Key Points
Steady-state and emergency granulopoiesis are both dependent on TLR signaling.
Abstract
Polymorphonuclear neutrophil granulocytes (neutrophils) are tightly controlled by an incompletely understood homeostatic feedback loop adjusting the marrow's supply to peripheral needs. Although it has long been known that marrow cellularity is inversely correlated with G-CSF levels, the mechanism linking peripheral clearance to production remains unknown. Herein, the feedback response to antibody induced neutropenia is characterized to consist of G-CSF–dependent shifts of marrow hematopoietic progenitor populations including expansion of the lin−/Sca-1+/c-kit+ (LSK) and granulocyte macrophage progenitor (GMP) compartments at the expense of thrombopoietic and red cell precursors. Evidence is provided that positive feedback regulation is independent from commensal germs as well as T, B, and NK cells. However, in vivo feedback is impaired in TLR4−/− and TRIF−/−, but not MyD88−/− animals. In conclusion, steady-state neutrophil homeostasis is G-CSF–dependent and regulated through pattern-recognition receptors, thereby directly linking TLR-triggering to granulopoiesis.
Introduction
Neutrophils are indispensable in generating the early inborn immunologic response to invading bacteria and fungi. Because both the lack of neutrophils and their increased or misguided activity contribute to human disease, neutrophil homeostasis is tightly regulated.1 The discovery and cloning of granulocyte colony stimulating factor (G-CSF), the principal cytokine stimulating neutrophil production and egress from the bone marrow, has opened the door for an understanding of neutrophil homeostasis.2
Pancytopenia because of bone marrow aplasia after myeloablative therapy and G-CSF levels have been described to be inversely correlated.3 The regulatory circuits determining plasma G-CSF in wild-type mice, however, have not been well determined. Although the physiologic response of granulopoiesis to infection, termed emergency granulopoiesis, has been characterized in more detail, steady-state granulopoiesis remains incompletely understood. Indeed, current evidence suggests that it may be regulated in a completely different manner.4 We therefore examined the effects of neutrophil depletion in an established mouse model of neutropenia. Our results show that there is specific sensing of neutropenia in the absence of inflammation. The positive feedback phenomena are characterized by typical quantitative shifts of hematopoietic marrow progenitors, dependence on up-regulated G-CSF, and down-regulated marrow CXCL12. Analyses of the underlying mechanisms suggest the existence of several, redundant pathways regulating G-CSF–dependent granulopoiesis, including IL-23 and IL-17, as previously described.5
Although TLR4-signaling has been implicated in emergency granulopoiesis,6-8 this highly conserved pathway may also be suitable for tailoring neutrophil production to prevailing needs in the steady-state.9 Our results suggest that TLR4-signaling represents a conditio sine qua non for the sensing of peripheral blood neutropenia in the steady-state.
Methods
Mice
C57BL/6, NOD.Cg-Prkdcscid IL2rgtmWjl/Sz (NSG), B6.129P2(SJL)-Myd88tm1.1Defr/J (MyD88−/−), C3H/HeJ and C3H/N mice were obtained from The Jackson Laboratory and maintained under specific pathogen-free conditions.
Germ-free (GF) C57BL/6 mice were maintained as previously described10 (University of Ulm, Germany). C3H/HeJ/TLR2−/− mice were provided by H.-G. Rammensee (Eberhard Karls University, Tuebingen, Germany). TRIF−/− and TLR4−/− mice by M. Radsak (Johannes Gutenberg University Medical Center, Mainz, Germany). All animals received amoxicillin in their drinking water. Animal experiments were performed with the authorization of the Institutional Animal Care and Use Committee of the University of Tuebingen according to German federal and state regulations.
Antibody-induced neutropenia
To induce neutropenia, anti–Gr-1 clone RB6-8C5 (BioXCell) was injected at a dose of 500 μg every other day. Alternatively, anti-mLy6G clone 1A8 (BioXCell) was injected at 1 mg every 36 hours. Antibodies were injected intraperitoneally in 500 μL of PBS for 8 days. Control groups received 500 μL of PBS intraperitoneally.
Antibody-induced depletion NK cells
To deplete NK cells, 0.3 mg anti-NK1.1, clone PK 136 (BioXCell) in 500 μL of PBS was injected intraperitoneally daily for 7 days. Five hundred milliliters of PBS was injected as control.
Flow cytometry
Antibodies were purchased from eBioscience (Natutec): CD34 (Pacific Blue); Sca-1, Gr-1 and IgG2a (PE); CD11b and c-kit (APC); streptavidin (PECy7); CD16/32 (PerCP-Cy5), CD127 (biotin). CD3, CD11b, B220, Gr-1, and Ter119 (biotinylated) were used as mouse lineage panel (BD Pharmingen).
Peripheral blood analysis
Retro-orbital blood was collected and differential blood counts were obtained using an automated Bayer Advia 120 MultiSpecies Analyzer (Bayer HealthCare). For flow cytometric analysis (FACS-Canto II; BD Bioscience), red blood cells (RBCs) were lysed with ammonium chloride buffer (0.150mM NH4Cl, 0.1mM EDTA, 0.150mM KHCO3) for 10 minutes on ice. Cells were stained to determine myeloid cells (CD11b, Gr-1). Isotype controls were used as indicated.
Bone marrow flow cytometry
After 8 days of continuous neutropenia, bones were harvested and flushed. In addition, the vertebral column was harvested and pestled to obtain a maximum of marrow cells. RBCs were lysed with ammonium chloride buffer for 10 minutes on ice. Then cells were washed with PBS and stained for flow cytometric analysis of progenitor cells.
Cytokine ELISA
Plasma levels of G-CSF, M-CSF, IL-17, and IL-23 were measured using Quantikine ELISA kits (R&D Systems) according to the recommendations of the manufacturer.
Quantitative real-time PCR
Whole marrow mRNA was isolated by RNeasy mini kit (QIAGEN). After reverse transcription (SuperScriptII, Invitrogen), quantitative PCR was performed in a LC480 (Roche). Primers for G-CSF and β-Actin were purchased from Applied Biosystems, Taqman gene expression assay. The ratio was calculated to β-Actin.
Hematoxylin-eosin staining of femora
Femora were harvested, fixed with 2% PFA, and embedded in paraffin after decalcification (Richard Allan Scientific). Sections were stained with H&E.
Statistics
Data are shown as mean ± SEM. Where indicated, statistical significance of results in paired t test analysis is given as P values (Microsoft Excel 2003). P < .05 was considered statistically significant.
