transmembrane and intracellular tyrosine kinase domains of VEGFR-3 (Figure 1B); we designated the molecule sVEGFR-3 (or sFLT-4). Cloning (supplemental Figure 1A) and sequencing (supplemental Figure 1B) showed a sVEGFR-3 cDNA of 1575 bp in mice and 1962 bp in humans, encoding 59.5 kDa and 73.1 kDa proteins, respectively (Figure 1C and supplemental Figure 1D). Mouse sVEGFR-3 has a 338-bp tail derived from intron 11 (supplemental Figure 1B). However, it contains an in-frame stop codon 24 bp downstream from the exon-intron 11 junction, which encodes 8 unique amino acids (supplemental Figure 1C). Human sVEGFR-3 has a 414-bp unique intron-derived tail downstream from the exon-11/intron-11 junction, resulting in a unique C-terminal tail of 137 amino acids (supplemental Figure 1B-C). A custom antibody (PA 4835; Thermo Scientific) was developed against this unique C-terminal tail of human sVEGFR-3 for further study.
sVEGFR-3 is expressed in the cornea. (A) Structure of the VEGFR-3 receptor. (B) sVEGFR-3 consists of 4 extracellular domains and a unique tail derived from intron 11. (C) Representative western blot of human cornea with antibody specific to the intron-derived unique C-terminal tail of human sVEGFR-3 (PA 4835; Thermo Scientific) demonstrates sVEGFR-3 at 70 kDa. (D) Genomic, exon-intron structure of sVEGFR-3 consisting of 11 exons (black boxes) and a tail derived from intron 11. Ab, antibody.
sVEGFR-3 is expressed in the cornea. (A) Structure of the VEGFR-3 receptor. (B) sVEGFR-3 consists of 4 extracellular domains and a unique tail derived from intron 11. (C) Representative western blot of human cornea with antibody specific to the intron-derived unique C-terminal tail of human sVEGFR-3 (PA 4835; Thermo Scientific) demonstrates sVEGFR-3 at 70 kDa. (D) Genomic, exon-intron structure of sVEGFR-3 consisting of 11 exons (black boxes) and a tail derived from intron 11. Ab, antibody.
Human and mouse cornea express sVEGFR-3, which binds VEGF-C
sVEGFR-3 mRNA (Figure 2A) and protein (Figures 1C and 2B) were identified in the cornea, primarily in the epithelium (Figure 2D and supplemental Figure 2A), and not in the sclera (Figure 2A-B). Membrane VEGFR-3 was present in sclera (Figure 2A-B) with minimal expression in cornea (Figure 2A,C and supplemental Figure 2B). We confirmed VEGF-C mRNA and protein expression in cornea (Figure 2E).
sVEGFR-3 is expressed in corneal epithelium and antagonizes VEGF-C. (A) Reverse-transcriptase PCRs (RT-PCRs) with intron-tail–specific reverse primer and exon-exon junction forward primers showing sVEGFR-3 in mouse cornea. Membrane VEGFR-3 mRNA expression in sclera only. (B) Western blot of corneal and scleral lysate (n = 5) with anti–VEGFR-3 N-terminal antibody demonstrates sVEGFR-3 at 60 kDa in cornea and membrane VEGFR-3 at 170 kDa in sclera. (C) Western blot of corneal and scleral lysate (n = 5) with anti–VEGFR-3 C-terminal antibody demonstrates the expression of membrane VEGFR-3 at 170 kDa in sclera only (none in cornea). (D) Immunolocalization of sVEGFR-3 (brown) in human cornea via an intron-derived C-terminal tail, human sVEGFR-3 antibody (PA 4835; Thermo Scientific). Isotype-negative control rabbit IgG. (E) RT-PCR and western blot of mouse cornea shows VEGF-C mRNA and protein. (F) Western blot of corneal lysate (n = 5) with anti–VEGFR-3 N-terminal antibody, blotted with anti–VEGF-C antibody under reducing (1) and native conditions (2), reveals binding of sVEGFR-3 to VEGF-C. (G) Sandwich ELISA with anti–VEGF-C–coated antibodies in 96-well plates, followed by the addition of corneal lysate (n = 5, each group; corneas from 3 mice in each sample), and then anti–VEGF-C, anti–VEGFR-3 N-terminal, and anti–N-terminal VEGFR-2 antibodies. (H) Competitive ELISA with human recombinant VEGF-C coated on 96-well plates and equimolar human recombinant VEGFR-3 and VEGFR-2 with extracellular domains added, showing affinity and binding to VEGF-C (n = 5). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP, immunoprecipitation; M, marker; mVEGFR-3, membrane VEGFR-3; WB, western blot.
