Abstract
We have recently identified T cells as important mediators of ischemic brain damage, but the contribution of the different T-cell subsets is unclear. Forkhead box P3 (FoxP3)–positive regulatory T cells (Tregs) are generally regarded as prototypic anti-inflammatory cells that maintain immune tolerance and counteract tissue damage in a variety of immune-mediated disorders. In the present study, we examined the role of Tregs after experimental brain ischemia/reperfusion injury. Selective depletion of Tregs in the DEREG mouse model dramatically reduced infarct size and improved neurologic function 24 hours after stroke and this protective effect was preserved at later stages of infarct development. The specificity of this detrimental Treg effect was confirmed by adoptive transfer experiments in wild-type mice and in Rag1−/− mice lacking lymphocytes. Mechanistically, Tregs induced microvascular dysfunction in vivo by increased interaction with the ischemic brain endothelium via the LFA-1/ICAM-1 pathway and platelets and these findings were confirmed in vitro. Ablation of Tregs reduced microvascular thrombus formation and improved cerebral reperfusion on stroke, as revealed by ultra-high-field magnetic resonance imaging at 17.6 Tesla. In contrast, established immunoregulatory characteristics of Tregs had no functional relevance. We define herein a novel and unexpected role of Tregs in a primary nonimmunologic disease state.
Key Points
Regulatory T cells are promoters of ischemic stroke by inducing dysfunction of the cerebral microvasculature.
Introduction
Ischemic stroke induces a profound local inflammatory response involving various types of immune cells that transmigrate across the activated blood-brain barrier to invade the brain in a timed fashion.1 Although previous research mainly focused on the role of innate immune cells,2 recent evidence suggests that T cells, which belong to the adaptive immune system, also contribute critically to stroke development, especially in the early phase.3 T cells have been identified in the postischemic brain as soon as 24 hours after reperfusion,4 and Abs directed against vascular adhesion receptors expressed on the brain endothelium or leukocyte very late antigen-4 (VLA-4) expressed on lymphocytes inhibited T-cell transmigration and reduced tissue damage in models of stroke.5
We and others showed recently that recombination activating gene (Rag1)–deficient mice, which lack functional T cells, are largely resistant against ischemic neurodegeneration.6-8 T cell–mediated brain damage became manifest by 24 hours after transient middle cerebral artery occlusion (tMCAO) and did not depend on antigen recognition or costimulation.8 This clearly argues against TCR-driven mechanisms of tissue damage and suggests instead that T cells act detrimentally in ischemic stroke through antigen-independent pathways, at least during the early phase. Moreover, Rag1−/− mice did not display a gross defect in thrombus formation after artificial vessel wall injury, which could easily explain the stroke protective phenotype in these animals.8
Although the deleterious effects of T cells in stroke pathophysiology are well accepted, the functional relevance of the different T-cell subsets for stroke progression is less clear, as is their pathologic contribution at the different stages of cerebral ischemia (ie, acute versus chronic). Using adoptive cell transfer in Rag1−/− mice, we could demonstrate that natural killer T cells (NKT cells) and γδ T cells do not mediate early ischemic brain damage after tMCAO.8 In contrast, transfer of CD4+ T cells into Rag1−/− mice fully restored the susceptibility for stroke.8 Within the population of CD4+ T cells, conventional Th cells and regulatory T cells (Tregs) can be distinguished. Tregs are phenotypically determined by different cell-surface expression markers such as CD25 and the intracellular transcription factor Forkhead box P3 (FoxP3).9 Tregs are usually considered as prototypic anti-inflammatory cells that control and limit antigen-specific immune reactions. Although it is likely that Tregs exert beneficial functions in inflammatory disorders of the CNS,10 their pathologic relevance for ischemic stroke, which is regarded a primary nonimmunologic disease, remains to be established. In the present study, we identify Tregs as important mediators of ischemic neurodegeneration. This is the first description of a detrimental Treg effect in a nonimmunologic disease state.
Methods
A detailed description of the methods used in the present study is provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mice
Animal experiments were approved by governmental authorities. A total of 687 male mice were included in the study. For the Treg-depletion experiments, we used 6- to 8-week-old male DEREG mice.11 Age- and sex-matched C57Bl/6 mice (Charles River Laboratories) served as controls. To ablate Tregs, DEREG mice were IP injected with 1 μg of diphtheria toxin (Merck) once daily for 3 consecutive days before tMCAO.11 Depletion and spontaneous reconstitution of Tregs were confirmed by flow cytometry (supplemental Figure 1). For adoptive transfer experiments, lymphocyte-deficient Rag1−/− mice (The Jackson Laboratory) were used as recipients. Il-17−/− mice,12 Ifnγ−/− mice (The Jackson Laboratory), Ccr6−/− mice (The Jackson Laboratory), DEREG mice,11 DEREG × scurfy mice,13 and C57Bl/6 wild-type mice functioned as cell donors. We blocked lymphocyte function-associated antigen 1 (LFA-1) in vivo by injecting an anti-CD11a Ab (5 mg/kg IP; Serotec). For platelet-depletion experiments, antiplatelet serum (10 μL in PBS IP; Accurate Chemical and Scientific) was applied (supplemental Figure 2). Removal of circulating neutrophils from Treg-depleted DEREG mice was achieved by IP injecting 75 μg of an anti–neutrophil serum (ANS, RB6-8C5; eBiosciences)14 or control serum (AIS403; Accurate Chemical and Scientific; supplemental Figure 3).
Ischemia model
Focal cerebral ischemia was induced for 30 minutes or 60 minutes by tMCAO.8 The detailed stroke study population is given in supplemental Table 1. DEREG mice, Rag1−/− mice, and C57Bl/6 wild-type mice were controlled for critical physiologic and anatomical parameters (supplemental Figures 4-7 and supplemental Table 2). We calculated infarct volumes from 2,3,5-triphenyltetrazolium chloride (TTC)–stained brain slices. All stroke experiments were performed in accordance with the ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). Mice were randomly assigned to the operators by an independent person not involved in data analysis. We performed surgery and evaluation of all readout parameters while being blinded to the experimental groups.
Functional outcome tests
We assessed the Bederson score and the grip test score to monitor neurologic function.8
MRI
To analyze infarct dynamics and to scan for possible intracerebral bleedings serial stroke assessment by magnetic resonance imaging (MRI) was performed as described previously.15 We used multimodal ultra-high-field MRI at 17.6 Tesla to measure cerebral blood flow (CBF), diffusion-weighted-imaging, and T2 relaxometry.16
Cell separation and adoptive transfer
CD4+CD25+ Tregs, CD4+CD25+FoxP3+ Tregs, and the non-Treg cell populations were isolated from single-cell suspensions of spleens and lymph nodes of donor mice, resuspended to 750 000 cells in 100 μL of PBS, and IV injected in Rag1−/− or C57Bl/6 recipient mice 24 hours before tMCAO. A purity of ≥ 80% was achieved for all experiments.