Results
Effective and durable antibody mediated peripheral blood neutropenia
C57BL/6 mice received either RB6-8C5 or 1A8 antibody versus PBS intraperitoneally. Within 12 hours, the Gr-1+/CD11b+ population was completely eradicated in the antibody treated, but not in the placebo injected mice (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Continuous peripheral blood neutropenia could be induced in all animals (supplemental Figure 1B). Forward versus side scatter FACS analysis of peripheral blood cells after red blood cell lysis revealed a relative increase of Gr-1−/CD11b+ cells after antibody administration (supplemental Figure 1A). This population showed immature myelocytic morphology (supplemental Figure 1D).11
Mature neutrophils express Ly6G, which was previously defined as myeloid differentiation antigen (Gr-1), and anti-Ly6G antibody clone RB6-8C5 depletes these cells.12 Numerous publications on neutrophil depleted states in murine models of infection have been published. However, RB6-8C5 antibody has been shown to also deplete dendritic cells and subsets of macrophages, lymphocytes, monocytes, and may be functionally active in stimulating Gr-1–positive myeloid precursors.13 Therefore, we also used the more selective anti-Ly6G antibody (clone 1A8) to deplete neutrophils in vivo.14
Automated as well as manual microscopic peripheral blood analysis revealed that both antibodies resulted in a highly significant reduction of neutrophils (supplemental Figure 1B-D). Peripheral blood counts showed that neutropenic mice had a concomitant decrease in absolute white cell numbers on day 8 (supplemental Figure 1C; control = 7330/μL, RB6-8C5 = 2260/μL, 1A8 = 2800/μL).
Antibody-induced neutropenia was previously shown to induce hematopoietic stem and progenitor cell (HSPC) proliferation in the marrow independent from complement or Fc receptor γ,15 which strongly argues against inflammation mediated by complement fixation or Fc receptor signaling as a mediator of Gr-1 antibody induced effects. Nevertheless, to prove the specificity of the observed changes after antibody-dependent neutrophil depletion, C57BL/6 mice received anti-NK cell antibody (NK1.1, clone PK136) at a dose of 0.3 mg once daily. After 7 days, mice underwent peripheral blood and bone marrow analysis. In contrast to neutrophil depletion, NK depletion did not cause LSK or GMP expansion. Moreover, G-CSF levels were unchanged (data not shown). Direct effects of 1A8-antibody on stromal G-CSF production as previously described for anti–Sca-1 antibodies16 could be ruled out: C57BL/6 mesenchymal stromal cells did not up-regulate G-CSF RNA on addition of 1A8 (data not shown). In addition, we analyzed whether residual endotoxin in the antibody was responsible for the changes. To this end, wild-type mice received 2 EU/LAL LPS every 36 hours. After 8 days, animals were analyzed, and no changes of marrow progenitors were detected (supplemental Figure 2A). We conclude that the observed changes after neutrophil depletion represent specific results.
Neutropenia induces expansion of lineage negative and myeloid lineage committed stem and progenitor cells in the bone marrow
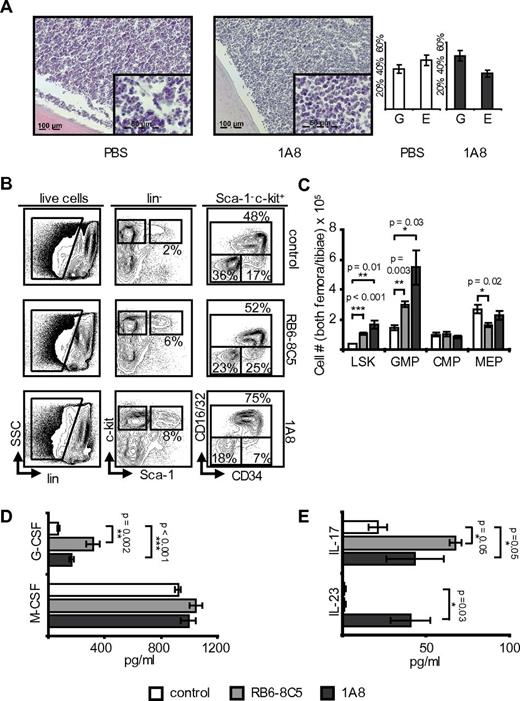
To establish the effects of neutropenia on hematopoietic marrow, mice underwent extensive analyses of their femoral marrows. For optimum comparability, analyses were done on day 8 of neutropenia. Histology demonstrated normocellularity with an increased granulopoiesis/erythropoiesis ratio (Figure 1A). Interestingly, progenitor frequencies were affected at all levels of hematopoietic differentiation in neutropenic animals: there were both relative and absolute increases in LSK cells (Figure 1B-C). Myeloid progenitors displayed a shift from lin−CD127− Sca-1− c-kit+ CD16/32− CD34− megakaryocyte/erythrocyte lineage restricted progenitor (MEP) toward the lin− CD127− Sca-1− c-kit+ CD16/32+ CD34+ granulocyte/macrophage lineage-restricted progenitor (GMP) phenotype (Figure 1B-C). Lin− CD127− Sca-1− c-kit+ CD16/32− CD34+ common myeloid progenitors (CMP) were not significantly affected.
Neutropenia induces expansion of myeloid hematopoietic progenitors and myelopoietic cytokines. (A) H&E-stained femora of neutropenic and control C57BL/6 mice on day 8 of treatment with granulocyte/erythrocyte ratio (microscope: Apotome, Zeiss; acquisition software: Axiovision Rel 4.8.2, Zeiss; magnification 63× and 20×). Note that marrow cellularity remains high in neutropenic animals and the increased granulocyte/erythrocyte ratio in antibody-treated mice. (B) Marrow flow cytometry including gating strategy in C57BL/6 wild-type mice. Myeloid progenitor cells (lin− CD127− Sca-1− c-kit+) were further differentiated into GMP (CD16/32+ CD34+), CMP (CD16/32− CD34+), and MEP (CD16/32− CD34−). Note expansion of the LSK population and increase of GMP at the cost of MEP. (C) Absolute marrow cell numbers calculated to reflect total cell counts in both hind limbs (n = 3). RB6-8C5: p(LSK) < 0.001; p(GMP) = 0.003; p(MEP) = 0.02; p(CMP) = not significant (NS); 1A8: p(LSK) = 0.01; p(GMP) = 0.03; p(MEP) = NS; p(CMP) = NS. (D) Plasma G-CSF and M-CSF levels in control and neutropenic C57BL/6 mice. Note the significant increase of G-CSF (n = 3). G-CSF: p(RB6-8C5) = 0.002; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS. (E) Plasma IL-17 and IL-23 levels in control and neutropenic C57BL/6 mice (n = 3 each group). IL-17: p(RB6-8C5) = 0.05; p(1A8) = 0.05; IL-23: p(RB6-8C5) = NS; p(1A8) = 0.03.