sVEGFR-3 is expressed in corneal epithelium and antagonizes VEGF-C. (A) Reverse-transcriptase PCRs (RT-PCRs) with intron-tail–specific reverse primer and exon-exon junction forward primers showing sVEGFR-3 in mouse cornea. Membrane VEGFR-3 mRNA expression in sclera only. (B) Western blot of corneal and scleral lysate (n = 5) with anti–VEGFR-3 N-terminal antibody demonstrates sVEGFR-3 at 60 kDa in cornea and membrane VEGFR-3 at 170 kDa in sclera. (C) Western blot of corneal and scleral lysate (n = 5) with anti–VEGFR-3 C-terminal antibody demonstrates the expression of membrane VEGFR-3 at 170 kDa in sclera only (none in cornea). (D) Immunolocalization of sVEGFR-3 (brown) in human cornea via an intron-derived C-terminal tail, human sVEGFR-3 antibody (PA 4835; Thermo Scientific). Isotype-negative control rabbit IgG. (E) RT-PCR and western blot of mouse cornea shows VEGF-C mRNA and protein. (F) Western blot of corneal lysate (n = 5) with anti–VEGFR-3 N-terminal antibody, blotted with anti–VEGF-C antibody under reducing (1) and native conditions (2), reveals binding of sVEGFR-3 to VEGF-C. (G) Sandwich ELISA with anti–VEGF-C–coated antibodies in 96-well plates, followed by the addition of corneal lysate (n = 5, each group; corneas from 3 mice in each sample), and then anti–VEGF-C, anti–VEGFR-3 N-terminal, and anti–N-terminal VEGFR-2 antibodies. (H) Competitive ELISA with human recombinant VEGF-C coated on 96-well plates and equimolar human recombinant VEGFR-3 and VEGFR-2 with extracellular domains added, showing affinity and binding to VEGF-C (n = 5). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP, immunoprecipitation; M, marker; mVEGFR-3, membrane VEGFR-3; WB, western blot.
To see if sVEGFR-3 can bind to VEGF-C, as VEGFR-3 is known to bind VEGF-C,15 in vivo interaction was confirmed by immunoprecipitation of corneal lysate with anti–N-terminal VEGFR-3 antibody and western blotting with anti–VEGF-C antibody. A 30-kDa band was observed under native and denaturation conditions (Figure 2F). A competitive sandwich ELISA was performed and demonstrated that 98.61% ± 3.8% of VEGF-C in the cornea is in the bound form while 1.5% ± 0.9% is in the free form (n = 5, Figure 2G). A total of 57.87% ± 2% of corneal VEGF-C was bound to sVEGFR-3 and 40.7% ± 1.8% to sVEGFR-2 (Figure 2G; n = 5). sVEGFR-3 had 3 times higher affinity for VEGF-C than sVEGFR-2, consistent with earlier findings17 (Figure 2H).
Corneal injury induces upregulation of membrane VEGFR-3 and VEGF-C
To characterize the cornea’s reaction to trauma, we performed suturing of the cornea, which induced development of blood and lymphatic vessels within 10 to 14 days. sVEGFR-3 and VEGF-C were present at the site of sutures (Figure 3A) with a dramatic increase in levels of VEGF-C mRNA (n = 5) (Figure 3B). Normal corneas expressed only sVEGFR-3, while sutured corneas expressed membrane VEGFR-3 (Figure 3C). Low levels of sVEGFR-3 and higher levels of VEGFR-3 were observed in sutured corneas from day 5 onward (Figure 3C). In tandem with these findings from trauma-induced lymphaticized corneas, we examined the spontaneously lymphatic corneas of Pax6+/− mice29 for the presence of sVEGFR-3. Corneas of Pax6+/− mice, unlike those of their background strain C57BL/6J, were deficient in sVEGFR-3 mRNA (supplemental Figure 2C-D).