Thrombosis assays
Platelet adhesion under flow conditions and intravital microscopy of thrombus formation in FeCl3-injured mesenteric arterioles was performed as described previously.17
Statistics
All values are expressed as means ± SD except for the neurologic scores, which are depicted as scatter plots including median. Numbers of animals (n = 10) necessary to detect a standardized effect size on infarct volumes ≥ 0.25 (DEREG vs DEREG + diphtheria toxin) were calculated via a priori sample size analysis. For statistical analysis, the Prism Version 5.0 software package (GraphPad) was used. Data were analyzed by 1- or 2-way ANOVA with posthoc Bonferroni adjustment for P values. If only 2 groups were compared, the 2-tailed Student t test was applied. P < .05 was considered statistically significant.
Results
Kinetics and localization of Tregs in ischemic stroke
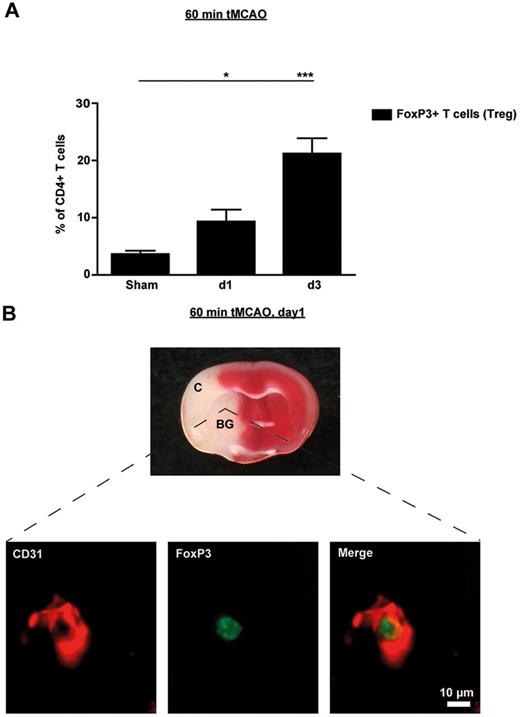
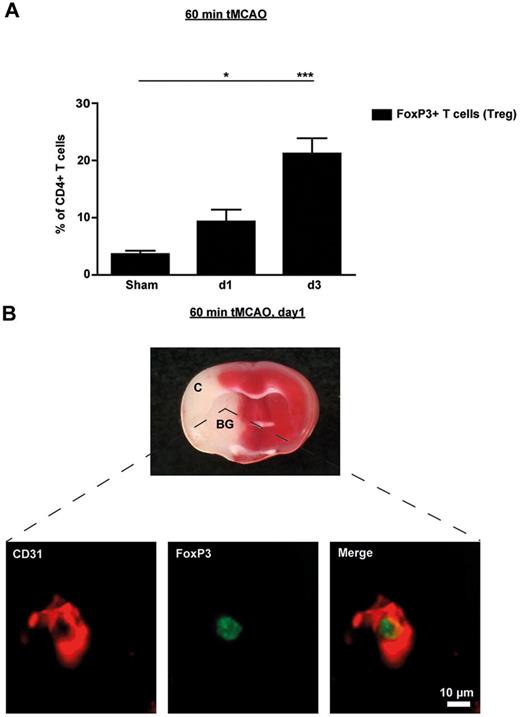
In a first set of experiments, we investigated the kinetics of FoxP3+ T-cell (Treg) infiltration into the brains of wild-type mice after tMCAO by flow cytometry. Frequencies of FoxP3-expressing Tregs in the brain among the total number of CD4+ T cells was more than doubled as early as 24 hours after tMCAO compared with sham operated mice (n = 5, P < .05) and further increased until day 3 (n = 5, P < .0001), thereby confirming previous results (Figure 1A).18 Perfusion of mice before brain sampling was omitted in these experiments, so these numbers also include FoxP3+ Tregs in the intravascular compartment. In contrast, the proportion of Tregs in the peripheral blood was significantly down-regulated on day 1 after tMCAO (n = 5, P < .05; supplemental Figure 8) which is consistent with recent observations in stroke patients.19 The decrease in blood Tregs was transient because normal values could be measured on day 3 after stroke. Therefore, the rise of Tregs observed in the ischemic hemispheres (Figure 1A) is probably not because of an overall increase of Tregs, but rather reflects redistribution of Tregs within different compartments and a relative accumulation of Tregs in the brain.
Tregs are present in the ischemic brain early after stroke and are mainly found in the vascular compartment. (A) Flow cytometric analysis of FoxP3+ Tregs counted in the ischemic hemispheres on day 1 (d1) and d3 after 60 minutes of tMCAO or sham-operated mice. Transcardial perfusion of animals was omitted before brain sampling. (B) Top panel is a macroscopic view of a representative TTC-stained brain section from a regular DEREG mouse without diphtheria toxin treatment on day 1 after 60 minutes of tMCAO showing that the immunohistochemical pictures shown at the bottom of the figure were taken from the basal ganglia (BG). Bottom panel is immunohistochemical brain sections from DEREG mice on day 1 after 60 minutes of tMCAO showing FoxP3+ Tregs predominantly in the cerebral vasculature (double staining with the endothelial marker CD31). The area of the basal ganglia is depicted. C indicates cortex. *P < .05; ***P < .0001.
Tregs are present in the ischemic brain early after stroke and are mainly found in the vascular compartment. (A) Flow cytometric analysis of FoxP3+ Tregs counted in the ischemic hemispheres on day 1 (d1) and d3 after 60 minutes of tMCAO or sham-operated mice. Transcardial perfusion of animals was omitted before brain sampling. (B) Top panel is a macroscopic view of a representative TTC-stained brain section from a regular DEREG mouse without diphtheria toxin treatment on day 1 after 60 minutes of tMCAO showing that the immunohistochemical pictures shown at the bottom of the figure were taken from the basal ganglia (BG). Bottom panel is immunohistochemical brain sections from DEREG mice on day 1 after 60 minutes of tMCAO showing FoxP3+ Tregs predominantly in the cerebral vasculature (double staining with the endothelial marker CD31). The area of the basal ganglia is depicted. C indicates cortex. *P < .05; ***P < .0001.
To further analyze the location of Tregs in the ischemic brain during the early phase of stroke, we performed immunohistochemistry of brain specimens taking advantage of genetically modified mice in which FoxP3-expressing cells are visible by a transgenic construct linking green fluorescent protein (GFP) and the diphtheria toxin receptor (DEREG mice)11 (Figure 1B). On day 1 after 60 minutes of tMCAO, Tregs were predominantly found within the vessel lumina but were absent within the brain parenchyma (the region of the basal ganglia is depicted in Figure 1B). This indicates that Tregs are recruited to the brain at a very early stage during cerebral ischemia but initially linger predominantly within the cerebral vasculature.