Neutropenia induces expansion of myeloid hematopoietic progenitors and myelopoietic cytokines. (A) H&E-stained femora of neutropenic and control C57BL/6 mice on day 8 of treatment with granulocyte/erythrocyte ratio (microscope: Apotome, Zeiss; acquisition software: Axiovision Rel 4.8.2, Zeiss; magnification 63× and 20×). Note that marrow cellularity remains high in neutropenic animals and the increased granulocyte/erythrocyte ratio in antibody-treated mice. (B) Marrow flow cytometry including gating strategy in C57BL/6 wild-type mice. Myeloid progenitor cells (lin− CD127− Sca-1− c-kit+) were further differentiated into GMP (CD16/32+ CD34+), CMP (CD16/32− CD34+), and MEP (CD16/32− CD34−). Note expansion of the LSK population and increase of GMP at the cost of MEP. (C) Absolute marrow cell numbers calculated to reflect total cell counts in both hind limbs (n = 3). RB6-8C5: p(LSK) < 0.001; p(GMP) = 0.003; p(MEP) = 0.02; p(CMP) = not significant (NS); 1A8: p(LSK) = 0.01; p(GMP) = 0.03; p(MEP) = NS; p(CMP) = NS. (D) Plasma G-CSF and M-CSF levels in control and neutropenic C57BL/6 mice. Note the significant increase of G-CSF (n = 3). G-CSF: p(RB6-8C5) = 0.002; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS. (E) Plasma IL-17 and IL-23 levels in control and neutropenic C57BL/6 mice (n = 3 each group). IL-17: p(RB6-8C5) = 0.05; p(1A8) = 0.05; IL-23: p(RB6-8C5) = NS; p(1A8) = 0.03.
In summary, antibody induced neutropenia specifically stimulated CMP and GMP expansion at the cost of MEP. Moreover, there was a strong effect on the LSK population, which increased by 3-fold. Importantly, these changes were dependent on G-CSF: concomitant administration of anti–G-CSF completely abrogated the previously described feedback phenomena in neutropenic mice (supplemental Figure 2B-C).
Positive feedback regulation of G-CSF in neutropenic mice
In adhesion-molecule deficient mice, neutrophil homeostasis was shown to be regulated through a feedback loop involving IL-23, IL-17, and G-CSF.5 IL-17 has been described to mediate G-CSF induced granulopoiesis.17 We therefore analyzed plasma concentrations of known modulators of granulopoiesis including G-CSF, M-CSF, IL-17, and IL-23. Within 8 days of neutrophil depletion with RB6-8C5, G-CSF concentration increased to a mean concentration of 317 pg/mL, corresponding to a 4.5-fold increase more than baseline levels (Figure 1D). 1A8 induced neutropenia resulted in a 2.5-fold increase more than baseline levels to 165 pg/mL. Interestingly, although RB6-8C5 eliminated monocytes in addition to neutrophils, M-CSF plasma levels remained unchanged (Figure 1D). Further studies will be necessary to elucidate the underlying regulatory mechanisms.
Interestingly, effects on IL-23 and IL-17 levels were not pronounced and dependent on the anti-Ly6G antibody used. In agreement with recent publications, IL-17 levels increased from 21 pg/mL to 67 pg/mL at day 8 of RB6-8C5–induced neutropenia (P = .05) and from 21 pg/mL to 43 pg/mL with 1A8 (P = .05; Figure 1E).
In contrast to control mice, IL-23 plasma levels increased from levels below the ELISA detection threshold to 40 pg/mL with 1A8 (P = .03; Figure 1E). RB6-8C5–treated mice did not display an increase in IL-23. IL-23 is assumed to be a monocyte/macrophage and dendritic cell secreted cytokine. Nonspecific depletion of these cells by RB6-8C5 may explain the absent increase of IL-23 in these animals.
In summary, induction of peripheral blood neutropenia in vivo induced strong increases of G-CSF, IL-17, and IL-23. These data are in line with results in leukocyte adhesion-molecule deficient mice, where a regulatory loop including TH17 cells was suggested.5
T-/B-/NK-deficient mice display steady-state neutropenia accompanied by an increase of noncommitted hematopoietic stem cells
We next hypothesized that deficiency in IL-17 producing T cells would result in steady-state neutropenia because of reduced G-CSF production. Comparative analysis of absolute neutrophil numbers and their degree of maturation in the peripheral blood of wild-type and lymphocytopenic mice should therefore reveal potential redundancy of neutrophil regulatory loops. NSG mice have been reported to be devoid of T, B, and NK cells because of a deficiency in common IL-2 receptor γ-chain.18
Steady-state analysis of the peripheral blood showed significantly decreased white blood counts in NSG mice compared with C57BL/6 mice (1920/μL in NSG; 7330/μL in C57BL/6). Of note, leukocytopenia in these mice was not only attributable to the total lack of lymphocytes in NSG mice, but also to a reduction of Gr-1+/CD11b+ mature polymorphonuclear and band neutrophils as well as Gr-1low/CD11b+ monocytes (supplemental Figure 3A-B).
Analysis of bone marrows showed increased absolute numbers of LSK cells. In contrast, CMP as well as GMP were significantly reduced in NSG mice (supplemental Figure 3C-D). These findings are in line with reduced stimulation of myelopoiesis by loss of TH17 cells. Accordingly, a significant reduction of plasma G-CSF was detected in NSG mice (supplemental Figure 3E).
In summary, differences at the marrow and peripheral blood level consistent with lymphocyte-dependent regulation of granulopoiesis can be observed in NSG. However, almost normal neutrophil counts and plasma G-CSF levels are maintained in NSG mice. These data confirm the contribution of lymphocytes to steady-state neutrophil granulopoiesis, but indicate the existence of additional, redundant pathways.
Lymphocytes are dispensable in neutropenia-induced G-CSF–mediated feedback granulopoiesis
To gain further insight into lymphocyte-independent granulopoiesis, NSG mice were made neutropenic. Analysis of the marrow revealed a pattern of changes over baseline identical to the findings described in C57BL/6 mice, including a massively increased LSK population in both RB6-8C5– and 1A8–treated animals as well as significantly increased GMP at the expense of MEP in RB6-8C5–treated mice (Figure 2A-B). A possible explanation for the more pronounced effect on LSK and GMP compared with CMP-levels may be the faster transit time of the smaller, more short-lived CMP subpopulation.19 Plasma G-CSF increased from a mean of 35 pg/mL to 370 pg/mL, corresponding to a 10.5-fold increase with RB6-8C5 antibody and nearly 30-fold up to 1064 pg/mL in NSG mice depleted with 1A8 antibody (Figure 2C). Plasma IL-17 as well as IL-23 levels remained below detection threshold (4.37 pg/mL for IL-17, 2.28 pg/mL for IL-23) in control and experimental groups throughout the experiment (data not shown).
