In this issue of Blood, Petersdorf et al report negative results of the phase 3 study designed by the Southwest Oncology Group (SWOG), which tested the addition of gemtuzumab ozogamicin (GO) to a 3+7 regimen in a randomized fashion in adult patients newly diagnosed with acute myeloid leukemia (AML) who are younger than 60 years.1 Also in this issue of Blood, Rowe and Löwenberg discuss results of this study in the context of 4 other controlled studies (1 negative and 1 positive in a predefined subset of patients, and 2 positive) performed during the same period in Europe.2
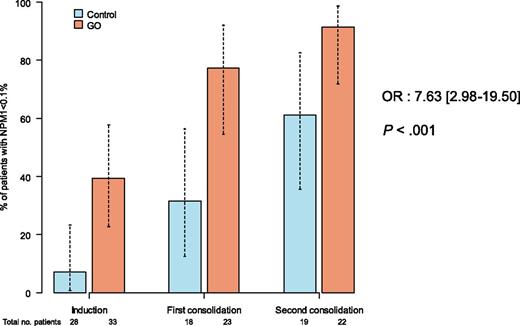
ALA 0701 study. Minimal residual response assessed by NPM1mut transcript levels in patients treated with or without GO.
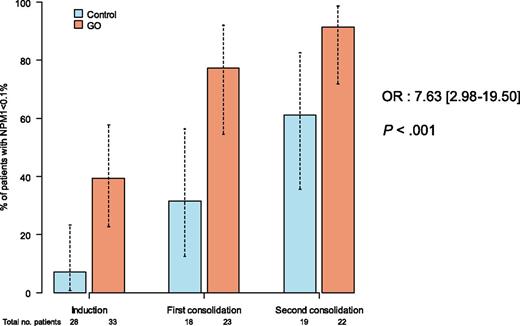
ALA 0701 study. Minimal residual response assessed by NPM1mut transcript levels in patients treated with or without GO.
Rowe and Löwenberg mainly explain observed contradictory results as being a result of the different doses of daunorubicin or GO used across the studies. They also underline the apparent paradox of better outcomes being observed in positive studies in patients treated with GO, despite there being no higher complete remission rate, which is likely explained by a better quality of remission. Indeed, results from minimal residual disease monitoring throughout the Randomized Study of GO With Daunorubicine and Cytarabine in Untreated AML Aged of 50–70 Years Old, known as the ALFA 0701 study, showed that a good MRD response was associated with an increase in overall survival and that NPM1 minimal residual disease (MRD) good responders were significantly more frequent in the GO group compared with in the control group (P < .0001; see figure).3
Some other comments can be added: The 2 negative studies from the SWOG and the Groupe Ouest Est d'Etude des Leucémies et Autres Maladies du Sang (GOELAMS) have used nearly the same design. They were performed in younger patients, and allogeneic transplant may be a confounding factor on outcome. They used a single dose of 6 mg/m2 GO, which is likely to be too toxic in combination with other chemotherapy. Indeed, the Medical Research Council (MRC) feasibility phase 2 study showed that a dose of 6 mg/m2 GO in addition to chemotherapy was associated with an excess of liver and hematological toxicity.4 In particular, profound and prolonged thrombocytopenia is observed with GO.
The specific toxicity of GO on the platelet lineage is not fully understood. It may be a result of calicheamicin, as thrombocytopenia has also been described with the use of inotuzumab ozogamicin, a monoclonal antibody against CD22.5 Prolonged thrombocytopenia appears dose-related, with less platelet toxicity in the MRC studies than in the ALFA 0701 study.6 ,7
The significant difference in early death rate observed in the SWOG study can be explained by a very low and unusual death rate in the control group and by an excess of death from hemorrhage in the GO group.
Finally, in the SWOG and GOELAMS studies, GO was added at day 4 of the induction course, perhaps when many of the CD33 antigens and blasts cells had been eliminated by the first 3 days of treatment with Daunorubicin and Cytarabine.
How can we explain the difficulties in finding the best way to use GO? Calicheamicin is a very toxic intercalating agent that is 1000-fold more potent in vitro than doxorubicin. Its conjugation with a monoclonal antibody allows the drug to be delivered directly to leukemic cells and explains its efficacy, but the favorable risk–benefit ratio of the conjugate may be only within a narrow dose range. The use of repetitive low doses is likely a way to enhance the drug’s efficacy without increasing toxicity.6 ,8 The ongoing study AML18, from the United Kingdom, is testing a single dose vs 2 doses of 3 mg/m2 of GO in addition to standard induction.
In addition, as underlined by Rowe and Löwenberg, the efficacy of GO is, unsurprisingly, observed in chemosensitive AML, as in AML with high CD33 expression. This was shown in acute promyelocytic leukemia and in the ALFA study in NPM1-positive AML.6 In this regard, future results from the ongoing controlled German study in NPM1-positive patients and detailed analysis of the interactions between the effect of GO and molecular AML subsets within the MRC studies will be of major interest.
Conflict-of-interest disclosure: S.C. received consultancy fees from Pfizer in 2012.