Elimination of Tregs improves outcome after ischemic stroke
DEREG mice are an excellent model with which to study the pathophysiologic role of Tregs in brain ischemia, because in these animals FoxP3-expressing Tregs can be selectively depleted by the application of diphtheria toxin (supplemental Figure 1).11 Brain infarct volumes at 24 hours after 60 minutes of tMCAO were significantly reduced in Treg-depleted DEREG mice compared with controls, as revealed by TTC staining (n = 10-14; P < .0001; Figure 2A). The reduction of infarct size was functionally relevant, because the Bederson score (n = 10-14, P < .0001) and the grip test (n = 10-14, P < .001 or P < .0001) were significantly better in the absence of Tregs (Figure 2B). To further prove that the observed neuroprotective effect in diphtheria toxin–treated DEREG mice was specifically related to the lack of Tregs, mice with diphtheria toxin–induced ablation of Tregs were allowed to reconstitute their Treg population over a period of 3 weeks11 (supplemental Figure 1) and underwent 60 minutes of tMCAO thereafter. These “spontaneously” Treg-reconstituted DEREG mice again developed infarcts (n = 6, P > .05; Figure 2A) and neurologic deficits (n = 6, P > .05; Figure 2B) similar to control mice. We next addressed the long-term consequences of Treg deficiency in stroke. Protection from tMCAO in Treg-depleted DEREG mice was sustained on day 4 after tMCAO, as indicated by significantly smaller infarct volumes (n = 5, P < .0001; Figure 2A) and a better Bederson score (n = 5, P < .05; Figure 2B) compared with naive DEREG mice. These observations exclude the possibility that Treg deficiency simply induces faster recovery from stroke but underline its long-lasting effect on stroke outcome. To further corroborate our findings, we also performed serial MRI. Consistent with the results from our TTC stainings, Treg-depleted DEREG mice developed significantly smaller brain infarctions on day 1 after 60 minutes of tMCAO (DEREG, n = 5; DEREG + diphtheria toxin, n = 7; P < .0001; Figure 2C). The size of the infarctions assessed in individual Treg-depleted animals by sequential MRI did not increase after 1 week (P > .05; Figure 2C), thus excluding delayed infarct growth. Because Treg numbers in the blood (n = 5; P < .0001) and spleens (n = 5; P < .001) from diphtheria toxin–treated DEREG mice were still significantly lower compared with untreated DEREG mice on day 7 after tMCAO, 8 days after the last diphtheria toxin injection (supplemental Figure 9), the potential beneficial effects of (spontaneously reconstituted) Tregs at this advanced time point appear unlikely.
Treg depletion protects from acute ischemic stroke. (A) Infarct volumes on day 1 or day 4 after 60 minutes of tMCAO in Treg-depleted DEREG mice (DEREG + DT) and controls or Treg-depleted DEREG mice treated with anti–neutrophil serum (ANS) or control serum as calculated from TTC-stained brain sections. DT indicates diphtheria toxin (B) Neurologic deficits after stroke (day 1 or day 4) were assessed by the Bederson score and the grip test score. Consistent with infarct volume reduction, Treg-depleted DEREG mice also had a significantly better functional outcome. (C) Serial coronal MR brain images (1.5 Tesla) confirmed smaller infarct volumes in Treg-depleted DEREG mice on day 1 after 60 minutes of tMCAO and excluded secondary infarct growth in these mice until day 7. (D) Delayed depletion of Tregs in DEREG mice (DEREG + DT) also did not lead to larger infarcts on day 7 (30 minutes of tMCAO). Alleged shrinkage of strokes between day 1 and day 7 is because of fogging effects occurring during infarct maturation. *P < .05, **P < .001, and ***P < .0001 between the indicated groups; ns indicates not significant.
Treg depletion protects from acute ischemic stroke. (A) Infarct volumes on day 1 or day 4 after 60 minutes of tMCAO in Treg-depleted DEREG mice (DEREG + DT) and controls or Treg-depleted DEREG mice treated with anti–neutrophil serum (ANS) or control serum as calculated from TTC-stained brain sections. DT indicates diphtheria toxin (B) Neurologic deficits after stroke (day 1 or day 4) were assessed by the Bederson score and the grip test score. Consistent with infarct volume reduction, Treg-depleted DEREG mice also had a significantly better functional outcome. (C) Serial coronal MR brain images (1.5 Tesla) confirmed smaller infarct volumes in Treg-depleted DEREG mice on day 1 after 60 minutes of tMCAO and excluded secondary infarct growth in these mice until day 7. (D) Delayed depletion of Tregs in DEREG mice (DEREG + DT) also did not lead to larger infarcts on day 7 (30 minutes of tMCAO). Alleged shrinkage of strokes between day 1 and day 7 is because of fogging effects occurring during infarct maturation. *P < .05, **P < .001, and ***P < .0001 between the indicated groups; ns indicates not significant.
We also performed experiments with a delayed depletion of Tregs. Diphtheria toxin was given on 3 consecutive days beginning from day 1 after 30 minutes of tMCAO, which induces small infarcts. DEREG mice with a delayed ablation of Tregs did not exhibit secondary infarct growth until day 7 compared with nondepleted DEREG mice (n = 5, P > .05; Figure 2D), thus arguing against a major role of Tregs for infarct reorganization. Thirty minutes of tMCAO was chosen in this experimental setup because an occlusion time of 60 minutes already produces fully mature infarctions after 24 hours, making it difficult to detect any additional infarct growth. The alleged shrinkage in stroke size after 1 week in both groups was because of the well-known “fogging effect” on MRI20 rather than true infarct size reduction.
Previous studies also suggested a substantial impact of polymorphonuclear (PMN) leukocytes on stroke development in mice.21,22 Therefore, to estimate the relative contribution of Tregs versus PMN leukocytes in our model system in more detail, we treated Treg-depleted DEREG mice with a neutrophil-depleting serum or control serum (supplemental Figure 3) before the induction of tMCAO. Removal of neutrophils in DEREG mice lacking Tregs did not further reduce infarct volumes (n = 6; P > .05) or improve functional outcomes (n = 6; P > .05) in these animals on day 1 (Figure 2A-B). These findings indicate that, at least in the DEREG mouse system, Tregs are obviously of greater importance for stroke formation than PMN leukocytes.