Neutropenia-induced feedback in NSG mice. (A) NSG mice received 1A8, RB6-8C5, or PBS. Flow cytometric analyses of marrows are shown. Note the significant changes of LSK, GMP, and MEP. (B) Absolute cell numbers in both femora and tibiae of NSG mice (n = 5). RB6-8C5: p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) < 0.001; p(MEP) = 0.02; 1A8: p(LSK) < 0.001; p(GMP) = NS; p(MEP) = NS; p(CMP) = NS. (C) Plasma G-CSF and M-CSF levels in control and antibody-treated NSG mice. Note significant change of G-CSF [n(control) = 4; n(RB6-8C5) = 3; n(1A8) = 3]. G-CSF: p(RB6-8C5) = 0.003; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS.
Neutropenia-induced feedback in NSG mice. (A) NSG mice received 1A8, RB6-8C5, or PBS. Flow cytometric analyses of marrows are shown. Note the significant changes of LSK, GMP, and MEP. (B) Absolute cell numbers in both femora and tibiae of NSG mice (n = 5). RB6-8C5: p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) < 0.001; p(MEP) = 0.02; 1A8: p(LSK) < 0.001; p(GMP) = NS; p(MEP) = NS; p(CMP) = NS. (C) Plasma G-CSF and M-CSF levels in control and antibody-treated NSG mice. Note significant change of G-CSF [n(control) = 4; n(RB6-8C5) = 3; n(1A8) = 3]. G-CSF: p(RB6-8C5) = 0.003; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS.
These data support the notion that neutropenia can be sensed and translated into profound changes in the composition of hematopoietic marrow in T-/NK-/B-cell–deficient mice as efficiently as in wild-type mice.
Transcriptional regulation of G-CSF is dependent on neutrophil mass
Peripheral blood neutrophils bear the highest expression of G-CSF receptor (colony stimulating factor 3 receptor, CSF3R) among hematopoietic cells.20 We therefore hypothesized that plasma G-CSF may be a function of total neutrophil mass, that is, G-CSF could be regulated by binding to its receptor on the neutrophil surface with consecutive effects on G-CSF plasma levels. In analogy to megakaryopoiesis,21 homeostatic granulopoiesis could thus be the result of an indirect regulation of G-CSF through the prevailing neutrophil mass. Alternatively, G-CSF may be regulated at the transcriptional level.
To test the direct effect of neutrophil mass on G-CSF protein levels, granulocyte transfusions were conducted. C57BL/6 mice received 1A8 antibody to deplete neutrophils for 8 days and subsequently received granulocyte transfusions, which resulted in measurable increases of peripheral blood neutrophils (supplemental Figure 4A). Plasma G-CSF in acceptor mice determined immediately before and 36 hours after transfusion showed no significant changes over baseline (supplemental Figure 4B). We conclude that Gr-1+ neutrophil cell mass may not be a direct regulator of plasma G-CSF. Total marrow cell derived G-CSF RNA showed an insignificant increase over baseline (Figure 3C).
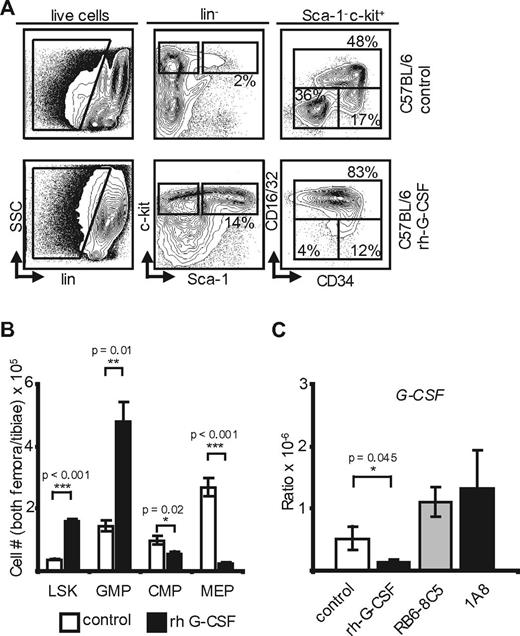
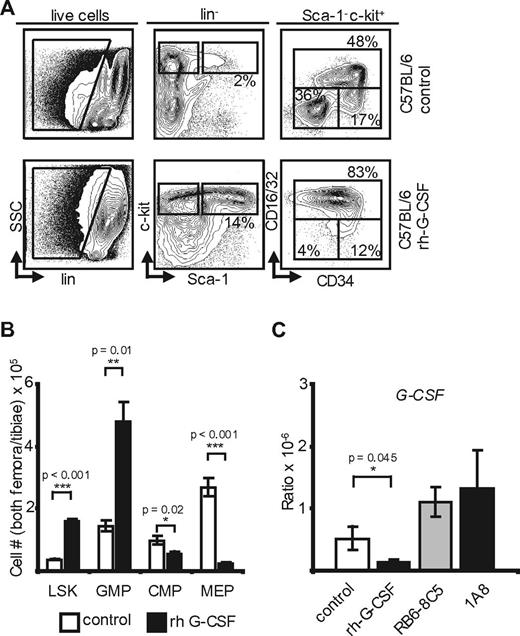
Exogenous G-CSF yields changes similar to neutropenia and reveals transcriptional regulation of G-CSF in the marrow. (A) Marrow flow cytometry in control and rh-G-CSF–treated C57BL/6 mice (n = 5). (B) Absolute marrow cell numbers in both hind limbs of C57BL/6 mice after rh-G-CSF (n = 5) versus PBS (n = 3). rh-G-CSF: p(LSK) < 0.001; p(GMP) = 0.01; p(CMP) = 0.02; p(MEP) < 0.001. (C) Transcriptional G-CSF levels in the marrow of control-, rh-G-CSF–, RB6-8C5–, and 1A8-treated C57BL/6 mice. Note the increase of G-CSF on neutropenia and the negative feedback at the RNA level after application of rh-G-CSF. p(rh-G-CSF) = 0.045; p(RB6-8C5) = NS; p(1A8) = NS.
Exogenous G-CSF yields changes similar to neutropenia and reveals transcriptional regulation of G-CSF in the marrow. (A) Marrow flow cytometry in control and rh-G-CSF–treated C57BL/6 mice (n = 5). (B) Absolute marrow cell numbers in both hind limbs of C57BL/6 mice after rh-G-CSF (n = 5) versus PBS (n = 3). rh-G-CSF: p(LSK) < 0.001; p(GMP) = 0.01; p(CMP) = 0.02; p(MEP) < 0.001. (C) Transcriptional G-CSF levels in the marrow of control-, rh-G-CSF–, RB6-8C5–, and 1A8-treated C57BL/6 mice. Note the increase of G-CSF on neutropenia and the negative feedback at the RNA level after application of rh-G-CSF. p(rh-G-CSF) = 0.045; p(RB6-8C5) = NS; p(1A8) = NS.