Corneal injury induces lymphangiogenesis, upregulation of VEGF-C, and membrane VEGFR-3 expression. (A) Immunostaining of normal and sutured cornea on day 3 reveals sVEGFR-3 and VEGF-C upregulation at the site of suture, indicating that sVEGFR-3 initially tends to capture VEGF-C and control VEGF-C surge. Negative controls are sections stained with isotype-control primary antibodies. (B) Real-time PCR of normal and sutured cornea on day 3 revealing mechanical trauma leads to instant increase in VEGF-C mRNA levels (n = 5). (C) Western blot of normal (1) and sutured cornea (2) on day 5 leads to expression of membrane VEGFR-3 (170 kDa) in sutured cornea that leads to signaling through VEGFR-3 and development of lymphatic vessels.
Corneal injury induces lymphangiogenesis, upregulation of VEGF-C, and membrane VEGFR-3 expression. (A) Immunostaining of normal and sutured cornea on day 3 reveals sVEGFR-3 and VEGF-C upregulation at the site of suture, indicating that sVEGFR-3 initially tends to capture VEGF-C and control VEGF-C surge. Negative controls are sections stained with isotype-control primary antibodies. (B) Real-time PCR of normal and sutured cornea on day 3 revealing mechanical trauma leads to instant increase in VEGF-C mRNA levels (n = 5). (C) Western blot of normal (1) and sutured cornea (2) on day 5 leads to expression of membrane VEGFR-3 (170 kDa) in sutured cornea that leads to signaling through VEGFR-3 and development of lymphatic vessels.
sVEGFR-3 is necessary for corneal alymphaticity
To determine if sVEGFR-3 preserved corneal alymphaticity, we employed RNA knockdown. shRNA were directed to two targets in the 3′ unique intron of sVEGFR-3 (supplemental Figure 3A). Controls included plasmids expressing an shRNA targeting a sequence in the unique carboxyl-terminus region of VEGFR-3 not present in sVEGFR-3 (pshRNA–VEGFR-3) and a scrambled sequence (pshRNA-CTR). Corneal injection of plasmid pshRNA–sVEGFR-3, but not pshRNA-CTR or pshRNA–VEGFR-3, reduced sVEGFR-3 mRNA (supplemental Figure 3B-C). This increased free VEGF-C in the cornea (supplemental Figure 3D), corroborating our hypothesis that sVEGFR-3 mediates VEGF-C sequestration.
pshRNA-sVEGFR-3 injection into cornea led to formation of lymphatic vessels 10 days postinjection (cornea area 8.73% ± 1.07%, n = 10) (Figure 4A-B). Interestingly, these corneas also induced blood vessels (cornea area 7.06% ± 1.71%, n = 10) (Figure 4A,C). A similar pattern of results was observed using siRNA oligos designed for another target site in the unique carboxyl terminal region of sVEGFR-3 (supplemental Figure 4A-C). This effect was abolished by injection of anti–VEGF-C antibodies 1 day prior to pshRNA-sVEGFR-3 treatment, which suppressed both lymphatic and blood vessels (Figure 4A). There was a 75% reduction in lymphatic vessel (total area 2.16% ± 0.46% (P < .01, n = 10) (Figure 4B) and a 56% reduction of blood vessel (total area 3.08% ± 0.44%, P < .01, n = 10) area (Figure 4C).