Adoptive transfer of Tregs worsens outcome after ischemic stroke
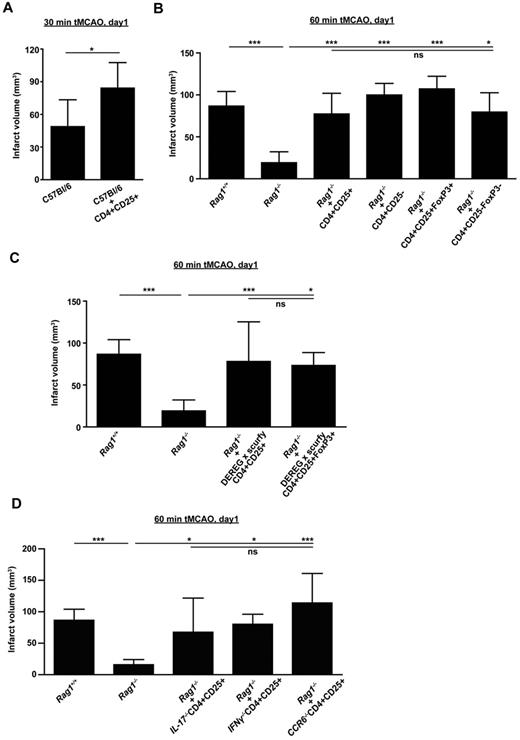
To further substantiate our hypothesis that Tregs are critical mediators of ischemic neurodegeneration, we performed adoptive transfer experiments with C57Bl/6 wild-type mice and Rag1−/− mice as recipients. First, adoptive transfer of 750 000 purified CD4+CD25+ Tregs into naive C57Bl/6 wild-type mice was performed 24 hours before tMCAO. A total of 750 000 cells accurately reflects the number of circulating Tregs under physiologic conditions in rodents23 and are in good accordance with other adoptive cell-transfer protocols in immunodeficient mice.24 The increase of circulating Treg numbers induced a significant increase in stroke size on day 1 after 30 minutes of tMCAO (n = 8, P < .05; Figure 3A). Thirty minutes of tMCAO is a well-accepted model of mild ischemic stroke, which under basal conditions causes infarctions of the deep basal ganglia but leaves the cortex intact.25
Tregs exacerbate ischemic brain damage in wild-type mice and Rag1−/− mice lacking T cells independently of Treg immunologic function. (A) Purified CD4+CD25+ Tregs (750 000) were transferred into regular C57Bl/6 mice 24 hours before 30 minutes of tMCAO and infarct volumes were determined on day 1. (B) The stroke-protective effect observed in Rag1−/− mice on day 1 after 60 minutes of tMCAO could be reversed by adoptive transfer of Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) or non-Tregs (CD4+CD25− or CD4+CD25−FoxP3−). (C) Adoptive transfer of “wannabe” Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) from DEREG × scurfy mice into Rag1−/− mice also induced infarctions of regular size on day 1 after 60 minutes of tMCAO, indicating that the immunologic function of Tregs is dispensable for stroke development. (D) CD4+CD25+ Tregs (750 000) were collected from Il-17−/− mice, Ifnγ−/− mice, and Ccr6−/− mice and transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO. Infarct volumes were determined on day 1. The detrimental effects of Tregs in ischemic stroke cannot be ascribed to one specific cytokine because infarct volumes after adoptive transfer were similar to those observed in Rag1+/+ mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
Tregs exacerbate ischemic brain damage in wild-type mice and Rag1−/− mice lacking T cells independently of Treg immunologic function. (A) Purified CD4+CD25+ Tregs (750 000) were transferred into regular C57Bl/6 mice 24 hours before 30 minutes of tMCAO and infarct volumes were determined on day 1. (B) The stroke-protective effect observed in Rag1−/− mice on day 1 after 60 minutes of tMCAO could be reversed by adoptive transfer of Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) or non-Tregs (CD4+CD25− or CD4+CD25−FoxP3−). (C) Adoptive transfer of “wannabe” Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) from DEREG × scurfy mice into Rag1−/− mice also induced infarctions of regular size on day 1 after 60 minutes of tMCAO, indicating that the immunologic function of Tregs is dispensable for stroke development. (D) CD4+CD25+ Tregs (750 000) were collected from Il-17−/− mice, Ifnγ−/− mice, and Ccr6−/− mice and transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO. Infarct volumes were determined on day 1. The detrimental effects of Tregs in ischemic stroke cannot be ascribed to one specific cytokine because infarct volumes after adoptive transfer were similar to those observed in Rag1+/+ mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
Rag1−/− mice completely lack functional T cells and are largely protected from focal cerebral ischemia6-8 (Rag1+/+, n = 11; Rag1−/−, n = 10, P < .0001; Figure 3B). Therefore, Rag1−/− mice represent an elegant tool for studying the pathophysiologic significance of distinct T-cell subsets during ischemic stroke. Reconstitution of Rag1−/− mice with both CD4+CD25+ Tregs (n = 15, P > .05) and CD4+CD25− lymphocytes (n = 11, P > .05) fully restored the susceptibility for brain ischemia on day 1 after 60 minutes of tMCAO (Figure 3B). This indicates that other CD4+ T-cell subpopulations apart from Tregs are also involved in stroke development, which confirms previous studies.6,8 CD25 is not exclusively expressed on Tregs, but is also up-regulated on activated T cells,26 whereas FoxP3 is regarded as a more specific marker for Tregs.27 Therefore, by taking advantage of GFP expression under the FoxP3 promoter in DEREG mice,11 CD4+CD25+FoxP3+ Tregs were collected from DEREG mice by cell sorting and transferred into Rag1−/− mice. The transfer of FoxP3+ Tregs into Rag1-deficient mice induced brain infarctions of regular size on day 1 after 60 minutes of tMCAO (n = 3, P > .05; Figure 3B). Again, reconstitution of Rag1−/− mice with CD4+CD25−FoxP3− cells was also able to reverse the reduction in stroke size in these animals (n = 3, P > .05), further underlining that Tregs represent a major, but not the only, detrimental T-cell subpopulation in acute ischemic stroke.
Tregs exacerbate stroke independently of their immunologic function
We next assessed whether the suppressive immunologic function of Tregs would be a prerequisite for their damaging effect in acute stroke. We analyzed mice that had been generated by cross-breeding of scurfy mice, a line that bears a defect in the FoxP3 gene that causes severe Treg dysfunction,28 with DEREG mice.13 Those crossings provide the unique opportunity to analyze the functional relevance of so-called “wannabe” Tregs, which are characterized by GFP expression in the absence of functional FoxP3. Adoptive transfer of CD4+CD25+ lymphocytes (n = 9, P > .05) and CD4+CD25+GFP+ Tregs (n = 4, P > .05) from DEREG × scurfy mice into Rag1−/− mice before stroke caused infarctions similar to that of Rag1+/+ mice on day 1 after 60 minutes of tMCAO (Figure 3C). This suggests that only the presence of “phenotypic” Tregs, not their suppressive immunologic properties, is required for mediating tissue damage in the ischemic brain.
The finding that Tregs act independently of their immunologic function during stroke was further corroborated by the fact that the mRNA expression levels of several prototypic pro- and anti-inflammatory cytokines involved in infarct development or relief1 such as TNFα, IL-17, IL-1β, and IL-10 in the ischemic basal ganglia did not differ between DEREG mice with or without Tregs (n = 5, P > .05; supplemental Figure 10).
It has been reported that FoxP3+ T cells can convert into proinflammatory Th17 cells under certain conditions and that subsets of FoxP3+ Tregs can secrete IL-17, at least in humans.29 In addition, a recent study suggested that IL-17 plays an important role in the development of ischemic brain injury in the delayed phase.30 Given this background, we investigated whether Treg-derived IL-17 accounts for the pathogenic effect of this cell type after tMCAO. Injection of CD4+CD25+ Tregs devoid of IL-17 (n = 5, P > .05) into Rag1−/− mice reversed the protection from stroke seen in naive Rag1−/− mice, indicating that Tregs act independently of IL-17 (Figure 3D). Moreover, CD4+CD25+ Tregs collected from IFNγ-deficient (n = 5, P > .05) or CCR6-deficient (n = 5, P > .05) mice also induced normal brain infarctions when transferred into Rag1−/− mice (Figure 3D). IFNγ is a major T-cell cytokine and has been shown to promote infarct development,3 whereas CCR6 regulates the recruitment of T cells during inflammation.31 These findings suggest that the deleterious properties of Tregs in focal cerebral ischemia cannot be ascribed to one or more distinct cytokines.