To examine transcriptional regulation of G-CSF in response to neutrophil leukocytosis, C57BL/6 mice received daily injections of rh-G-CSF.22 On day 5, peripheral blood was obtained and animals were killed. Bone marrow flow cytometry (Figure 3A-B) revealed changes indistinguishable from mice with antibody induced neutropenia. Specifically, marrow hematopoietic precursors showed a 4-fold increase of LSK cells and a 3-fold increase of GMP at the expense of MEP (Figure 3A-B). Although hematopoiesis in neutropenic and rhG-CSF–treated mice appeared phenotypically indistinguishable, whole bone marrow–derived G-CSF mRNA in G-CSF–treated mice plummeted (Figure 3C). Thus, there is a negative feedback loop, whereby G-CSF–induced neutrophil leukocytosis induces down-regulation of marrow G-CSF transcription.
In summary, G-CSF is down-regulated on rh-G-CSF–induced neutrophilia, showing negative feedback on the RNA level. On the other hand, G-CSF plasma levels are not affected by neutrophil transfusions. We therefore conclude that transcriptional regulation of G-CSF is indeed dependent on neutrophil mass. However, in contrast to TPO, G-CSF receptor scavenging does not seem to play a role in G-CSF regulation. In lymphocyte deficient mice, this pathway is robust and therefore independent of IL-17 secreting NK, NK-like, and T cells.
Reduced steady-state granulopoiesis with conserved feedback homeostatic regulation in germ-free mice
Bacterial colonization was established to influence development of both innate and adaptive immunity.23 Granulopoiesis is hypothesized to be highly dependent on peripheral needs. Although results obtained in G-CSF– and CSF3R-deficient mice, who display strongly reduced neutrophil numbers have revealed the importance of G-CSF signaling in steady-state neutrophil homeostasis,24,25 “emergency granulopoiesis” is mediated by microbial compounds binding to pattern recognition receptors.26 Bacterial flora was also shown to enhance the ability of neutrophils to kill pathogenic bacteria.26
We hypothesized that steady-state granulopoiesis could indirectly be influenced by microbial colonization and therefore analyzed GF mice in the steady-state.
Steady-state comparative analyses of C57BL/6 mice kept under SPF conditions versus GF C57BL/6 mice revealed reduced total white blood counts (7300/μL versus 3400/μL) and a significantly smaller proportion of Gr-1+/CD11b+ neutrophils in GF mice (1424/μL versus 374/μL). Indeed, neutrophil counts in GF mice were lower than described in G-CSF– or CSF3R-deficient animals.24,25 Manual differential blood counts confirmed reduced relative proportions of band and polymorphonuclear neutrophils. These results may indicate that steady-state granulopoiesis could be at least partly dependent on pattern recognition receptors.
Toll-like receptors (TLRs) have been found to be expressed on hematopoietic stem cells and myeloid progenitors of the granulocyte lineage, and microbial compounds may provide cues for hematopoiesis including neutrophil production.27 Because neutropenia may feedback stimulate hematopoiesis via commensal bacteria invading mucosal membranes unopposed by neutrophils thereby triggering TLR-signaling, we used GF mice to study the influence of commensal microbiotes on neutrophil homeostasis. To this end, GF mice either received 1A8 antibody or PBS, and effects on hematopoiesis were analyzed as described (Figure 4A). Comparable with SPF mice, neutropenia induced absolute and relative increases of the LSK and GMP populations: the number of LSK cells in neutropenic GF mice increased from 0.31 × 105 ± 0.14 × 105 to 1.72 × 105 ± 0.54 × 105. Myeloid progenitors were enriched in the marrows of neutropenic GF mice, GMP increased from 3.18 × 105 ± 0.28 × 105 to 9.25 × 105 ± 0.49 × 105 (Figure 4B). Moreover, analysis of cytokine plasma levels revealed significant increases of plasma G-CSF in the antibody treated group from 34 pg/mL to 293 pg/mL (Figure 4C).
Commensal germs and MyD88 are dispensable in G-CSF–mediated feedback. (A) Flow cytometry of peripheral blood from neutropenic GF C57BL/6 mice and controls (n = 3). (B) Absolute marrow cell numbers in both hind limbs of GF C57BL/6 mice. Note the similarity of changes in GF mice compared with SPF-kept C57BL/6 (n = 3). p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) = NS; p(MEP) = NS. (C) Plasma G-CSF levels in C57BL/6 and GF mice after neutrophil depletion with 1A8 versus controls. Note the significant increase of G-CSF in neutropenic mice and decreased baseline G-CSF levels in GF mice compared with animals maintained under SPF conditions [n(GF) = 3, n(C57BL/6) = 3; p(C57BL/6) = 0.04; p(GF C57BL/6) = 0.02].
Commensal germs and MyD88 are dispensable in G-CSF–mediated feedback. (A) Flow cytometry of peripheral blood from neutropenic GF C57BL/6 mice and controls (n = 3). (B) Absolute marrow cell numbers in both hind limbs of GF C57BL/6 mice. Note the similarity of changes in GF mice compared with SPF-kept C57BL/6 (n = 3). p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) = NS; p(MEP) = NS. (C) Plasma G-CSF levels in C57BL/6 and GF mice after neutrophil depletion with 1A8 versus controls. Note the significant increase of G-CSF in neutropenic mice and decreased baseline G-CSF levels in GF mice compared with animals maintained under SPF conditions [n(GF) = 3, n(C57BL/6) = 3; p(C57BL/6) = 0.04; p(GF C57BL/6) = 0.02].
Taken together, analysis of GF C57BL/6 mice shows that a total lack of microbial colonization is associated with extraordinarily decreased neutrophil numbers in the steady-state as well as decreased baseline G-CSF levels. However, on antibody-induced neutropenia, GF mice generate responses both at the marrow and peripheral blood level indistinguishable from wild-type or NSG animals.