sVEGFR-3 knockdown leads to growth of corneal lymphatic and blood vessels and expression and phosphorylation of membrane VEGFR-3. (A) Immunofluorescent staining and confocal imaging of cornea flat mounts (n = 10 each group) injected with 2 μg pshRNA–sVEGFR-3, pshRNA-CTR, pshRNA–sVEGFR-3 + anti–VEGF-C immunoglobulin, and IgG. pshRNA–sVEGFR-3 injection leads to lymphangiogenesis in 10 days, with vessels oriented to injection site. Anti–VEGF-C antibody administration 1 day before pshRNA-sVEGFR-3 controls lymphangiogenesis. (B-C) Corneal area of blood and lymphatic vessels 10 days after pshRNA–sVEGFR-3, pshRNA-CTR, pshRNA–sVEGFR-3 + anti–VEGF-C immunoglobulin, and IgG antibody injection. Anti–VEGF-C immunoglobulin injection 1 day prior to pshRNA–sVEGFR-3 shows 75% and 56% reduction of lymphatic and blood vessels (n = 10 each group). (D) sVEGFR-3 knockdown with pshRNA–sVEGFR-3 injection into cornea is associated with expression of membrane VEGFR-3 and downregulation of sVEGFR-3 as seen in western blot with anti–N-terminal VEGFR-3 antibody. (E) sVEGFR-3 knockdown with pshRNA–sVEGFR-3 (samples harvested on day 5 after injection) is associated with phosphorylation of membrane VEGFR-3 as demonstrated via immunoprecipitation with anti–N-terminal VEGFR-3 antibody followed by western blotting with anti-phosphotyrosine antibody. (F) Stripping and reblotting the membrane with anti–N-terminal end VEGFR-3 antibody confirms that sVEGFR-3 is present after control shRNA treatment but not after pshRNA–sVEGFR-3. *P < .01 by paired Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP, immunoprecipitation; WB, western blot.
sVEGFR-3 knockdown leads to growth of corneal lymphatic and blood vessels and expression and phosphorylation of membrane VEGFR-3. (A) Immunofluorescent staining and confocal imaging of cornea flat mounts (n = 10 each group) injected with 2 μg pshRNA–sVEGFR-3, pshRNA-CTR, pshRNA–sVEGFR-3 + anti–VEGF-C immunoglobulin, and IgG. pshRNA–sVEGFR-3 injection leads to lymphangiogenesis in 10 days, with vessels oriented to injection site. Anti–VEGF-C antibody administration 1 day before pshRNA-sVEGFR-3 controls lymphangiogenesis. (B-C) Corneal area of blood and lymphatic vessels 10 days after pshRNA–sVEGFR-3, pshRNA-CTR, pshRNA–sVEGFR-3 + anti–VEGF-C immunoglobulin, and IgG antibody injection. Anti–VEGF-C immunoglobulin injection 1 day prior to pshRNA–sVEGFR-3 shows 75% and 56% reduction of lymphatic and blood vessels (n = 10 each group). (D) sVEGFR-3 knockdown with pshRNA–sVEGFR-3 injection into cornea is associated with expression of membrane VEGFR-3 and downregulation of sVEGFR-3 as seen in western blot with anti–N-terminal VEGFR-3 antibody. (E) sVEGFR-3 knockdown with pshRNA–sVEGFR-3 (samples harvested on day 5 after injection) is associated with phosphorylation of membrane VEGFR-3 as demonstrated via immunoprecipitation with anti–N-terminal VEGFR-3 antibody followed by western blotting with anti-phosphotyrosine antibody. (F) Stripping and reblotting the membrane with anti–N-terminal end VEGFR-3 antibody confirms that sVEGFR-3 is present after control shRNA treatment but not after pshRNA–sVEGFR-3. *P < .01 by paired Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP, immunoprecipitation; WB, western blot.
Corneal injection with pshRNA–sVEGFR-3 induced increased expression of membrane VEGFR-3 (Figure 4D) and phosphorylation of VEGFR-3 (Figure 4E). sVEGFR-3 was present after control shRNA treatment, but membrane VEGFR-3 was expressed after pshRNA–sVEGFR-3 treatment (Figure 4F). This suggests that ablation of sVEGFR-3 and the subsequent VEGF-C surge leads to upregulation of membrane VEGFR-3, with unbound VEGF-C binding to and inducing membrane-bound VEGFR-3 signal transduction.
