Key Points
Human CD141+ cDCs not only produce IL-12 but also yield large amounts of IFN-α after TLR3 stimulation with synthetic dsRNA.
Targeting of antigen to DEC-205 and synthetic dsRNA as adjuvant for CD141+ cDCs maturation induces CD4+ T cell responses in humanized mice.
Abstract
Functional differences between human dendritic cell (DC) subsets and the potential benefits of targeting them with vaccines remain poorly defined. Here we describe that mice with reconstituted human immune system components (huNSG mice) develop all human conventional and plasmacytoid DC compartments in lymphoid organs. Testing different Toll-like receptor agonists for DC maturation in vivo, we found that IL-12p70 and interferon (IFN)-α production correlated with the maturation of CD141+ (BDCA3+) conventional DCs in huNSG mice. Furthermore, depletion of CD141+ DCs before stimulation significantly reduced IFN-α levels in vivo. This DC subset produced similar total amounts but different subtypes of IFN-α in response to synthetic double-stranded RNA compared with plasmacytoid DCs in response to a single-stranded RNA equivalent. Moreover, synthetic double-stranded RNA as adjuvant and antigen targeting to the endocytic receptor DEC-205, a combination that focuses antigen presentation for T-cell priming on CD141+ DCs, stimulated antigen-specific human CD4+ T-cell responses. Thus, the human CD141+ DC subset is a prominent source of IFN-α and interleukin-12 production and should be further evaluated for vaccine development.
Introduction
Dendritic cells (DCs) are antigen-presenting cells that get activated upon the sensing of pathogens and induce robust innate and adaptive immune responses. This so-called maturation leads to major histocompatibility complex and costimulatory molecule up-regulation, enhanced migration to secondary lymphoid tissues, and cytokine production by DCs.1,2 In humans, the major DC subsets in the steady state are conventional DCs (cDCs) and plasmacytoid DCs (pDCs),3 which differ in their expression of surface markers, Toll-like receptors (TLRs), and in the cytokines produced after activation. cDCs are positive for CD11c and carry either CD1c (BDCA1) or CD141 (BDCA3).4,5 CD1c+ cDCs express TLR1 through TLR8 and TLR10, which enable them to respond to pathogen-associated molecular patterns such as double-stranded (ds) and single-stranded (ss) RNA.6-8 The small subset of CD1c−CD141+ cDCs expresses TLR1, 2, 3, 6, 8, and 10 and efficiently cross-presents antigens to CD8+ T cells.8-12 Upon activation, cDCs are primarily known to secrete interleukin (IL)-12, tumor necrosis factor (TNF)-α, and IL-6. pDCs, on the other hand, are CD11c negative and CD123, CD303 (BDCA2), and CD304 (BDCA4) positive.5 In contrast to cDCs, they sense ssRNA and unmethylated DNA by TLR7 and TLR9, respectively.6 In response, they secrete type I interferons (IFNs) and play an important role in immune reactions to viral infection. pDCs also express TLR1, 6 and 10.7 Although the TLR expression of human DC subsets has been characterized in vitro, human pDC and cDC responses to TLR agonists have just begun to be investigated in vivo. Of note, efficient maturation of human DCs by adjuvants that induce signaling of TLRs or pathogen-associated molecular pattern receptors seems necessary for successful vaccination.
Mice reconstituted with human immune system components are a valuable tool to study human immune cells in vivo as well as to characterize the infection and immune response to pathogens with a strictly human tropism in a small animal model.13,14 The 2 major strains that are used for these studies are either mice deficient in recombination activating gene 2 (Rag2) and the cytokine receptor common γ chain on BALB/c background (BALB/c Rag2−/−γc−/−) or γc-deficient, nonobese diabetic (NOD) mice with the Prkdcscid mutation (NOD-scid γc−/−, [NSG]). On both genetic backgrounds, injection of CD34+ human hematopoietic stem or progenitor cells reconstitutes all major leukocyte subsets, including CD11c+ cDCs as well as CD123+ pDCs.15-18 Although the in vivo presence of reconstituted human DC subsets in NSG mice reconstituted with human immune system components (huNSG) has been described,19 the functions of these human DC subsets in these models have not been explored in detail.
Here, we describe the composition and organ distribution of the DC compartment in huNSG mice. Furthermore, we study their maturation by different TLR agonists in vivo by monitoring maturation marker expression and cytokine secretion. Surprisingly, we found that maturation of CD141+ cDCs correlates with the greatest production of both IL-12p70 and IFN-α in vivo and that enriched CD141+ DC populations produce similar amounts of IFN-α upon synthetic dsRNA exposure as pDCs after recognition of a ssRNA surrogate. Depletion of CD141+ cDCs in huNSG mice before stimulation significantly reduced IFN-α production in vivo. Accordingly, human donor-derived CD141+ cDCs produced IFN-α after stimulation with synthetic dsRNA. Furthermore, synthetic dsRNA as adjuvant and targeting antigen to the DEC-205 receptor, a combination that matures antigen-loaded CD141+ DCs, led to the priming of antigen-specific human CD4+ T-cell responses after vaccination. Our study, therefore, identifies the CD141+ cDC subset as a prominent source of IFN-α after dsRNA recognition and competent to prime T-cell responses.
Materials and methods
Generation of HuNSG mice and cell isolation
NOD, Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), mice were obtained from The Jackson Laboratory. HuNSG mice were generated as described by reconstitution with human fetal liver−derived CD34+ hematopoietic progenitor cells (Advanced Bioscience Resources).20 Animal protocols were approved by the Cantonal Veterinary Office Zürich.
Cells from heparinized blood were obtained after centrifugation for 10 minutes at 400g and erythrocyte lysis. Serum was processed on BD Microtainers (BD Biosciences). Spleens were digested in Hanks balanced salt solution with CaCl2/MgCl2 containing 0.4 mg/mL collagenase D (Roche Diagnostics) and 20 μg/mL DNAse (Roche Diagnostics) before mashing and erythrocytes lysis. Bone marrow (BM) cells were flushed from excised femurs.
TLR agonists
CpG ODN 2216 was purchased from InvivoGen, glycopyranosyl lipid adjuvant (GLA), IDC 1001 from the Infectious Disease Research Institute, and R848 from Enzo Life Sciences. PolyICLC was obtained from Oncovir Inc. and protamine/RNA from S. Pascolo (RNA: AGUGUUAUUCUUGUAUGG, 1:4 protamine).
Flow cytometry
Per sample, 4 × 106 cells were stained. Antibodies used are described in the supplemental information; see the Blood website). For intracellular cytokine staining, we used the BD Biosciences Cytofix/Cytoperm Plus kit. Samples were acquired on a BD LSRFortessa with the use of FACS Diva software (BD Biosciences). Analysis was performed with FlowJo Software (Tristar).
Histology
Spleens were frozen in OCT medium, sectioned at 6-8 μm, and immunostained for 1 hour. Sections were washed in phosphate-buffered saline (PBS) and mounted in Aqua Poly/Mount (Polysciences).
Cytokine ELISAs
IFN-α pan, IFN-α2, IL-12p70, and IFN-γ enzyme-linked immunosorbent assay (ELISA) kits were purchased from Mabtech.
Preparation of human PBMCs and DC isolation
Preparation of human PBMCs and DC isolation are described in the supplemental Methods.