In support of an immune-independent Tregs mode of action, we also found that the number of CD4+ T cells or CD11b+ microglia/macrophages infiltrating the ischemic brain on day 1 after 60 minutes of tMCAO did not differ between diphtheria toxin–treated or untreated DEREG mice (n = 6-9, P > .05; supplemental Figure 11A). Lack of Tregs also did not influence the amount of invading immune cells at a more advanced stage after tMCAO, on day 4 (n = 5, P > .05; supplemental Figure 11B). In view of these findings, a functionally relevant activation of other immune cell populations by Tregs appears unlikely.
Tregs promote ischemic brain damage by causing microvascular dysfunction and thrombosis
Because propagation of tissue damage in acute ischemic stroke does not require “classic” Treg immune function, other mechanisms must be operative. In consideration of the observation that Tregs are harmful already 24 hours after stroke (Figure 2) and are predominantly found within the vessel lumina (Figure 1B), we speculated that mechanisms acting at the brain-vasculature interface could be of pathologic relevance. We showed recently that progressive thrombus formation in the cerebral microvasculature critically mediates secondary infarct growth.15 Indeed, the amount of fibrin(ogen) in the cortex and basal ganglia detected by Western blot was significantly reduced in the absence of Tregs on day 1 after tMCAO (n = 4, P < .0001, P < .001; Figure 4A). Accordingly, the patency of the brain microvessels was greater in DEREG mice receiving diphtheria toxin compared with naive DEREG mice (n = 3, P < .05; Figure 4B).
Depletion of Tregs reduces intracerebral thrombosis and improves cerebral blood flow after stroke. (A) Accumulation of fibrin(ogen) in the infarcted (I) and contralateral (C) cortices and basal ganglia of naive DEREG mice and Treg-depleted DEREG mice (DEREG + DT) was analyzed by immunoblotting 23 hours after 60 minutes of tMCAO and quantified. Three representative immunoblots of each group are shown. The higher fibrin(ogen) signal detectable also in the healthy contralateral (= left) hemisphere of nondepleted DEREG mice is probably a consequence of increased thrombotic activity in this region because of massive ipsilateral infarct swelling. DT indicates diphtheria toxin; AU, arbitrary units. (B) The number of thrombotic vessels (thrombosis index) in the ischemic basal ganglia of DEREG mice and Treg-depleted DEREG mice was counted from H&E-stained brain sections on day 1 after 60 minutes of tMCAO. Although numerous occlusions of vessel lumina were found in naive DEREG mice (arrows), the microvascular patency was significantly increased in the absence of Tregs (arrowheads). (C) Reduced intracerebral thrombosis was related to improved CBF, fewer tissue infarctions as reflected by lower apparent diffusion coefficient (ADC) values, and a lower infarct probability in Treg-depleted DEREG mice as revealed by MRI at ultra-high-field strength (17.6 Tesla) on day 1 after 60 minutes of tMCAO. *P < .05, **P < .001, and ***P < .0001 between the indicated groups.
Depletion of Tregs reduces intracerebral thrombosis and improves cerebral blood flow after stroke. (A) Accumulation of fibrin(ogen) in the infarcted (I) and contralateral (C) cortices and basal ganglia of naive DEREG mice and Treg-depleted DEREG mice (DEREG + DT) was analyzed by immunoblotting 23 hours after 60 minutes of tMCAO and quantified. Three representative immunoblots of each group are shown. The higher fibrin(ogen) signal detectable also in the healthy contralateral (= left) hemisphere of nondepleted DEREG mice is probably a consequence of increased thrombotic activity in this region because of massive ipsilateral infarct swelling. DT indicates diphtheria toxin; AU, arbitrary units. (B) The number of thrombotic vessels (thrombosis index) in the ischemic basal ganglia of DEREG mice and Treg-depleted DEREG mice was counted from H&E-stained brain sections on day 1 after 60 minutes of tMCAO. Although numerous occlusions of vessel lumina were found in naive DEREG mice (arrows), the microvascular patency was significantly increased in the absence of Tregs (arrowheads). (C) Reduced intracerebral thrombosis was related to improved CBF, fewer tissue infarctions as reflected by lower apparent diffusion coefficient (ADC) values, and a lower infarct probability in Treg-depleted DEREG mice as revealed by MRI at ultra-high-field strength (17.6 Tesla) on day 1 after 60 minutes of tMCAO. *P < .05, **P < .001, and ***P < .0001 between the indicated groups.
To further address whether reduced clot formation after the elimination of Tregs also translates into better cerebral (re)perfusion and less tissue damage, we used multimodal MRI at 17.6 Tesla.16 Although measurement of local CBF with high spatial resolution by continuous arterial spin labeling does not provide direct information on the intraluminal structure of the cerebral microvasculature, it can be considered as a useful indirect marker of microvascular function because it indicates the degree of patency in vivo by quantitating blood flow through the brain microcirculation. Cortical (DEREG + diphtheria toxin: 63.8 ± 28.1 mL/100 g/min vs DEREG: 32.9 ± 6.7 mL/100 g/min) and subcortical (DEREG + diphtheria toxin: 60.0 ± 25.2 mL/100 g/min vs DEREG: 31.2 ± 5.0 mL/100 g/min) CBF measured by continuous arterial spin labeling in the territory of the middle cerebral artery was higher after Treg depletion versus controls 23 hours after 60 minutes of tMCAO (n = 5, P = .0076; Figure 4C). In both regions, CBF findings in diphtheria toxin–treated DEREG mice were related to a lower probability of infarction, as reflected by significantly improved quantitative T2 relaxation times (n = 5, cortical, subcortical: P = .01) and lower apparent diffusion coefficient values indicative of tissue infarction (n = 5, cortical: P = .01, subcortical: P = .003; Figure 4C).
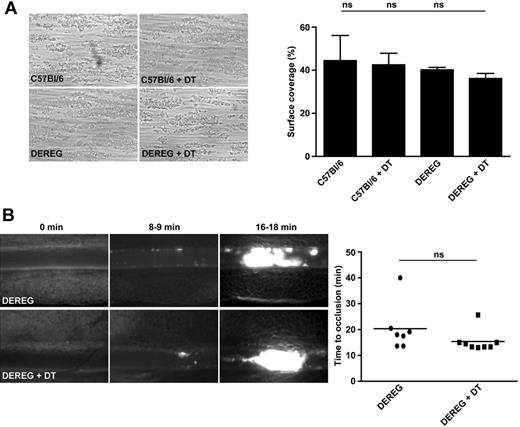
To exclude that Treg depletion or diphtheria toxin application causes a general defect in platelet function that could account for the profound protection observed in ischemic stroke, we analyzed the ability of diphtheria toxin–treated DEREG mice to form stable thrombi in standardized thrombus formation assays. First, we studied platelet adhesion and aggregate formation on a collagen-coated surface in an ex vivo whole-blood perfusion system under high shear conditions (1000/s).17 In this setting, platelets from naive C57Bl/6 mice, C57Bl/6 mice treated with diphtheria toxin, naive DEREG mice, and DEREG mice pretreated with diphtheria toxin adhered to collagen fibers and formed aggregates within 2 minutes, which consistently grew into large thrombi (Figure 5A). By the end of the perfusion period, the surface area covered by platelets did not differ significantly between the groups (n = 3-5, P > .05; Figure 5A). We also studied the effects of Treg deficiency on occlusive thrombus formation in vivo after FeCl3-induced injury on mesenteric arterioles.8 In untreated DEREG mice, the formation of small platelet aggregates was observed approximately 8 minutes after injury, with progression to complete vessel occlusion in 6 of 7 mice within 20 minutes (Figure 5B). No significant differences in the mean occlusion time of damaged arterioles were observed between DEREG mice and Treg-depleted DEREG mice (n = 7-8, P > .05; Figure 5B). Finally, DEREG with or without Tregs did not differ with respect to platelet counts and volumes (supplemental Table 3).