Overexpression of sVEGFR-3 suppresses growth of lymphatic and blood vessels
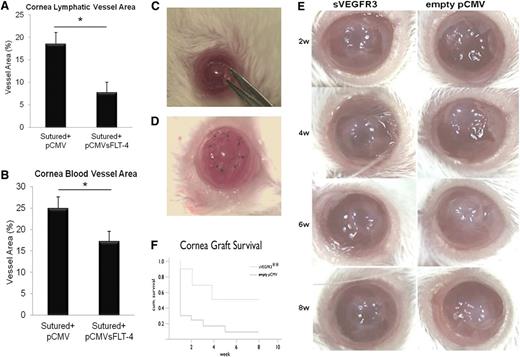
To determine if sVEGFR-3 overexpression could preserve corneal alymphaticity and avascularity, a plasmid overexpressing sVEGFR-3 (pCMV.sVEGFR-3) was injected 1 day before suturing the cornea. This injection suppressed the growth of lymphatic and blood vessels compared with control pCMV (pCMV.CTR). Corneas were harvested 10 days after injection. Sutured corneas injected with pCMV.CTR had a mean fractional lymphatic area of 18.55% ± 2.54% (n = 10) (Figure 5A) and a mean fractional hemangiogenic area of 24.92% ± 2.69% (n = 10) (Figure 5B). Injection with pCMV.sVEGFR-3 into sutured cornea led to a 58% decrease in lymphatic area (7.78% ± 2.24%, P < .01, n = 10) and a 31% decrease in blood vessel area (17.20% ± 2.37%, P < .01, n = 10) (Figure 5A-B).
sVEGFR-3 overexpression results in regression of corneal lymphatic and blood vessels; overexpression of sVEGFR-3 is protective of transplant graft survival. (A-B) Lymphatic and blood vessel area 10 days after pCMV.sVEGFR-3 and pCMV.CTR injection, 1 day prior to corneal suturing. pCMV.sVEGFR-3 injection resulted in a 58% decrease in lymphatic area and a 31% decrease in blood vessel area compared with pCMV.CTR (n = 10 each group). (C-D) Penetrating corneal transplantation performed using female mice (8 to 12 weeks old) of the BALB/c strain as graft recipients and mice of the C57BL/6 strain as graft donors. (E) Representative corneas of transplant experiment at 2-, 4-, 6-, and 8-week intervals. (F) Subconjunctival injection with sVEGFR-3–overexpressing plasmid (pCMV.sVEGFR-3) showed that corneal transplant graft survival was 40.0% compared with empty pCMV with 8.3% graft survival in BALB/c recipient mice (n = 9-12). **P < .05 Kaplan-Meier survival analysis. IP, immunoprecipitation; w, weeks.
sVEGFR-3 overexpression results in regression of corneal lymphatic and blood vessels; overexpression of sVEGFR-3 is protective of transplant graft survival. (A-B) Lymphatic and blood vessel area 10 days after pCMV.sVEGFR-3 and pCMV.CTR injection, 1 day prior to corneal suturing. pCMV.sVEGFR-3 injection resulted in a 58% decrease in lymphatic area and a 31% decrease in blood vessel area compared with pCMV.CTR (n = 10 each group). (C-D) Penetrating corneal transplantation performed using female mice (8 to 12 weeks old) of the BALB/c strain as graft recipients and mice of the C57BL/6 strain as graft donors. (E) Representative corneas of transplant experiment at 2-, 4-, 6-, and 8-week intervals. (F) Subconjunctival injection with sVEGFR-3–overexpressing plasmid (pCMV.sVEGFR-3) showed that corneal transplant graft survival was 40.0% compared with empty pCMV with 8.3% graft survival in BALB/c recipient mice (n = 9-12). **P < .05 Kaplan-Meier survival analysis. IP, immunoprecipitation; w, weeks.