Quantitative polymerase chain reaction
RNA was isolated with the RNeasy Micro Kit (QIAGEN). Reverse-transcription and quantitative polymerase chain reactions were performed as described21 and run on a BIO-RAD iCycler IQ.
Vaccination experiments
αDEC-205-Alexa647 or isotype-Alexa647 monoclonal antibodies (mAbs) were a kind gift of Dr. C. Cheong (Montreal, Canada). αDEC-205-EBNA1 or isotype-EBNA1, IFN-γ enzyme enzyme-linked immunospot, and peptide libraries have been described.22
Generation of EBNA1 specific T-cell clones
IFN-γ−secreting cells were enriched from splenocytes of vaccinated huNSG mice by an IFN-γ−secretion assay/cell enrichment and detection kit (Miltenyi Biotec) and cloned by limiting dilution as described.23 Functional T-cell assays are described in the supplemental Methods.
Statistical analysis
Mann-Whitney U tests were performed for analysis in Figures 2, 3, 4, and 7. Data in Figures 5 and 6 were analyzed by paired t test using GraphPad Prism Software (Version 5.0a).
Results
Robust reconstitution of all human DC subsets in huNSG mice
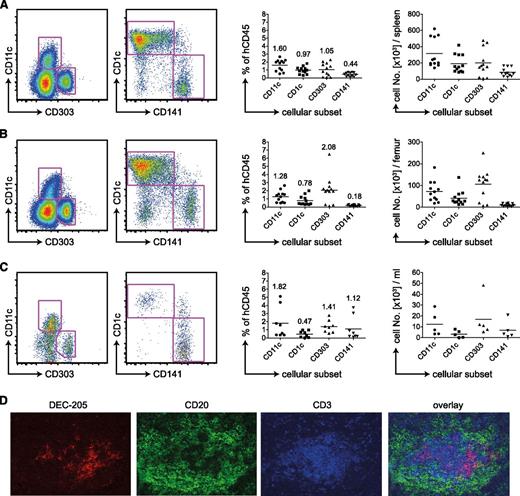
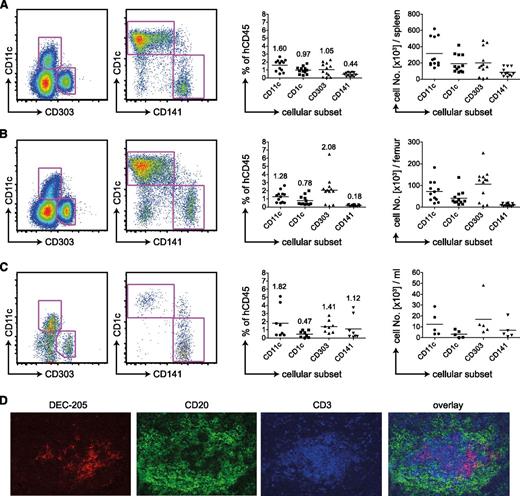
We reconstituted newborn NSG mice with CD34+ human fetal liver−derived hematopoietic progenitor cells and analyzed the DC compartment 4 months later. By flow cytometry staining, we defined human DCs as being positive for human CD45 and HLA-DR but negative for lineage markers CD3 (T cells), CD19 (B cells), CD56 (NK cells), CD14 (mainly monocytes), and CD16 (mainly NK cells; supplemental Figure 1A). We subdivided the CD11c+ cDC population into CD1c or CD141-positive cells. The pDC subset was defined as CD11c−CD303+ (Figure 1A). We detected all 3 human DC subsets in spleen (Figure 1A), BM (Figure 1B), and blood (Figure 1C). On average, DCs comprised 3% of reconstituted cells. In spleen and BM, most cDCs were CD1c+, whereas in blood CD141+ cDCs predominated. The greatest percentage of pDCs was located in the BM, exceeding 2% of human CD45+ cells in most mice. Those trends reflected the absolute numbers of DC subsets per spleen (Figure 1A), femur (Figure 1B), or milliliters of blood (Figure 1C). Per spleen, we detected on average 300 000 cDCs and 200 000 pDCs.
Human DC subsets in huNSG mice. (A) Flow cytometric staining of CD11c−, CD303+ pDCs, or CD1c+ and CD141+ on CD11c+ cDCs of the spleen (left). Samples were pregated as singlet, live cells positive for hCD45 and HLA-DR and negative for lineage, CD14, and CD16. Percentages of those subsets in relation to live, singlet, human CD45+ cells are shown in the middle. The numbers on top of the data points indicate the average percentage of human CD45-positive cells for each analyzed DC subset. Absolute numbers of DC populations are shown on the right. Graphs represent data from mice from 4 independent reconstitutions. Each data point represents one individually analyzed mouse. (B) Same as in panel A for DC subsets of the BM. (C) Same as in panel A for DC subsets of the blood. (D) Immunofluoresence microscopy for DC markers on spleen sections. DEC-205 in red as DC-marker, CD20 in green as B-cell marker, and CD3 in blue as T cell marker.
Human DC subsets in huNSG mice. (A) Flow cytometric staining of CD11c−, CD303+ pDCs, or CD1c+ and CD141+ on CD11c+ cDCs of the spleen (left). Samples were pregated as singlet, live cells positive for hCD45 and HLA-DR and negative for lineage, CD14, and CD16. Percentages of those subsets in relation to live, singlet, human CD45+ cells are shown in the middle. The numbers on top of the data points indicate the average percentage of human CD45-positive cells for each analyzed DC subset. Absolute numbers of DC populations are shown on the right. Graphs represent data from mice from 4 independent reconstitutions. Each data point represents one individually analyzed mouse. (B) Same as in panel A for DC subsets of the BM. (C) Same as in panel A for DC subsets of the blood. (D) Immunofluoresence microscopy for DC markers on spleen sections. DEC-205 in red as DC-marker, CD20 in green as B-cell marker, and CD3 in blue as T cell marker.
We analyzed the distribution of human DCs in spleens of huNSG mice by immunofluorescence microscopy on histological sections. As in flow cytometric staining (supplemental Figure 1B-C), we detected both DEC-205 high and low cells. They were of DC morphology and in T-cell zones of white pulp areas (Figure 1D). We could not subdivide those by CD1c and CD141 because the staining for these markers was unspecific.