Depletion of Tregs does not induce a general defect in platelet function or activation. (A) Platelets in whole blood from Treg-depleted DEREG mice (DEREG + DT) form stable thrombi when perfused over a collagen-coated surface at a shear rate of 1000/s. On the left are representative phase-contrast images. On the right is the mean surface coverage by thrombi in the indicated mouse groups. DT indicates diphtheria toxin; and ns, not significant. (B) In vivo analysis of thrombus formation in Treg-depleted DEREG mice (DEREG + DT) and controls. Mesenteric arterioles were treated with FeCl3 and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Representative images (left) and the time to vessel occlusion (right) are shown. Each symbol represents one individual. ns indicates not significant. Data are presented as means ± SD or scatter plots showing the median (panel B).
Depletion of Tregs does not induce a general defect in platelet function or activation. (A) Platelets in whole blood from Treg-depleted DEREG mice (DEREG + DT) form stable thrombi when perfused over a collagen-coated surface at a shear rate of 1000/s. On the left are representative phase-contrast images. On the right is the mean surface coverage by thrombi in the indicated mouse groups. DT indicates diphtheria toxin; and ns, not significant. (B) In vivo analysis of thrombus formation in Treg-depleted DEREG mice (DEREG + DT) and controls. Mesenteric arterioles were treated with FeCl3 and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Representative images (left) and the time to vessel occlusion (right) are shown. Each symbol represents one individual. ns indicates not significant. Data are presented as means ± SD or scatter plots showing the median (panel B).
These findings demonstrate that Treg deficiency does not impair the general ability of platelets to form stable thrombi, at least in the setting of severe artificial vessel wall injury leading to the exposure of tissue factor and prothrombotic subendothelial matrix proteins. However, the extent of vascular damage during the early phase of brain ischemia/reperfusion injury (tMCAO) is more subtle. At the initial phase, ischemia only induces activation of the cerebral endothelium, leading to the up-regulation of cell adhesion receptors (selectins and integrins).32 Lymphocytes can bind these receptors, for example, via the ICAM-1 (expressed on endothelial cells)–LFA-1 (expressed on T cells) pathway. As a consequence, brain capillaries remain obstructed even after successful recanalization of large proximal vessels, a phenomenon commonly referred to as “no reflow.”33
In an attempt to delineate the pathophysiologic relevance of Treg-endothelium interactions in acute stroke, Rag1−/− mice received a blocking anti–LFA-1 Ab or isotype control before reconstitution with CD4+CD25+ or CD4+CD25− T cells (Figure 6A). Blocking of LFA-1 in Rag1−/− mice without adoptive transfer of CD4+ T cells could not further reduce infarct volumes on day 1 after tMCAO in these animals (Rag1+/+ mice: n = 11, Rag1−/− mice: n = 10, Rag1−/− mice + anti–LFA-1: n = 5, P > .05). As expected (Figure 3), adoptive transfer of CD4+CD25+ Tregs under control conditions (ie, without anti–LFA-1 Ab) reversed protection from stroke in Rag1−/− mice (n = 11, P < .0001). After blocking of LFA-1, however, the detrimental effect of CD4+CD25+ Tregs in Rag1−/− mice was overcome and infarctions remained nearly as small as in naive Rag1−/− mice (n = 10, P > .05; Figure 6A left). Most importantly, LFA-1 block was ineffective in Rag1−/− mice receiving CD4+CD25− T cells, because this group developed large infarctions (n = 4, P < .0001), as did Rag1−/− mice after the application of control Abs in combination with CD4+CD25− T cells (n = 5, P < .0001). This indicates that within the subgroup of CD4+ T cells, the ability of Tregs to interact with the ischemic brain endothelium (in an LFA-1–dependent manner) is especially pronounced. Indeed, circulating natural CD4+CD25+ Tregs expressed significantly higher amounts of LFA-1 than CD4+CD25− non-Tregs on day 1 after tMCAO (n = 6, P < .05; Figure 6A right), which confirms our previous data under nonischemic conditions.34 Mechanistically, blocking of LFA-1 in CD4+CD25+–reconstituted Rag1−/− mice using anti–LFA-1 Abs resulted in reduced thrombotic activity in the cortical microcirculation (n = 5, P < .05) and basal ganglia (n = 5, P < .05) 24 hours after tMCAO (supplemental Figure 12).
Tregs interact with cerebral endothelial cells and platelets to promote ischemic neurodegeneration after stroke. (A left) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO in the presence of anti–LFA-1 blocking Abs or control Abs. Infarct volumes were determined on day 1. Blocking of LFA-1 reversed the stroke-enhancing effect of CD4+CD25+ Tregs but not of CD4+CD25− non-Tregs in Rag1−/− mice. Right, circulating CD4+CD25+ Tregs express higher amounts of LFA-1 than CD4+CD25− non-Tregs on day 1 after 60 minutes of tMCAO. MFI indicates mean fluorescence intensity. (B) In vitro binding capacity to ICAM-1 (left) but not VCAM-1 (right) is more pronounced in CD4+CD25+ Tregs than in CD4+CD25− non-Tregs both under basal conditions and after stimulation with CXCL12. (C) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO. Rag1−/− mice were treated with antiplatelet serum to deplete circulating platelets or controls serum and infarct volumes were determined on day 1. Depletion of platelets reversed the stroke-enhancing effect of CD4+CD25+ Tregs and also of CD4+CD25− non-Tregs in Rag1−/− mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
Tregs interact with cerebral endothelial cells and platelets to promote ischemic neurodegeneration after stroke. (A left) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO in the presence of anti–LFA-1 blocking Abs or control Abs. Infarct volumes were determined on day 1. Blocking of LFA-1 reversed the stroke-enhancing effect of CD4+CD25+ Tregs but not of CD4+CD25− non-Tregs in Rag1−/− mice. Right, circulating CD4+CD25+ Tregs express higher amounts of LFA-1 than CD4+CD25− non-Tregs on day 1 after 60 minutes of tMCAO. MFI indicates mean fluorescence intensity. (B) In vitro binding capacity to ICAM-1 (left) but not VCAM-1 (right) is more pronounced in CD4+CD25+ Tregs than in CD4+CD25− non-Tregs both under basal conditions and after stimulation with CXCL12. (C) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into Rag1−/− mice 24 hours before 60 minutes of tMCAO. Rag1−/− mice were treated with antiplatelet serum to deplete circulating platelets or controls serum and infarct volumes were determined on day 1. Depletion of platelets reversed the stroke-enhancing effect of CD4+CD25+ Tregs and also of CD4+CD25− non-Tregs in Rag1−/− mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
We were able to exclude that alterations in the cerebral microvascular beds of genetically modified Rag1−/− mice and DEREG mice account for the stroke-protective phenotype in these animals. Leakage of the vascular tracer Evan blue into the brain parenchyma was unchanged in healthy (without stroke) Rag1−/− mice and healthy DEREG mice compared with healthy C57Bl/6 wild-type mice, indicating a similar tightness and composition of the blood-brain barrier (n = 5, P > .05; supplemental Figure 13A). Moreover, we prepared primary cerebral endothelial cells from healthy Rag1−/− mice, DEREG mice, and wild-type controls and compared the mRNA expression levels of the vascular adhesion molecules ICAM-1, VCAM-1, and PECAM-1, as well as of the tight junction proteins occludin, claudin-5, and zonula occludens protein-1 (ZO-1; supplemental Figure 13B). Again, no differences were observed among the 3 groups for either molecule (n = 4; P > .05).