Overexpression of sVEGFR-3 results in increased corneal transplant survival
To determine if these effects were clinically relevant, penetrating corneal transplantation was performed using C57BL/6J mice as graft donors and BALB/c mice as graft recipients (Figure 5C-D). Subconjunctival injection of plasmid overexpressing sVEGFR-3 (pCMV.sVEGFR-3) and control empty pCMV was done on the day of keratoplasty and postoperatively at 1, 2, 3, and 4 weeks in BALB/c recipient mice (Figure 5E). Graft protection was 40.0% in the sVEGFR3 group and 8.3% in the empty pCMV group (P = .032; Figure 5F).
sVEGFR-3 suppressed hemangiogenesis by blocking VEGF-C–induced VEGFR-2 phosphorylation
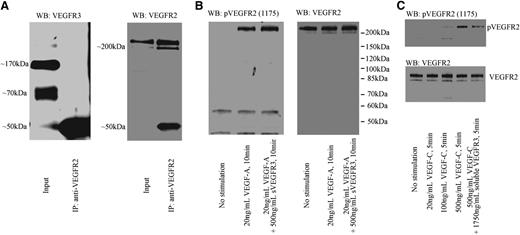
To determine why sVEGFR-3 suppressed hemangiogenesis, we examined whether endogenous soluble or membrane VEGFR-3 heterodimerizes with VEGFR-2 by immunoprecipitation of VEGFR-2. After the immunoprecipitation of VEGFR-2, soluble or membrane VEGFR3 was not detected by western blotting with anti–N-terminal VEGFR-3 antibody (Figure 6A). Next, we tested whether recombinant sVEGFR-3 could inhibit VEGF-A–induced VEGFR-2 phosphorylation. We found that recombinant sVEGFR-3 did not inhibit VEGFR-2 phosphorylation, even at a 25-fold-higher concentration than VEGF-A (Figure 6B). Finally, we tested whether sVEGFR-3 inhibits VEGF-C–induced VEGFR-2 phosphorylation. We found that recombinant sVEGFR-3 inhibited VEGFR-2 phosphorylation mediated by VEGF-C (Figure 6C). These data indicate that sVEGFR-3 suppressed hemangiogenesis through the blocking of VEGF-C–induced VEGFR-2 phosphorylation, but not through heterodimerization or blockade of VEGF-A.
sVEGFR-3 inhibits VEGF-C–induced VEGFR-2 phosphorylation but not VEGF-A–induced VEGFR-2 phosphorylation. (A) Immunoprecipitation by VEGFR-2 antibody from HUVEC lysate. The input shows soluble and membrane VEGFR-3 bands. However, VEGFR-3 bands were not detected after precipitation, indicating that the two receptors do not heterodimerize. (B) After 24 hours of serum starvation, HUVEC was stimulated with 20 ng/mL VEGF-A or 20 ng/mL VEGF-A + 500 ng/mL recombinant sVEGFR-3. Recombinant sVEGFR-3 did not block VEGF-A–induced VEGFR-2 phosphorylation. (C) After 24 hours of serum starvation, HUVEC was stimulated with 20 ng/mL, 100 ng/mL, or 500 ng/mL VEGF-C or 500 ng/mL VEGF-C + 1750 ng/mL recombinant sVEGFR-3. Recombinant sVEGFR-3 treatment inhibits VEGF-C–induced VEGFR-2 phosphorylation. IP, immunoprecipitation; WB, western blot.
sVEGFR-3 inhibits VEGF-C–induced VEGFR-2 phosphorylation but not VEGF-A–induced VEGFR-2 phosphorylation. (A) Immunoprecipitation by VEGFR-2 antibody from HUVEC lysate. The input shows soluble and membrane VEGFR-3 bands. However, VEGFR-3 bands were not detected after precipitation, indicating that the two receptors do not heterodimerize. (B) After 24 hours of serum starvation, HUVEC was stimulated with 20 ng/mL VEGF-A or 20 ng/mL VEGF-A + 500 ng/mL recombinant sVEGFR-3. Recombinant sVEGFR-3 did not block VEGF-A–induced VEGFR-2 phosphorylation. (C) After 24 hours of serum starvation, HUVEC was stimulated with 20 ng/mL, 100 ng/mL, or 500 ng/mL VEGF-C or 500 ng/mL VEGF-C + 1750 ng/mL recombinant sVEGFR-3. Recombinant sVEGFR-3 treatment inhibits VEGF-C–induced VEGFR-2 phosphorylation. IP, immunoprecipitation; WB, western blot.