In conclusion, we identified the main constitutive human DC subsets in primary and secondary lymphoid organs of huNSG mice. Interestingly, the frequency of the CD141+ DCs in blood and BM of huNSG mice is greater than in human blood and BM.8 Furthermore, the reconstituted cDCs in huNSG mice home to similar areas as their counterparts in human spleen.24
Ligation of RNA receptors matures human conventional and plasmacytoid DCs in vivo
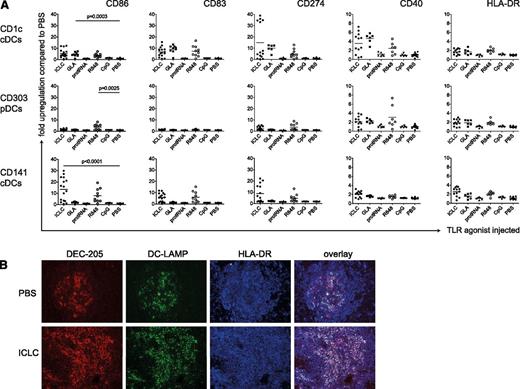
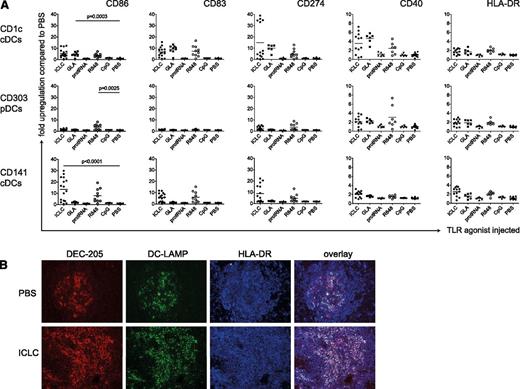
To test the functional competence of reconstituted DCs in huNSG mice, we analyzed whether intraperitoneal injection of TLR agonists matured human DCs in vivo. We used the TLR3 ligand polyICLC,25,26 the TLR4 ligand GLA,27 the TLR7/8 ligands R848,28 and protamine/RNA,29 as well as CpG as a TLR9 agonist.30 After 14 hours (supplemental Figure 2A), we sacrificed the mice and assessed surface levels of maturation markers (CD86, CD83, CD274, CD40, and HLA-DR) on the different splenic DC subsets (supplemental Figure 1D). Both cDC subsets significantly up-regulated maturation markers when injected with polyICLC, in line with TLR3 expression on CD1c+ and CD141+ cDCs in human (Figure 2A and supplemental Figure 2B). In contrast, only CD1c+ cDCs should have TLR4. Accordingly, only this cDC subset matured significantly in response to GLA. R848 not only matured cDCs but also up-regulated CD40, CD86, CD274, and HLA-DR expression on pDCs significantly, albeit to a lesser extent (Figure 2A). Neither protamine/RNA nor CpG ODN2216 phenotypically matured human DCs (Figure 2A), even though those compounds induced, albeit weak, murine cytokine production by DCs in nonreconstituted NSG mice (supplemental Figure 3A). With both being particulate formulations, however, their bioactivity might improve with intravenous compared with intraperitoneal injection. Changes in CD83, CD86, and CD274 expression were greatest after the injection of polyICLC and R848, whereas CD40 and HLA-DR expression was less affected by the TLR agonists (Figure 2A). Furthermore, CD86 was most dramatically up-regulated on CD141+ DCs after polyICLC stimulation (>10-fold). In summary, the effect of a given TLR agonist depends on the DC subset as well as on the maturation marker analyzed.
Maturation of human DC subsets upon TLR ligand injection in vivo. (A) Fold up-regulation of CD86, CD83, CD274, CD40, and HLA-DR on CD1c+ cDCs (top), CD303+ pDCs (middle), and CD141+ cDCs (bottom). HuNSG mice were injected intraperitoneally with 50 μg/mouse polyICLC, 20 μg/mouse GLA IDC 1001, 25 μg/mouse protamine/RNA, 20 μg/mouse R848, and 50 μg/mouse CpG ODN 2216 or PBS. At 14 hours after injection, splenocytes were isolated and stained for flow cytometry. Fold up-regulation was calculated from the mean fluorescence intensity (MFI) in relation to the mean of the corresponding PBS samples. The graph represents composite data from 5 independent experiments. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test. (B) Immunohistochemistry for DC markers on spleen sections. HuNSG mice were injected with PBS (top) or with polyICLC (bottom) and euthanized after 14 hours. DEC-205 serves as a DC marker and DC-LAMP and HLA-DR as DC maturation markers.
Maturation of human DC subsets upon TLR ligand injection in vivo. (A) Fold up-regulation of CD86, CD83, CD274, CD40, and HLA-DR on CD1c+ cDCs (top), CD303+ pDCs (middle), and CD141+ cDCs (bottom). HuNSG mice were injected intraperitoneally with 50 μg/mouse polyICLC, 20 μg/mouse GLA IDC 1001, 25 μg/mouse protamine/RNA, 20 μg/mouse R848, and 50 μg/mouse CpG ODN 2216 or PBS. At 14 hours after injection, splenocytes were isolated and stained for flow cytometry. Fold up-regulation was calculated from the mean fluorescence intensity (MFI) in relation to the mean of the corresponding PBS samples. The graph represents composite data from 5 independent experiments. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test. (B) Immunohistochemistry for DC markers on spleen sections. HuNSG mice were injected with PBS (top) or with polyICLC (bottom) and euthanized after 14 hours. DEC-205 serves as a DC marker and DC-LAMP and HLA-DR as DC maturation markers.
In addition, we chose the stimulus most efficacious for reconstituted cDCs in this setting, polyICLC, to study DC maturation in tissue sections. We observed an increase of DEC-205+ cells in spleens of polyICLC-treated mice (Figure 2B). Whereas unstimulated spleens only displayed small areas of DEC-205+ DC-LAMP+ cDCs, DC-LAMP+ mature cDC numbers increased dramatically after the injection of polyICLC (Figure 2B). Of note, mature cDCs were preferentially found in T-cell zones of splenic white pulp.
In general, all reconstituted constitutive DC compartments of huNSG mice matured in response to TLR agonists in vivo. However, only those targeting RNA receptors (TLR3/7/8) induced robust maturation of cDCs and pDCs.
Human conventional DC populations mature slowly in response to TLR agonists in vivo
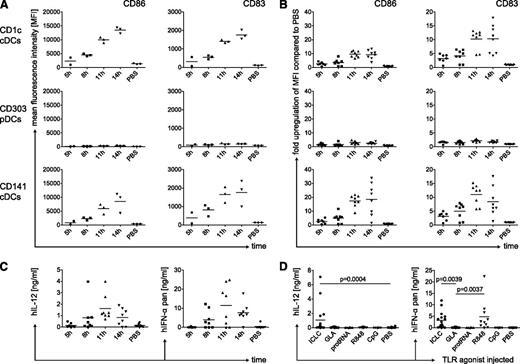
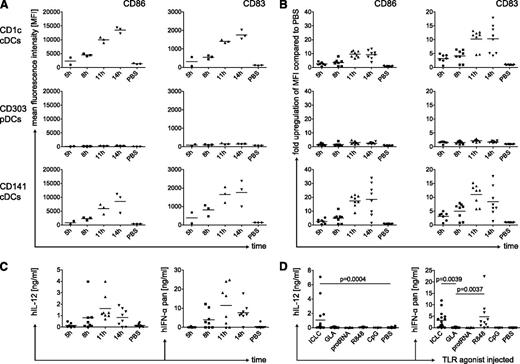
Next, we characterized the kinetics of DC maturation in huNSG mice after the injection of polyICLC. Maturation markers were maximally up-regulated at 11 to 14 hours (Figure 3A-B). Again, we detected the greatest up-regulation of CD86 on CD141+ cDCs, and pDCs were only minimally activated (Figures 2A and 3A). In general, the kinetics of DC maturation in huNSG mice resemble activation of human DCs in vitro, whereas TLR agonists activate mouse DCs considerably faster.25
Kinetics of human DC maturation and cytokine production in vivo. (A) Time-course for the up-regulation of maturation markers CD86 and CD83 on splenic DC subsets. HuNSG mice were injected with polyICLC and euthanized after the indicated time points. PBS-injected mice were sacrificed after 14 hours. Splenocytes were stained for flow cytometry and MFI for CD86 and CD83 is shown for CD1c+ cDCs (top), CD303+ pDCs (middle), and CD141+ cDCs (bottom). (B) Same as in panel A, showing fold up-regulation over the mean MFI of the PBS samples. (C) Time-course for serum cytokine levels in huNSG mice after the injection of polyICLC. HuNSG mice were injected and euthanized as in panel A. Serum cytokine levels were determined for human IL-12p70 (left) and human pan-specific IFN-α (right) by ELISA. Data represent 3 independent experiments. (D) Cytokine levels in the serum of huNSG mice injected with different TLR agonists. HuNSG mice were injected as in Figure 2A. At 11 hours after injection, mice were euthanized, and human IL-12p70 (left) as well as human pan-specific IFN-α (right) were determined in the serum by ELISA. Composite data from 5 independent experiments are shown. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test.