Neutropenia-induced feedback regulation is dependent on TLR4 and TRIF, but not TLR2 and MyD88
GF-maintained mice may receive TLR stimuli through LPS in their food and drinking water.28 We therefore analyzed, whether interference with downstream signaling pathways might abrogate feedback neutrophil granulopoiesis in mice resistant to endotoxin (Tlr4Lps-d) by a spontaneous mutation29 and mice deficient in both TLR2 and TLR4.30
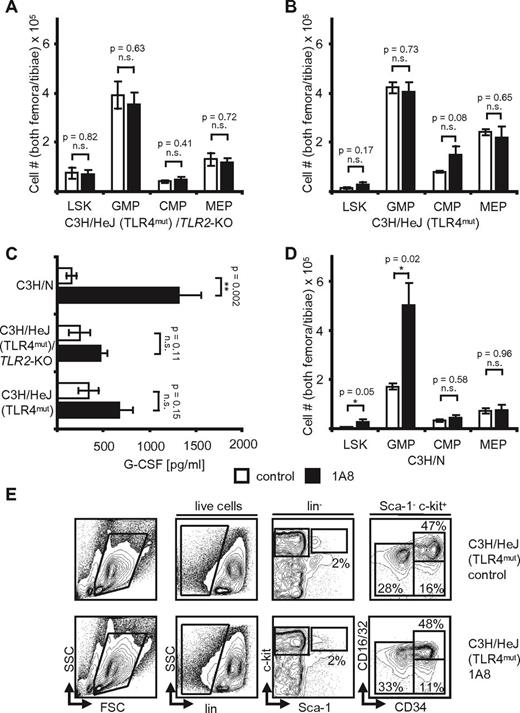
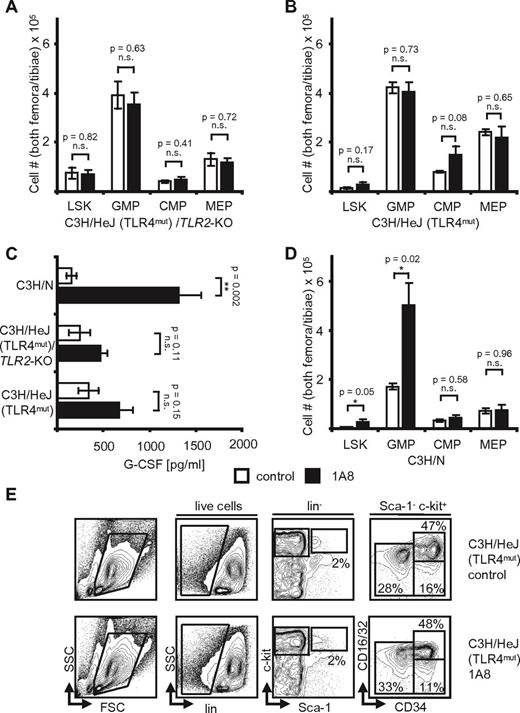
When C3H/HeJ(TLR4mut) /TLR2−/− mice, C3H/HeJ (TLR4mut) mice, and C3H/N wild-type control mice were challenged with 1A8 antibody, we found only insignificant changes from baseline in the knockout mice (Figure 5A-C). Indeed, whereas wild-type mice showed an increase of G-CSF by 8.5-fold from 156 pg/mL to 1318 pg/mL, there was a nonsignificant increase in the knockout animals (Figure 5C). Moreover, all neutropenia induced changes at the hematopoietic progenitor cell level were to be observed in control C3H/N, but not in knockout mice (Figure 5A-B,D-E). Numbers of marrow hematopoietic progenitors were not significantly different in neutropenic C3H/HeJ(TLR4mut) /TLR2−/− mice versus control mice, and LSK cells remained unchanged (Figure 5A). Interestingly, steady-state G-CSF levels differed between mouse strains, but were identical within a given background, that is, C57BL/6 or C3H/N.
Neutropenia-induced feedback regulation is TLR-dependent. (A) Absolute cell numbers in hind limb marrows of C3H/HeJ/TLR2-KO mice. Note that control and neutropenic mice (n = 5, each) are identical. p(LSK, GMP, CMP, and MEP) = NS. (B) Absolute cell numbers in hind limb marrows of C3H/HeJ mice after 8 days of 1A8-induced neutropenia. There are no significant differences in control versus neutropenic mice (n = 5). p(LSK, GMP, CMP, and MEP) = NS. (C) Plasma G-CSF levels in control and neutropenic C3H/N, C3H/HeJ/TLR2-KO, and C3H/HeJ mice. Note the significant increase of G-CSF levels in C3H/N and the insignificant differences in C3H/HeJ mice (n = 5). p(C3H/N) = 0.002; p(C3H/HeJ/TLR2-KO) = NS; p(C3H/HeJ) = NS. (D) Absolute cell numbers calculated to reflect total cell counts in both femora and tibiae of n = 3 C3H/N wild-type mice after treatment with 1A8 for 8 days. Note the significant changes of LSK and GMP. p(LSK) = 0.05; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = NS. (E) Flow cytometric analyses of marrow cells in control and 1A8-treated C3H/HeJ mice. Note equal numbers in control and neutropenic mice.
Neutropenia-induced feedback regulation is TLR-dependent. (A) Absolute cell numbers in hind limb marrows of C3H/HeJ/TLR2-KO mice. Note that control and neutropenic mice (n = 5, each) are identical. p(LSK, GMP, CMP, and MEP) = NS. (B) Absolute cell numbers in hind limb marrows of C3H/HeJ mice after 8 days of 1A8-induced neutropenia. There are no significant differences in control versus neutropenic mice (n = 5). p(LSK, GMP, CMP, and MEP) = NS. (C) Plasma G-CSF levels in control and neutropenic C3H/N, C3H/HeJ/TLR2-KO, and C3H/HeJ mice. Note the significant increase of G-CSF levels in C3H/N and the insignificant differences in C3H/HeJ mice (n = 5). p(C3H/N) = 0.002; p(C3H/HeJ/TLR2-KO) = NS; p(C3H/HeJ) = NS. (D) Absolute cell numbers calculated to reflect total cell counts in both femora and tibiae of n = 3 C3H/N wild-type mice after treatment with 1A8 for 8 days. Note the significant changes of LSK and GMP. p(LSK) = 0.05; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = NS. (E) Flow cytometric analyses of marrow cells in control and 1A8-treated C3H/HeJ mice. Note equal numbers in control and neutropenic mice.
Therefore, we also analyzed TLR4 knockout mice on the C57BL/6 background. As expected from the results obtained in C3H/HeJ mice, TLR4-deficient animals showed no significant changes in plasma G-CSF levels (78 pg/mL in control mice versus 77 pg/mL in 1A8-treated mice; Figure 6A). Moreover, analysis of the marrows showed that treatment with 1A8 resulted in minor, nonsignificant changes at the progenitor level: LSK cells increased insignificantly from 1.26 × 105 (control) to 1.75 × 105 cells (1A8). GMP increased from 2.49 × 105 to 3.0 × 105 cells, and there was a slight decrease of MEP from 2.32 × 105 in control mice to 1.87 × 105 in 1A8-treated mice (Figure 6B). We conclude from these findings that TLR4 is essential in homeostatic, G-CSF–dependent feedback regulation of neutrophil granulopoiesis.