We performed in vitro adhesion assays to characterize the cross-talk between Tregs and endothelial cells in more detail. Binding of CD4+CD25+ Tregs to ICAM-1 was already significantly increased under baseline conditions compared with CD4+CD25− T cells (n = 5, P < .05; Figure 6B left). After stimulation with CXCL12, this difference in the adhesion capability became even more prominent (n = 5, P < .05), suggesting that chemokine-induced (inside-out) LFA-1 activation occurs more effectively in Tregs than in other T-cell subsets. No difference in the adhesion rates of CD4+CD25+ Tregs or CD4+CD25− T cells was found when coating the plates with VCAM-1 (n = 5, P > .05; Figure 6B right), underpinning the critical importance of the LFA-1/ICAM-1 pathway for mediating Treg-endothelial cell interactions.
Apart from endothelial cells, T cells can also bind platelets to form occlusive microaggregates, for example, via CD40-CD40 ligand (CD40L), and this cross-talk has been shown to facilitate microvascular obstruction in brain ischemia/reperfusion injury.3 To assess whether this also applies to the subset of Tregs, Rag1−/− mice dedicated to undergo 60 minutes of tMCAO were pretreated with platelet-depleting serum (supplemental Figure 2) and reconstituted with CD4+CD25+ Tregs or CD4+CD25− T cells afterward (Figure 6C). Depletion of platelets in Rag1−/− mice without adoptive transfer of CD4+ T cells had no further impact on reduced infarct volumes in these animals (Rag1+/+ mice: n = 11, Rag1−/− mice: n = 10, Rag1−/− mice + antiplatelet serum: n = 5, P > .05). Rag1−/− mice receiving CD4+CD25+ Tregs (n = 6) or CD4+CD25− T cells (n = 6) in the presence of platelets developed fully matured infarctions of regular size (P < .0001). However, removal of platelets before injecting CD4+CD25+ Tregs (n = 6) or CD4+CD25− T cells (n = 6) preserved the stroke-protective phenotype in Rag1-deficient mice (P > .05; Figure 6C). This finding, together with our results of LFA-1 blockade, indicates that during the early course of tMCAO, Tregs interact with activated endothelial cells and platelets, leading to impaired tissue reperfusion (Figure 4C), which causes neuronal damage. Application of the platelet-depleting serum did not alter complement activation in the blood of Rag1−/− mice compared with Rag1−/− mice receiving control serum (n = 4; P > .05; supplemental Figure 14). Controlling for complement activation is essential because previous studies have shown that manipulation of the complement cascade can strongly alter stroke outcome in different animal species.35,36
Discussion
In the present study, we demonstrate for the first time that Tregs are strong mediators of acute ischemic stroke. Selective depletion of Tregs dramatically reduced stroke size 24 hours after a severe insult (ie, 60 minutes of tMCAO) and this protective effect persisted into the later stages of infarct development. These observations could be confirmed by another independent approach using adoptive transfer of Tregs into Rag1−/− mice. Tregs also behaved detrimental in a model of mild ischemic stroke (ie, 30 minutes of tMCAO), as demonstrated by enhanced infarct progression in wild-type mice supplemented with Tregs. We could also elucidate the mechanisms responsible for the deleterious Treg effects in stroke. In contrast to primary resting T cells, natural Tregs have a higher adhesive propensity, predestining them to interact functionally with activated cerebral endothelial cells and platelets. This interaction causes microvascular dysfunction, leading to increased thrombus formation and subsequently impaired cerebral reperfusion after tMCAO. In contrast, mechanisms related to the “immunologic” functions of Tregs do not appear to be of major importance in stroke pathophysiology. The findings report herein are novel and interesting for several reasons compared with our prevous study published in 2010.8 In that study, we could only show that T cells act detrimentally in acute ischemic stroke (day 1) in an antigen-independent and thrombosis-independent way, but at that time we were unable to unravel the underlying cause of this detrimental T-cell effect. Moreover, the specific contribution of different T-cell subsets, for example, Tregs, and the situation at later time points after tMCAO (eg, at days 4 and 7) had not been analyzed back then.
The functional role of Tregs has already been addressed in ischemia/reperfusion models in other organs. Recruitment of Tregs via CCR5 suppressed inflammation and reduced adverse remodeling after experimental myocardial infarction.37 Tregs also attenuated ischemia/reperfusion injury in the liver38 and kidney.39 During the acute phase of reversible ischemia, this protective effect was mainly ascribed to the relief of inflammation, whereas at more advanced stages, Tregs have also been described to support tissue repair.40 The findings in other organ systems mainly point toward a beneficial contribution of Tregs during ischemia, which is in contrast to our results in the brain. Tregs clearly promoted neuronal damage after tMCAO in mice by impairing reperfusion of the cerebral microvasculature. The exact reasons for these brain-specific Treg effects are unclear at present, but differences in the mounted inflammatory response and the structural characteristics of the blood-brain barrier might play a role.
In contrast to our study, Liesz et al recently reported that Tregs are key modulators of cerebroprotection in brain ischemia in mice.7 However, the neuroprotective effect of Tregs was relatively modest compared with the dramatic injurious effect size described herein and manifest only in the late phase after an ischemic insult. These discrepant findings can be explained in part by technical differences in the stroke models used (ie, transient vs permanent MCAO and different ischemia times). Moreover, the extent of brain damage (large infarctions vs small infarctions) might have differentially influenced the function of Tregs and, therefore, stroke outcome.41-43 Finally, Liesz et al predominantly focused on the contribution of Tregs for tissue reorganization and repair at later stages of infarct development,7 whereas our study above all was intended to analyze the acute effects of Tregs in stroke pathogenesis.