Kinetics of human DC maturation and cytokine production in vivo. (A) Time-course for the up-regulation of maturation markers CD86 and CD83 on splenic DC subsets. HuNSG mice were injected with polyICLC and euthanized after the indicated time points. PBS-injected mice were sacrificed after 14 hours. Splenocytes were stained for flow cytometry and MFI for CD86 and CD83 is shown for CD1c+ cDCs (top), CD303+ pDCs (middle), and CD141+ cDCs (bottom). (B) Same as in panel A, showing fold up-regulation over the mean MFI of the PBS samples. (C) Time-course for serum cytokine levels in huNSG mice after the injection of polyICLC. HuNSG mice were injected and euthanized as in panel A. Serum cytokine levels were determined for human IL-12p70 (left) and human pan-specific IFN-α (right) by ELISA. Data represent 3 independent experiments. (D) Cytokine levels in the serum of huNSG mice injected with different TLR agonists. HuNSG mice were injected as in Figure 2A. At 11 hours after injection, mice were euthanized, and human IL-12p70 (left) as well as human pan-specific IFN-α (right) were determined in the serum by ELISA. Composite data from 5 independent experiments are shown. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test.
Only stimulation of RNA receptors elicits cytokine production by human DCs in vivo
As a functional readout for DC activation, we measured human cytokines in the serum of huNSG mice at different time points after polyICLC injection. We analyzed IL-12p70 as a cDC signature cytokine and IFN-α, which is secreted at high levels by activated pDCs.6 Concentrations of those cytokines peaked 11 hours after injection (Figure 3C). Remarkably, they consistently reached approximately 1 ng/mL for IL-12p70 and up to 25 ng/mL for IFN-α.
In addition, we monitored cytokine production after we injected different TLR agonists. In line with DC maturation, injection of polyICLC or R848 induced strong cytokine production, whereas protamine/RNA and CpG did not (Figure 3D). Serum IL-12 and IFN-α concentrations varied after polyICLC injection between experiments, possibly resulting from hematopoietic progenitor cell donor variation or differences in the used TLR agonist batches. These cytokines, however, were coregulated in individual huNSG mice, and reconstitution levels of DC subsets were more homogeneous than these variations in cytokine production. Surprisingly, GLA did not elicit even low human cytokine levels, despite its capacity to mature CD1c+ cDCs (Figure 2A).
This finding suggests that RNA receptor (TLR3/7/8) ligation fully matures human cDCs and pDCs with up-regulation of costimulatory molecules and cytokine production, whereas TLR4 and 9 agonists only partially mature DCs in this experimental setting. Particularly, TLR4 agonists seem to be poor activators of cytokine production by constitutive human DC subsets but potently activate mouse DCs to produce IL-6 and IL-12 (supplemental Figure 3A).
Depletion of CD141+ cDCs reduces IFN-α levels in the serum of huNSG mice
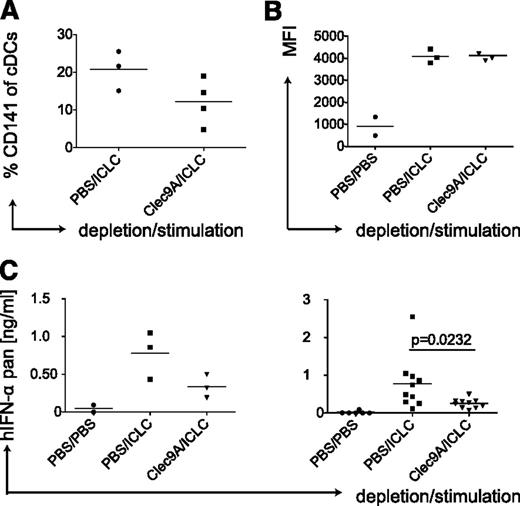
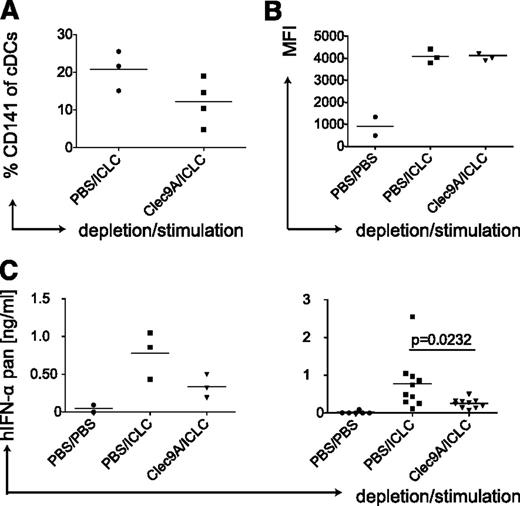
Because polyICLC, which mainly matures cDCs (Figure 2A), induced similar serum levels of IFN-α as detected after injection of the pDC activating TLR ligand R848 (Figure 3D), we hypothesized that human cDCs could be a nonrecognized source for considerable amounts of IFN-α. Furthermore, polyICLC matured both human cDC subsets and led to IFN-α production in vivo, whereas GLA matured only CD1c+ cDCs without detectable cytokine secretion (Figures 2A and 3D), suggesting that the CD141+ cDC subset could be producing the IFN-α. To test this, we depleted CD141+ cDCs in huNSG mice by injecting an antibody recognizing the CD141+ cDC-specific molecule Clec9A.31,32 Depletion of CD141+ cDCs was transient and partial (Figure 4A) and left the other DC populations mostly unaltered (supplemental Figure 3B). Subsequently, we injected polyICLC and analyzed serum IFN-α levels, only considering mice showing equal CD86 expression on splenic CD1c+ cDCs (Figure 4B). A decrease in CD141+ cDCs resulted in significantly diminished IFN-α levels (Figure 4C). Taken together, data generated in huNSG mice strongly suggest that the CD141+ cDC subset is a main source of IFN-α production after stimulation with synthetic dsRNA.