TLR4 and TRIF are necessary for feedback granulopoiesis. (A) Plasma G-CSF levels in control and antibody treated C57BL/6 (n = 3, each), TLR4−/− (n = 3, each), MyD88−/− (n = 5, each), and TRIF−/− mice [n(1A8) = 5, n(PBS) = 4]. Note the significant increase of G-CSF in C57BL/6 and MyD88−/− and nonsignificant changes in the TLR4−/− and TRIF−/− mice. p(C57BL/6) = 0.04; p(TLR4−/−) = NS; p(MyD88−/−) < 0.001; p(TRIF−/−) = NS. (B) Absolute marrow cell numbers in TLR4−/− hind limbs after 8 days of 1A8-induced neutropenia. Note the insignificant changes of LSK, GMP, and MEP (n = 3). p(LSK, GMP, CMP, and MEP) = NS. (C) Absolute cell numbers in hind limbs of n = 5 MyD88−/− mice. LSK and GMP are significantly increased in neutropenia. p(LSK) = 0.03; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = 0.03. (D) Hind limb marrow cell numbers of n = 5 TRIF−/− mice after 8 days of 1A8-induced neutropenia versus PBS (n = 4). Although there is an increase of LSK, changes at progenitor level are nonsignificant. p(LSK) = 0.02; p(GMP) = NS; p(CMP) = NS; p(MEP) = NS.
TLR4 and TRIF are necessary for feedback granulopoiesis. (A) Plasma G-CSF levels in control and antibody treated C57BL/6 (n = 3, each), TLR4−/− (n = 3, each), MyD88−/− (n = 5, each), and TRIF−/− mice [n(1A8) = 5, n(PBS) = 4]. Note the significant increase of G-CSF in C57BL/6 and MyD88−/− and nonsignificant changes in the TLR4−/− and TRIF−/− mice. p(C57BL/6) = 0.04; p(TLR4−/−) = NS; p(MyD88−/−) < 0.001; p(TRIF−/−) = NS. (B) Absolute marrow cell numbers in TLR4−/− hind limbs after 8 days of 1A8-induced neutropenia. Note the insignificant changes of LSK, GMP, and MEP (n = 3). p(LSK, GMP, CMP, and MEP) = NS. (C) Absolute cell numbers in hind limbs of n = 5 MyD88−/− mice. LSK and GMP are significantly increased in neutropenia. p(LSK) = 0.03; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = 0.03. (D) Hind limb marrow cell numbers of n = 5 TRIF−/− mice after 8 days of 1A8-induced neutropenia versus PBS (n = 4). Although there is an increase of LSK, changes at progenitor level are nonsignificant. p(LSK) = 0.02; p(GMP) = NS; p(CMP) = NS; p(MEP) = NS.
TLR-signaling involves the recruitment of 1 or several TIR domain-containing adaptor proteins such as MyD88, TIRAP, TRIF, or TRAM.31 Therefore, MyD88−/−32 and TRIF−/− mice33 were analyzed in addition. MyD88−/− and C57BL/6 control mice received 1A8 antibody as described. Analysis of MyD88−/− peripheral blood, plasma, and marrow displayed changes indistinguishable from results obtained in C57BL/6 animals (Figure 6A-C). Moreover, plasma G-CSF increased significantly up to 6-fold in neutropenic MyD88−/− mice from 56 pg/mL to 347 pg/mL. Analysis of neutropenic versus control TRIF−/− mice showed a nonsignificant increase of plasma G-CSF from 184 pg/mL (PBS) to 422 pg/mL (1A8; Figure 6A). Moreover, LSK expansion was detectable with a significant increase of LSK cells from 1.06 × 105 in control to 2.54 × 105 in antibody treated animals. GMP numbers as well as MEP levels, however, remained unchanged (Figure 6D). Expressional analysis of TLR4, MyD88, and TRIF RNA in the marrow of all tested mouse types revealed unchanged expression levels (data not shown).
Taken together, TLR4 is essential for feedback regulation in neutropenia. Interestingly, absence of TRIF partially abrogates feedback G-CSF up-regulation and respective changes in the marrow, whereas TLR2 and MyD88 are completely dispensable. We conclude that undisturbed signaling via TLR4 represents necessary elements of the signaling pathway sensing neutropenia and initiating the appropriate response.
Neutropenia inhibits bone marrow CXCL12 transcription
Neutrophil supply is not only dependent on production, but also on release from the marrow. Down-regulation of CXCL12 within the marrow milieu has been described to be associated with facilitated neutrophil egress.34 We therefore analyzed marrow CXCL12 under various experimental conditions.
C57BL/6 mice received rh-G-CSF at a dose of 300 μg/kg body weight for 5 consecutive days.22 Peripheral blood revealed leukocytosis with 82% neutrophils (13 260/μL ± 2180/μL leukocytes, 10 864/μL ± 1810/μL neutrophils). As previously described, whole marrow CXCL12 RNA levels were significantly reduced by approximately 10-fold (supplemental Figure 5A). Interestingly, neutropenia down-regulated CXCL12 RNA at a comparable magnitude both in spf-kept and in GF wild-type mice (supplemental Figure 5B). In addition, flow cytometric surface expression levels of CXCR4 were determined in LSK, GMP, CMP, MEP, and Gr1low/CD11b+ cells. CXCR4 was slightly down-regulated on granulocyte depletion in all examined populations except from MEPs (data not shown). Thus, antibody induced neutropenia may result in facilitated egress through suppression of marrow CXCL12.
Analysis of marrow CXCL12 in NSG mice in the steady-state demonstrated a significant increase by approximately 3-fold compared with C57BL/6 mice (supplemental Figure 5A). Therefore, steady-state neutropenia in NSG mice may also be because of reduced egress of neutrophils. On neutropenia, however, down-regulation of CXCL12 in NSG mice was similar to wild-type mice (supplemental Figure 5C).
To establish the importance of TLR-signaling, TLR4−/−, C3H/HeJ, C3H/HeJ/TLR2−/−, and C3H/N control mice received PBS or 1A8, and CXCL12 RNA was analyzed. Neutropenic C3H/HeJ and C3H/N mice displayed significantly decreased CXCL12 RNA. However, in C3H/HeJ/TLR2−/−and C57BL/6 TLR4−/− mice, the CXCL12 decrease did not reach a significant extent (supplemental Figure 5D).
In conclusion, increased marrow granulopoiesis, whether driven by exogenous rh-G-CSF or neutropenia, results in decreased CXCL12 levels. In addition, peripheral blood neutropenia in NSG mice is not only because of loss of the IL-17 signaling axis, but may also be ascribed to CXCL12 mediated retention of neutrophils.