Apart from aspects related to the modeling and timing of stroke, several other factors might account for our contrary results. First, Liesz et al used an anti-CD25 mAb to deplete CD4+CD25+ T cells,7 whereas we used a more precise approach to specifically target FoxP3+ Tregs. The DEREG mouse model allows ablation of FoxP3+ cells with very high specificity and has been used in several recent studies to delineate the functional role of Tregs in vivo.11,44-46 Kim et al did not find evidence for FoxP3 expression in nonhematopoietic cells44 and diphtheria toxin injection did not lead to a loss of epithelial integrity in the DEREG model, making the “nonimmune” effects of diphtheria toxin treatment in the tMCAO model unlikely. In addition, Treg depletion is reversible in DEREG mice after several weeks, thus providing the unique possibility of using intrinsic phenotype rescue as a control. The fact that CD25 is also up-regulated on activated T cells,9 the existence of a CD25− Treg subpopulation,47 and the results of a study claiming that the abovementioned anti-CD25 Ab induces shedding of the binding epitope rather than depletion of the cells48 limits the interpretation of the data by Liesz et al.7 In addition, it has been shown that the anti-CD25 Ab (clone PC61) is detectable in the circulation for several days and sterically impairs binding of the fluorochrome-conjugated anti-CD25 Ab necessary for later analysis.49
Another important difference between our study and that of Liesz et al is that our results could be validated by an independent approach: Adoptive transfer of purified Tregs into Rag1−/− mice, which are largely protected from ischemic stroke,6,8 induced fully matured cerebral infarctions. Moreover, excess of circulating Tregs exacerbated infarct growth in wild-type mice. Interestingly, adoptive transfer of both CD4+CD25+ Tregs and CD4+CD25− lymphocytes reversed the protection from stroke in Rag1−/− mice. This indicates that other CD4+ T-cell populations apart from Tregs are also involved in stroke development, which confirms previous studies.6,8
Finally, we used MRI to assess infarct progression in individual animals over time. In doing so, we could exclude delayed infarct growth between day 1 and day 7 in the absence of Tregs, as suggested by Liesz et al.7 Further, Liesz et al measured infarct volumes on a merely histologic basis,7 which means that different animals for each time point are required, which unavoidably increases the variability of the results.50
Our consistent findings in different models strongly argue for a detrimental rather than a protective role of Tregs, at least during the acute phase of brain ischemia/reperfusion injury. This is further strengthened by a recent study using a transgenic mouse model similar to DEREG mice, which also failed to confirm increased infarct volumes in the absence of Tregs in the later phase after stroke.51 Nevertheless, this study is rather preliminary in that only one time point after stroke was investigated and information regarding functional outcome or mechanistic findings are lacking.
Tregs promote stroke progression within a few hours after cessation of cerebral blood flow. At this early stage, they are mainly found within cerebral blood vessels. These rapid effects clearly argue against a prominent role of Treg-mediated adaptive immune responses in ischemic brain damage. Indeed, Tregs were no major determinants of local cytokine production or immune cell infiltration after tMCAO on day 1 or day 4 and reconstitution of Rag1−/− mice with functionally compromised wannabe Tregs13 induced fully mature infarctions.
It is widely accepted that lymphocytes not only interact with other immune cells, but can also bind to platelets and endothelial cells.33,52 During the reperfusion phase of transient brain ischemia, this interaction can become deleterious because it prevents sufficient blood flow restoration through the formation of microaggregates that plug the cerebral microvasculature (the no reflow phenomenon). PMN leukocytes have previously been suggested to play an important role in inducing capillary no reflow in cerebral ischemia.21,22 However, at least in the DEREG mouse model, the detrimental effects of Tregs seem to prevail over PMN leukocytes, because depletion of PMN leukocytes in DEREG mice already lacking Tregs did not further improve stroke outcome on day 1. Whether this observation is specific for the DEREG system or if it reflects a more general finding awaits clarification. It is also important to emphasize that according to our results and those of others, Tregs are not the only harmful T-cell subset in acute ischemic stroke. For example, Yilmaz et al were able to show that reconstitution of Rag1−/− mice with CD4+ Th cells or CD8+ cytotoxic T cells reversed the protection from stroke in these animals,6 which is consistent with our results after adoptive transfer of CD4+CD25− non-Tregs. Moreover, other studies recently stressed the stroke-promoting function of so-called γΔ T cells, a specialized subpopulation of T cells placed at the intersection between the innate and the adaptive immune system, at least during later stages of infarct development,30,53 whereas these cells do not appear to play a major pathophysiologic role early after tMCAO.8
We recently showed that natural Tregs bear an intrinsic propensity for migration, thus increasing the probability of a Treg-endothelial cell interaction compared with non-Tregs.34 In the present study, we extended these findings by demonstrating that Tregs are especially prone to adhere to the vascular endothelium via LFA-1/ICAM-1 binding under ischemic conditions, and blocking of this pathway abolished the stroke-enhancing activity of Tregs by reducing intravascular thrombosis and improving tissue reperfusion. Moreover, depletion of platelets also prevented Treg-driven infarct progression in Rag1−/− mice. This is consistent with the novel definition of ischemic stroke being a “thromboinflammatory” disease in which thrombotic and inflammatory processes are closely intertwined.54,55
In summary, the present study is the first description of a detrimental Treg effect in a primary nonimmunologic disease model, which could lead to a revision of the longstanding concept claiming that Tregs without exception are beneficial players of the immune system, acting by maintaining tolerance and limiting inflammation. Interesting from a translational point of view, we provide evidence herein that Tregs are key mediators of ischemic stroke. Short-term inhibition of Tregs might provide an effective therapy for this devastating neurologic condition. However, the true pathophysiologic relevance of Tregs in stroke patients still needs to be established and findings from animal studies should not be uncritically transferred to the human situation. Moreover, additional Treg subsets than those investigated here could also account for the detrimental effects in stroke.56 Further studies in relevant disease models are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Glaser, G. Köllner, and A. Sauer for excellent technical assistance; Christian Linden (Institute of Immunology, University of Würzburg) for cell sorting; and Tobias Tischer-Zeitz (Department of Anesthesiology and Critical Care, University of Würzburg) for performing the blood gas analysis.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 688 TP A12, A13, B1, and Z02; KL 2323/6-1 to C.K.; and WI1722/13-1 to H.W.), the Excellence Cluster Cells in Motion Münster (Area B and C to H.W. and S.G.M.); and the Bundesministerium für Bildung und Forschung (NEURON-ERANET/NANOSTROKE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: C.K. and H.W. conceived, directed, and funded the entire study, designed the experiments, analyzed the data, and wrote the manuscript; P.K. designed and performed the experiments, analyzed the data, and wrote the manuscript; A.D. provided specific input on the flow cytometric analysis and interpreted the data; I.H., K.G., M.K.S., F.L., T. Schwartz, S.B., C.T.M., M.B., T.M., and S.G.M. designed and performed the experiments, analyzed the data, and wrote the manuscript; X.H., C.V., M.P., M.B., and P.J. and provided specific input on the MRI experiments, including experimental design and data analysis; Y.I., A.Z., and T. Sparwasser provided transgenic mice, designed the experiments, and commented on the manuscript at all stages; and B.N. and G.S. funded the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Kleinschnitz, MD, University of Würzburg, Department of Neurology, Josef-Schneider Strasse 11, 97080 Würzburg, Germany, e-mail: christoph.kleinschnitz@uni-wuerzburg.de; or Heinz Wiendl, MD, University of Münster, Department of Neurology and Inflammatory Disorders of the Nervous System and Neurooncology, Albert-Schweitzer-Campus 1, 48149 Münster, Germany; e-mail: heinz.wiendl@ukmuenster.de.
References
Author notes
C.K. and P.K. contributed equally to this work.