Depletion of human CD141-positive DCs reduces IFN-α levels after polyICLC stimulation in huNSG mice. (A) CD141+ cDC depletion efficiency in huNSG mice. HuNSG mice were injected with PBS or 10 μg of αClec9A antibody intraperitoneally on 3 consecutive days. At 10 hours after the last injection, mice were injected with polyICLC and euthanized 11 hours later. Splenocytes were stained for flow cytometry, and the percentage of CD141+ cDCs within the CD11c-positive gate was determined. (B) CD1c+ cDC maturation in huNSG mice without and with CD141+ cDC depletion. Same as in panel A with flow cytometry staining for CD86 on CD1c+ cDCs. (C) Serum levels of IFN-α in huNSG mice without and with CD141+ cDC depletion. After injections, as in panel A, serum cytokine levels were determined for human pan-specific IFN-α by ELISA. Left, a representative experiment; right, plot: composite data from 3 experiments. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test.
Depletion of human CD141-positive DCs reduces IFN-α levels after polyICLC stimulation in huNSG mice. (A) CD141+ cDC depletion efficiency in huNSG mice. HuNSG mice were injected with PBS or 10 μg of αClec9A antibody intraperitoneally on 3 consecutive days. At 10 hours after the last injection, mice were injected with polyICLC and euthanized 11 hours later. Splenocytes were stained for flow cytometry, and the percentage of CD141+ cDCs within the CD11c-positive gate was determined. (B) CD1c+ cDC maturation in huNSG mice without and with CD141+ cDC depletion. Same as in panel A with flow cytometry staining for CD86 on CD1c+ cDCs. (C) Serum levels of IFN-α in huNSG mice without and with CD141+ cDC depletion. After injections, as in panel A, serum cytokine levels were determined for human pan-specific IFN-α by ELISA. Left, a representative experiment; right, plot: composite data from 3 experiments. Each data point represents one individually analyzed mouse. Statistical analysis was performed with the Mann-Whitney U test.
Human donor-derived CD141+ cDCs are potent producers of IFN-α
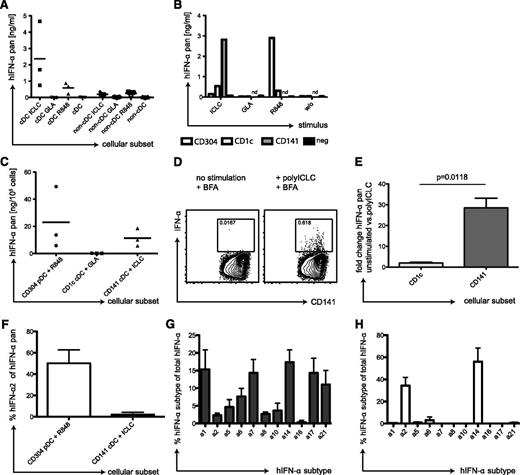
To confirm this hypothesis in a strictly human setting, we isolated cDCs from peripheral blood mononuclear cells (PBMCs) of healthy volunteers. We stimulated the cDC and the non-cDC fractions with GLA, polyICLC, or R848 and analyzed IFN-α in the supernatants. Indeed, cDCs produced considerable amounts of IFN-α in response to polyICLC (Figure 5A). They secreted much less IFN-α in response to R848 and, in line with huNSG data, none after GLA stimulation. The non-cDC fraction produced only low IFN-α levels in response to polyICLC (Figure 5A), probably originating from residual cDCs. For R848 stimulation of non-cDCs, we attributed at least part of the IFN-α to pDCs by intracellular cytokine staining using flow cytometry (not shown).
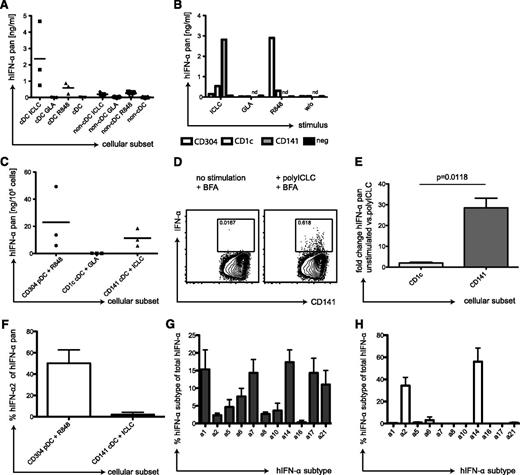
IFN-α production by CD141+ cDCs in response to TLR3 ligand. (A) Human cDCs produce IFN-α in response to polyICLC. PBMCs of 3 donors were separated into a cDC and a non-cDC fraction by magnetic-activated cell sorting (MACS) separation. Then, 2 × 105 cells were plated and stimulated for 14 hours with 25 μg/mL polyICLC, 5 μg/mL GLA, or 4 μg/mL R848. Pan IFN-α levels were determined in the cell supernatants after 14 hours by the use of ELISA. (B) IFN-α production by DC subsets after TLR stimulation. PBMCs were sequentially separated into CD141+ cDCs, CD304+ pDCs, and CD1c+ cDCs by MACS separation. A total of 0.25 × 105 cells of the positive fractions as well as the final negative fraction were plated, stimulated, and IFN-α levels were determined as in panel A. (C) Same as in panel B but showing composite data from 3 donors. (D) Intracellular staining for IFN-α in CD141+ cDCs stimulated with polyICLC. cDCs were isolated from PBMCs by MACS separation and stimulated with polyICLC for 9 hours with 10 μg/mL brefeldin A. Cells were stained for surface markers followed by intracellular cytokine staining for IFN-α. Gating was performed as described in Figure 1A, and plots display the CD141+ cDCs. Numbers indicate the percentage of IFN-α producing cells with (right) and without (left) polyICLC stimulation. (E) Fold change of intracellular levels of IFN-α in 4 donors. Same as in panel D but shown for the CD1c+ and CD141+ cDCs fractions. Statistical analysis was performed with a paired t test. (F) IFN-α secretion by DC subsets. Cellular supernatants shown in panel C were run on pan-IFN-α−specific and IFN-α2−specific ELISAs in parallel. The plot shows composite data for the percentage of IFN-α2 of the pan IFN-α amount for 3 donors. Error bars indicate SD. (G) Transcriptional profiles for IFN-α subtypes in CD141+ cDCs. CD141+ cDCs were isolated from 3 donors by MACS and stimulated with polyICLC for 8 hours before RNA isolation. Sybr-green based Q-PCR was performed for the different IFN-α subtypes and transcript numbers were related to 1000 GAPDH copies. Results are displayed as percentage of the indicated IFN-α subtype transcript number in relation to the total IFN-α count. Error bars indicate SD. (H) Same as in panel H but for CD304+ pDCs stimulated with R848 for 1 hour.