Discussion
We demonstrate that expression of endogenous sVEGFR-3 plays a key role in maintaining an alymphatic cornea, which, in turn, establishes the immune privilege of the cornea. The protein, similar to and along with the previously described soluble VEGFR-2,11 acts as an endogenous antagonist by serving as a decoy receptor for free monomeric VEGF-C and preventing lymphangiogenesis, consistent with previous findings that blockade of membrane-bound VEGFR-3 suppressed dendritic cell trafficking and rejection of corneal transplants.30 The binding of VEGF-C to VEGFR-3 is well established, and its signaling through membrane-bound VEGFR-3 is important for lymphangiogenesis.15,31 Spontaneous lymphangiogenesis is present in mice with a destrin mutation, which is inhibited with an anti–VEGFR-3 antibody.32 The previous work relied on antibodies targeting solely the N-terminal VEGFR-3 and was not able to distinguish the activity or presence of the soluble vs membrane isoforms.
Lymphangiogenesis and angiogenesis occur in concert. Previously, VEGF-C has been demonstrated to crosstalk between VEGFR-3 and VEGFR-2, though the signaling is not well understood.16,33 Here, our data directly demonstrate that upregulation or inhibition of VEGF-C via the presence of sVEGFR-3 directly controls signaling through VEGFR-3 and VEGFR-2, influencing lymphatic and blood vessel growth or inhibition.
VEGF-C is an essential chemotactic and survival factor during lymphangiogenesis.5 Proinflammatory cytokines induce VEGF-C mRNA transcription, presumably through nuclear factor ĸΒ–mediated promoter activation, suggesting a regulatory role in lymphatic vessel growth during inflammation.34 The strong expression of natural sVEGFR-3 in the corneal epithelium provides a defense mechanism for the cornea to counter inflammatory stimuli induced by eye exposure to foreign particles, allergens, microorganisms, and mechanical rubbing.
Overexpression of the extracellular domain of VEGFR-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer.35 A fusion protein consisting of 3 extracellular domains of VEGFR-3 tagged to the IgG Fc domain inhibits fetal lymphangiogenesis and regression of already formed lymphatic vessels.36 We believe that vision-threatening injuries and infections of the cornea shift the VEGFR-3 splicing mechanism toward the membrane-bound isoform. Expression of natural sVEGFR-3 by the corneal epithelium likely provides a counterinflammatory defense against immune reaction and vascularization induced by eye exposure to foreign particles, allergens, microorganisms, and mechanical rubbing. The discovery of sVEGFR-3 provides a new molecular tool to control or regress lymphangiogenesis, inflammation, lymphangioma (which occurs in roughly 1 out of 50 children37 ), and corneal transplant rejection.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by an RPB Physician Scientist Award, a VA Merit Award, and National Institutes of Health National Eye Institute grant R01EY017950.
Authorship
Contribution: N.S. and B.K.A. designed the study and wrote the manuscript. Z.O. edited the manuscript. N.S. designed and performed experiments. N.S. and M.T. made constructs. N.S., M.T., and R.W. conducted in vitro experiments. Y.W. did the immunohistochemistry experiments. T.W. and T.O. performed the animal work. Y.C. and L.L. performed corneal injections and the corneal suture model. Y.C. performed corneal transplant experiments. H.U. provided support for in vitro experiments and technical aspects. B.K.A. provided vital support with suggestions and experiment plans. All authors had an equal opportunity to discuss the results and comment on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Balamurali K. Ambati, Moran Eye Center, University of Utah, 65 Mario Capecchi Dr, Salt Lake City, UT, 84132; e-mail: bala.ambati@utah.edu; and Nirbhai Singh, Moran Eye Center, University of Utah, 65 Mario Capecchi Dr, Salt Lake City, UT, 84132; e-mail: nirbhais@gmail.com.