IFN-α production by CD141+ cDCs in response to TLR3 ligand. (A) Human cDCs produce IFN-α in response to polyICLC. PBMCs of 3 donors were separated into a cDC and a non-cDC fraction by magnetic-activated cell sorting (MACS) separation. Then, 2 × 105 cells were plated and stimulated for 14 hours with 25 μg/mL polyICLC, 5 μg/mL GLA, or 4 μg/mL R848. Pan IFN-α levels were determined in the cell supernatants after 14 hours by the use of ELISA. (B) IFN-α production by DC subsets after TLR stimulation. PBMCs were sequentially separated into CD141+ cDCs, CD304+ pDCs, and CD1c+ cDCs by MACS separation. A total of 0.25 × 105 cells of the positive fractions as well as the final negative fraction were plated, stimulated, and IFN-α levels were determined as in panel A. (C) Same as in panel B but showing composite data from 3 donors. (D) Intracellular staining for IFN-α in CD141+ cDCs stimulated with polyICLC. cDCs were isolated from PBMCs by MACS separation and stimulated with polyICLC for 9 hours with 10 μg/mL brefeldin A. Cells were stained for surface markers followed by intracellular cytokine staining for IFN-α. Gating was performed as described in Figure 1A, and plots display the CD141+ cDCs. Numbers indicate the percentage of IFN-α producing cells with (right) and without (left) polyICLC stimulation. (E) Fold change of intracellular levels of IFN-α in 4 donors. Same as in panel D but shown for the CD1c+ and CD141+ cDCs fractions. Statistical analysis was performed with a paired t test. (F) IFN-α secretion by DC subsets. Cellular supernatants shown in panel C were run on pan-IFN-α−specific and IFN-α2−specific ELISAs in parallel. The plot shows composite data for the percentage of IFN-α2 of the pan IFN-α amount for 3 donors. Error bars indicate SD. (G) Transcriptional profiles for IFN-α subtypes in CD141+ cDCs. CD141+ cDCs were isolated from 3 donors by MACS and stimulated with polyICLC for 8 hours before RNA isolation. Sybr-green based Q-PCR was performed for the different IFN-α subtypes and transcript numbers were related to 1000 GAPDH copies. Results are displayed as percentage of the indicated IFN-α subtype transcript number in relation to the total IFN-α count. Error bars indicate SD. (H) Same as in panel H but for CD304+ pDCs stimulated with R848 for 1 hour.
We next tested which cDC subset in the human samples produces the IFN-α. Therefore, we enriched CD141+ cDCs, CD304+ pDCs, and CD1c+ cDCs sequentially from PBMCs (supplemental Figure 4A-D); stimulated them with TLR agonists; and measured IFN-α in the supernatants. Because of the low CD141+ cDC recovery, we only tested polyICLC stimulation for this subset, whereas the other DC populations were incubated with all TLR stimuli. CD141+ cDC supernatants contained by far the greatest concentration of IFN-α after polyICLC stimulation (Figure 5B). Despite donor variation, CD141+ cDCs of all donors tested produced high amounts of IFN-α in response to polyICLC. Only with R848 did pDCs produce IFN-α copiously (Figure 5B-C). In contrast, CD1c+ cDCs did not secrete IFN-α after stimulation with GLA (Figure 5B-C), which is the TLR agonist inducing strong up-regulation of maturation markers on CD1c+ cDCs in huNSG mice (Figure 2A). We also established an intracellular cytokine staining for IFN-α in CD141+ cDCs. In enriched, polyICLC-stimulated cDCs, we detected an IFN-α−specific signal in CD141+ cDCs (Figure 5D), which was significantly greater than the fold-increase in CD1c+ cDCs (Figure 5E). Moreover, we compared the IFN-α subtypes produced by pDCs after stimulation with R848 in contrast to polyICLC-stimulated CD141+ cDCs. Although pDCs produced 50% of IFN-α2, this subtype only accounts for 5% of the IFN-α produced by CD141+ cDCs (Figure 5F). This finding was reflected in the transcriptional profiles of CD141+ cDCs, which revealed a much broader IFN-α repertoire than pDCs, with very low IFN-α2 transcription (Figure 5G-H). Thus, CD141+ cDCs from human blood produce significant amounts of IFN-α, in response to TLR3 stimulation, that consist of other IFN-α subtypes than those produced by pDCs in response to TLR7 ligation.
Priming of human CD4+ T-cell responses after antigen targeting to DEC-205
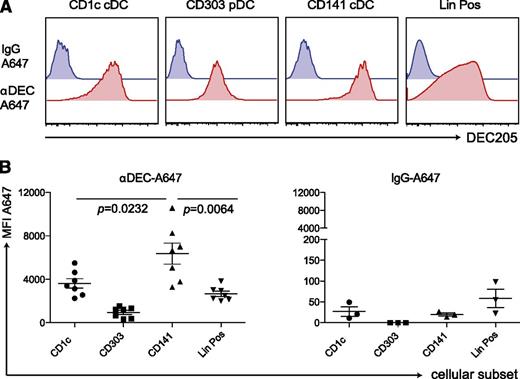
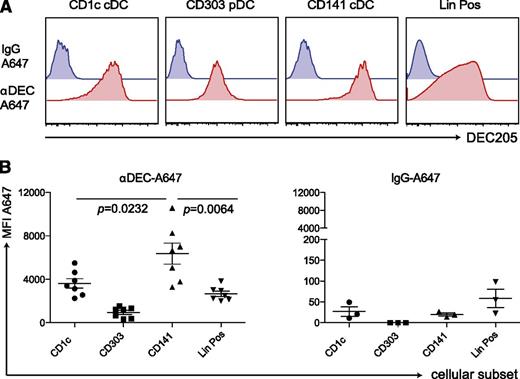
Finally, we tested the ability of this unique CD141+ cDC subset to prime immune responses in vivo. Because they highly express DEC-205, we aimed to target them by an αDEC-205 mAb with polyICLC as an adjuvant that mainly elicits immunostimulatory cytokine production by this DC subset. To validate specific targeting, we injected 5 μg of Alexa-647−labeled αDEC-205 or the labeled isotype control mAb into huNSG mice. After 3 to 5 hours, we examined antibody uptake by different splenic DC subsets (Figure 6A-B). Unlike the isotype mAb, the αDEC-205 mAb labeled most of the CD141+ cDCs. αDEC-205 also bound other DC subsets and leukocyte populations, as shown in mice33 and in line with DEC-205 expression on other human cell populations.34,35 However, labeling by αDEC-205 mAb reached the greatest levels in CD141+ cDCs among different DC subsets and lineage-positive cells. This finding infers that antigens targeted to DEC-205 are most efficiently taken up by CD141+ cDCs and that combining this antigen formulation with polyICLC that triggers immunostimulatory cytokine production by this DC subset should only render CD141+ DCs capable of priming T-cell responses.
αDEC-A647 is taken up most efficiently by CD141+ cDCs in vivo. (A) 5 μg of αDEC-A647 or IgG-A647 was injected intraperitoneally into huNSG mice. After 3 hours, the mice were euthanized, and antibody uptake into the different cellular subsets in the spleen was analyzed by fluorescence-activated cell sorting. (B) Same as in panel A but shown for mice from 2 independent experiments, including 3- and 5-hour time points, which showed similar results. Each data point represents one individually analyzed mouse. Statistical analysis was performed with paired t tests.
αDEC-A647 is taken up most efficiently by CD141+ cDCs in vivo. (A) 5 μg of αDEC-A647 or IgG-A647 was injected intraperitoneally into huNSG mice. After 3 hours, the mice were euthanized, and antibody uptake into the different cellular subsets in the spleen was analyzed by fluorescence-activated cell sorting. (B) Same as in panel A but shown for mice from 2 independent experiments, including 3- and 5-hour time points, which showed similar results. Each data point represents one individually analyzed mouse. Statistical analysis was performed with paired t tests.
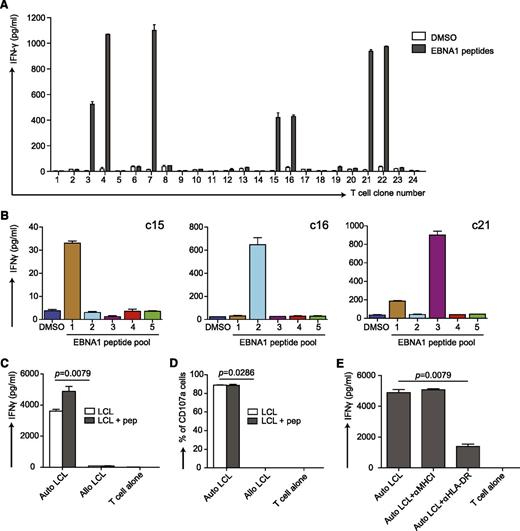
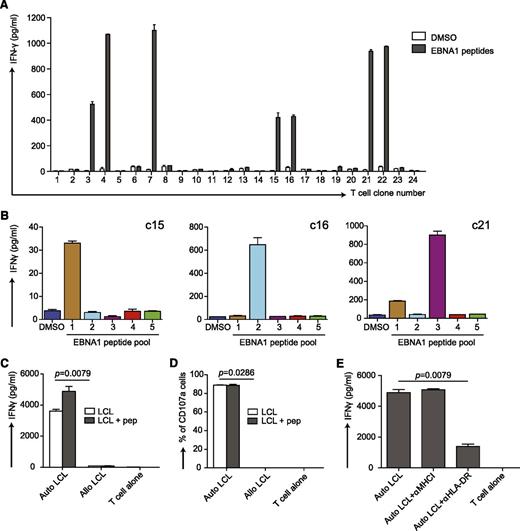
We have previously shown that the combination of poly(I:C) and DEC-205−targeted antigen elicits peptide-specific, T-cell responses against the Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) in huNSG mice.22 To further characterize this T-cell response, we repeated the immunization experiment with αDEC-205-EBNA1 mAb but used the more stable polyICLC instead to preferentially mature CD141+ DCs (supplemental Figure 5A). From the splenocytes of the responding huNSG mice, we cloned the EBNA1-specific T cells. Several clones responded to EBNA1 peptides (Figure 7A), and all were positive for human CD45, CD3, and CD4. Some reacted to only 1 of 5 EBNA1 peptide subpools (Figure 7B). Notably, these clones were specific for different epitopes as they reacted to different subpools (Figure 7B). This finding indicates that targeting CD141+ cDCs by linking EBNA1 to DEC-205 with polyICLC as adjuvant induces T-cell responses with broad epitope specificities in huNSG mice.
Characterization of T-cell responses induced by vaccination with αDEC-205-EBNA1 and polyICLC in huNSG mice. (A) αDEC-205-EBNA1 plus polyICLC vaccination primes specific T cells in huNSG mice. HuNSG mice were vaccinated with 5 μg of IgG-EBNA1 or αDEC-205-EBNA1 with the use of 50 μg of polyICLC as adjuvant and boosted with the same dose of antibodies and adjuvant 4 weeks later. The mice were euthanized 6 to 8 weeks after the boost. T-cell clones were generated by limiting dilution cloning of specific T cells after an IFN-γ capture assay after restimulation with 5μM EBNA1 peptide library for 12 hours. IFN-γ secretion was analyzed by ELISA in the supernatant. (B) The specificity of 3 expanded T-cell clones (c15, c16, c21) are shown after restimulation with 5 different peptide subpools of EBNA1 by measuring IFN-γ secreted into the supernatant. Data are represented as mean ± SD from duplicate or triplicate wells of IFN-γ ELISA. (C) The EBNA1 specific T-cell clone c16 recognizes autologous LCLs. Autologous LCLs (Auto LCL) or allogeneic LCLs (Allo LCL), unmanipulated or loaded for 1 hour with 5μM EBNA1 peptides, were incubated with the expanded EBNA1-specific T-cell clone c16 (E:T=1:2), T-cell activity was determined after 18 hours by measuring released IFN-γ by ELISA. (D) Same as in panel C but analyzing T-cell function by CD107a staining after 6 hours of coculture. (E) The T-cell response of clone c16 is MHC class II-restricted. T-cell clone c16 was exposed to the autologous LCLs in the absence or presence of the indicated HLA blocking antibodies and IFN-γ was measured in the supernatants by ELISA. One representative experiment of 2 is shown. Statistical analysis was performed by Mann-Whitney U tests, and data are represented as mean ± SD.
Characterization of T-cell responses induced by vaccination with αDEC-205-EBNA1 and polyICLC in huNSG mice. (A) αDEC-205-EBNA1 plus polyICLC vaccination primes specific T cells in huNSG mice. HuNSG mice were vaccinated with 5 μg of IgG-EBNA1 or αDEC-205-EBNA1 with the use of 50 μg of polyICLC as adjuvant and boosted with the same dose of antibodies and adjuvant 4 weeks later. The mice were euthanized 6 to 8 weeks after the boost. T-cell clones were generated by limiting dilution cloning of specific T cells after an IFN-γ capture assay after restimulation with 5μM EBNA1 peptide library for 12 hours. IFN-γ secretion was analyzed by ELISA in the supernatant. (B) The specificity of 3 expanded T-cell clones (c15, c16, c21) are shown after restimulation with 5 different peptide subpools of EBNA1 by measuring IFN-γ secreted into the supernatant. Data are represented as mean ± SD from duplicate or triplicate wells of IFN-γ ELISA. (C) The EBNA1 specific T-cell clone c16 recognizes autologous LCLs. Autologous LCLs (Auto LCL) or allogeneic LCLs (Allo LCL), unmanipulated or loaded for 1 hour with 5μM EBNA1 peptides, were incubated with the expanded EBNA1-specific T-cell clone c16 (E:T=1:2), T-cell activity was determined after 18 hours by measuring released IFN-γ by ELISA. (D) Same as in panel C but analyzing T-cell function by CD107a staining after 6 hours of coculture. (E) The T-cell response of clone c16 is MHC class II-restricted. T-cell clone c16 was exposed to the autologous LCLs in the absence or presence of the indicated HLA blocking antibodies and IFN-γ was measured in the supernatants by ELISA. One representative experiment of 2 is shown. Statistical analysis was performed by Mann-Whitney U tests, and data are represented as mean ± SD.
To evaluate the effector functions of these clones, we generated autologous lymphoblastoid cell lines (LCLs) because they serve as an in vitro model for EBV-positive tumors. This was achieved by infecting huNSG mice from the same reconstitution as the immunized mice with EBV and subsequent isolation of LCLs from their splenocytes. The T-cell clones were incubated with those autologous or allogeneic LCLs loaded with or without EBNA1 peptides, and we monitored IFN-γ production. Autologous LCLs induced strong responses of the EBNA1-specific T-cell clones, whereas allogeneic LCLs did not (Figure 7C and supplemental Figure 5B). In addition, we evaluated the cytotoxic potential of these clones toward LCLs. In line with IFN-γ secretion, they expressed the degranulation marker CD107a in autologous LCL cocultures (Figure 7D and supplemental Figure 5B). Furthermore, we could block responses of the CD4+ T-cell clone c16 by αHLA-DR mAb but not αHLA-class I mAb, indicating that the T-cell response toward LCLs is strictly HLA class II restricted (Figure 7E). Taken together, this finding suggests that targeting antigen to DEC-205 in combination with a strong stimulus for CD141+ cDC activation as adjuvant elicits CD4+ T-cell responses with protective function against autologous EBV-transformed B cells. In addition, it reports for the first time at the clonal-level, antigen-specific CD4+ T-cell responses in mice with reconstituted human immune system components.
Discussion
This study shows that DCs reconstituted in huNSG mice resemble their counterparts in humans. Human CD1c+ cDCs respond to the TLR3 agonist polyICLC, the TLR4 agonist GLA, and the TLR7/8 agonist R848 like in huNSG mice. In contrast, CD141+ cDCs lack TLR4 and are therefore unresponsive to GLA. Because high IL-12p70 production is a hallmark of CD141+ conventional DCs,8,9 we mainly observed it in response to dsRNA that matures this particular subset of human cDCs. As anticipated, pDCs up-regulate maturation markers after TLR7 stimulation with R848. However, in our hands weaker TLR7/8 agonists, such as protamine/RNA, and TLR9 agonists, such as CpG, did not mature pDCs. Therefore, RNA receptor engagement seems to be most efficient for human DC stimulation in vivo.
Interestingly, we observed not only robust human IL-12p70 production in response to TLR3 ligation but also efficient IFN-α production, which could not be enhanced upon R848-mediated maturation of pDCs (data now shown). Although murine IFN-α in response to poly(I:C) stimulation is mainly produced by nonhematopoietic cells,25,36 huNSG mice lack human nonhematopoietic tissues and, therefore, produce significant human IFN-α amounts with their hematopoietic cells. Among human hematopoietic cells, CD141+ cDCs have been proposed as type I and III IFN sources, producing IFN-β and -λ, respectively.8,37
In addition, we report here that this subset is also a prominent source of IFN-α after dsRNA stimulation in vitro and in vivo. In vivo, polyICLC stimulation leads to similar serum IFN-α levels in huNSG mice as observed in healthy volunteers, namely 5 ng/mL.38 This finding correlates with phenotypic maturation of CD141+ cDC maturation and reaches similar levels as R848 stimulation. Of note, extrapolating from all analyzed organs, the total cell number of CD141+ cDCs should be 4× lower than for pDCs in huNSG mice, yet these cells raise serum IFN-α concentrations to similar levels. Furthermore, in peripheral blood of healthy human donors, IFN-α production upon polyICLC stimulation resides mainly in cDCs, especially after CD141 enrichment. Of note, CD141+ cell-enriched populations produce similar IFN-α levels as pDCs after R848 stimulation. Therefore, CD141+ cDCs have the dual capacity to produce IL-12p70 and type I IFN, which makes them an attractive adjuvant target. RNA receptor ligands could serve as agonists to mature this cDC subset during vaccination.
Apart from harnessing CD141+ cDCs for vaccination, it would be interesting to assess their contribution to IFN-α production in an infection model. However, this is hampered by the transient nature of CD141+ cDC depletion in huNSG mice. Of note, this finding is in line with the fast repopulation of DCs after diphtheria toxin injection in CD11c-DTR transgenic mice.39 Furthermore, most infectious agents activate multiple cell types by various TLRs, which would mask the effect of CD141+ cDC after stimulation by dsRNA molecules. However, one could also envision a role of CD141+ cDC in resolving viral infection independent of absolute IFN-α production. As shown (Figure 5F-H), pDCs mainly produce IFN-α2 and IFN-α14. CD141+ cDCs, on the other hand, secrete several IFN-α subtypes, which could be needed to orchestrate a protective immune response against certain viral infections.
Although it remains so far unclear whether functional CD141+ cDCs also reconstitute in other immune-compromised mouse strains, reconstituted NOD-scid mice have cDCs (lin–CD11c+HLA-DR+) and pDCs (lin–IL-3R+HLA-DR+).40-42 Although they develop less DCs outside the BM than huNSG mice (this study and Ishikawa et al43 ), human DCs from reconstituted NOD-scid mice can stimulate alloreactive human T-cell responses in vitro.40-43 Furthermore, they up-regulate maturation markers and secrete IFN-α upon influenza virus infection40 and produce IL-12 after the administration of lipopolysaccharide41 or poly(I:C).42 Because functional human DCs reconstitute in immune compromised mice but NSG mice reconstitute especially high numbers of all human constitutive DC populations in spleen and blood, huNSG mice should be further explored for vaccine development.
We had previously observed that antigen targeted to DEC-205 on DCs with poly(I:C) as adjuvant induced IFN-γ−producing T cells in huNSG mice.22 Similarly, polyICLC plus DEC-205-targeted antigen elicited T-cell responses in nonhuman primates,44 and in this study, CD4+ T-cell responses in huNSG mice. Although antigen-specific CD8+ T-cell responses have been documented in mice with reconstituted human immune system components,20,45-47 our study shows for the first time antigen-specific CD4+ T-cell responses, which could be verified at the clonal level, in one of these models. Interestingly, both DEC-205 and TLR3 expression are greatest on human CD141+ cDCs (Robbins et al10 and this study), and, therefore, this antigen/adjuvant formulation might preferentially harnesses this small, but potent DC population for vaccination. Indeed, DEC-205 targeting of antigens to the mouse counterpart of CD141+ cDCs, CD8α+ cDCs, enhances priming of T-cell responses 100-fold, and the targeting to other receptors like Langerin and CLEC9A on CD8α+ cDCs similarly augments vaccine-induced immune responses.33 With the additional IFN-α production by this DC subset in humans, it becomes even more attractive for vaccine development. Furthermore, the role of CD141+ cDCs in innate and adaptive immunity to important human pathogens as well as in inducing tolerance in autoimmune settings could be explored in huNSG mice. In addition, they should be important tools to test vaccine regiments in a preclinical setting with hopefully improved predictive value for immunogenicity in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants to C.M. from the National Cancer Institute (R01CA108609), the Sassella Foundation (10/02, 11/02 and 12/02), Cancer Research Switzerland (KFS-02652-08-2010), the Association for International Cancer Research (11-0516), KFSPMS and KFSPHLD of the University of Zurich, the Vontobel Foundation, the Baugarten Foundation, the EMDO Foundation, the Sobek Foundation, Fondation Acteria, Novartis, and the Swiss National Science Foundation (310030_143979 and CRSII3_136241). S.M., C.S.L., and P.C.R. were supported by junior research fellowships from the University of Zürich. C.S.L. was also supported by the Croucher Foundation Hong Kong.
Authorship
Contribution: S.M. and C.S.L. designed and performed research; P.C.R., M.P., and L.D.V. performed experiments; R.M.S. and C.M. designed research; G.B., S.P., A.M.S., A.D., and J.S. contributed essential information or vital reagents; and S.M., C.S.L., and C.M. wrote the manuscript.
Conflict-of-interest disclosure: A.M.S. heads Oncovir Inc., which provided polyICLC. A.D. and J.S. work for Miltenyi Biotec, which provided the anti-Clec9A antibody. The remaining authors declare no competing financial interests.
Ralph M. Steinman died on September 30, 2011.
Correspondence: Christian Münz, Viral Immunobiology, Institute of Experimental Immunology, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; e-mail: christian.muenz@uzh.ch.
References
Author notes
S.M. and C.S.L. contributed equally to this study.