Discussion
In contrast to feedback regulation of erythropoiesis and megakaryopoiesis, knowledge on mechanisms of neutrophil granulopoiesis is fragmentary. Early observations of up-regulated G-CSF levels in neutropenic patients after myelosuppressive chemotherapy and in cyclic neutropenia have led to the conclusion that either a “neutrostat” would sense peripheral neutrophil levels or a neutrophil turnstile located at the marrow-blood interface would enumerate released neutrophils to provide feedback regulation of G-CSF and adapt marrow neutrophil production to peripheral needs.35
To elucidate feedback mechanisms, we used a model of neutropenia that includes minimum inflammation.15 Animals were kept SPF and received amoxicillin in their drinking water. During prolonged neutropenia, mice were closely monitored for signs of infection. Therefore, infection induced changes, also known as “emergency granulopoiesis” did not play a role in our study of neutrophil homeostasis.
Our findings show that 2 different anti Ly6G-antibodies effectively and specifically induced neutropenia in all studied mice (supplemental Figures 6 and 7). Neutropenia resulted in identical changes of hematopoietic marrow composition and G-CSF feedback stimulation independent from the binding epitope.
G-CSF is the principal neutrophil regulating cytokine, and the absence of G-CSF in humans or in knockout mice results in severe neutropenia.1,24 Based on observations in adhesion-molecule deficient mice, IL-23 and IL-17, secreted by dendritic cells/macrophages and TH17−CD4− T cells, respectively, have been described as upstream regulators of G-CSF.5,36 Although G-CSF seems to be necessary for the maintenance of neutrophils in steady-state mice, interference with IL-17 did not reduce neutrophil numbers.37 In our model of antibody-induced neutropenia, IL-17 increased significantly, confirming previous results.5 However, neutropenia in lymphocytopenic NSG-mice displaying baseline IL-17 levels without any variation resulted in positive feedback regulation of marrow hematopoiesis and increased G-CSF expression at the transcriptional level. Therefore, we conclude that the IL-17–dependent pathway is redundant.
Because feedback regulation of G-CSF was efficient in NSG-mice, we hypothesized that highly conserved sensing mechanisms of neutropenia may perceive indirect sequelae of neutropenia, such as mucosal membrane invasion by commensal germs which have been shown to regulate systemic immunity.38 Sensing receptors of pathogen-associated molecules include TLR2, TLR4, NOD1, and NOD2. TLR4 is known as the major LPS receptor, TLR2 is a mediator of responses to gram-positive bacteria. TLR2 and -4 agonists regulate neutrophil functions and contribute to inhibition of neutrophil apoptosis in states of antimicrobial response.39,40 Furthermore, several molecules including hyaluronan, surfactant protein-A, β-defensin, and byglycan represent endogenous TLR2 and TLR4 ligands.41 Moreover, heat shock proteins, such as Gp96, may represent endogenous potentiating factors allowing minute concentrations of TLR-ligands to generate a TLR-dependent signal.42 To study the influence of the mucosal bacterial flora on feedback granulopoiesis, we took advantage of germ-free C57BL/6 mice.38 These animals do not harbor commensal microbiotes, but they have also been shown to lack TH17− cells in the colonic bowel wall. However, when germ-free mice were made neutropenic we found that their ability to mount the typical response was preserved in the absence of commensal germs. Therefore, intestinal microflora is not a necessary prerequisite for the observed feedback regulation of neutrophil granulopoiesis. A potential disturbing factor in this model are effects of lipopolysaccharide (LPS) contained in the experimental animals' autoclaved food,28 which exert TLR2 and TLR4 agonist activity,39 enabling these mice to create feedback granulopoiesis on neutropenia. Thus, we examined TLR4-deficient, TLR4-mutated, and TLR2-deficient/TLR4-mutated animals. All of these genetically modified mice displayed markedly disturbed feedback up-regulation of G-CSF, LSK and GMP expansion.9 Indeed, absence of TLR4 resulted in a complete loss of feedback to neutropenia with a marrow composition and peripheral G-CSF levels identical to control treated mice. Therefore, we conclude that TLR4 is required for positive feedback signaling in antibody-induced neutropenia. Moreover, TRIF, but not MyD88 signaling is involved in G-CSF up-regulation in vivo, because MyD88−/− display an undisturbed feedback both at the G-CSF level and the marrow progenitor cell level. In contrast, TRIF−/− mice showed an insignificant increase of G-CSF over baseline and no GMP expansion.
Although binding of G-CSF to its cognate receptor (CSF3R) expressed on committed hematopoietic progenitors of the granulocyte lineage mediates proliferative and differentiative effects,35 G-CSF–mediated mobilization of hematopoietic stem and progenitor cells, as well as neutrophils from the marrow, has been elegantly shown to be mediated indirectly by CSF3R+ monocytes.43,44 Our data clearly show that neutropenia per se up-regulates plasma G-CSF levels. Moreover, we demonstrate for the first time that administration of exogenous rh-G-CSF down-regulates marrow G-CSF expression. Although negative feedback mechanisms of G-CSF stimulated neutrophilia on granulopoiesis have been described to be in part dependent on enzymatic cleavage of G-CSF by neutrophil elastase,45 our results demonstrate that there is feedback on the transcriptional level. Interestingly, infusion of CSF3R+ neutrophils did not significantly influence plasma G-CSF. The latter finding provides evidence against a model of indirect regulation of plasma G-CSF by neutrophil pool size.
Neutrophil numbers in peripheral blood are determined by their production rate, half-life, and their positioning (margination and marrow release); only 1% to 2% of neutrophils circulate in the periphery in the steady-state.46 Emergency granulopoiesis results in both increased neutrophil production and release to circulation.47 There is evidence that G-CSF releases neutrophils from the marrow by disrupting their anchoring to a CXCL12-positive marrow niche through CXCR4 expressed on the neutrophil surface.48 Our results are in line with previous observations that marrow CXCL12 expression is reduced during neutrophil depletion.46 Similar to emergency granulopoiesis, neutropenia-induced feedback granulopoiesis in our model is regulated by production and release. However, G-CSF may be responsible for both effects.
Regulation of myelopoiesis has been demonstrated to be dependent on its own downstream cellular components as well as on stromal cells. Loss or depletion of both conventional dendritic cells and macrophages was shown to increase myelopoiesis.49 Both cell types may function as negative regulators of myelopoiesis. Thus, neutrophil mass may either be sensed by macrophages as previously suggested5 or function as a negative regulator of myelopoiesis similar to macrophages and dendritic cells. Our findings suggest that, indeed, there is a “neutrostat,“ which may be located on the marrow level. In fact, emergency granulopoiesis has been shown to be dependent on TLR4 expressed on the nonhematopoietic marrow compartment.7 Preliminary results show that marrow-derived mesenchymal stromal cells potently up-regulate G-CSF expression on TLR4 triggering and that up-regulation of G-CSF RNA on neutropenia is to be detected in the CD45− marrow population (data not shown).
Our results challenge the current dogmatic distinction of steady-state versus emergency granulopoiesis: we suggest a mechanism relying on highly conserved signaling pathways that constantly adapt neutrophil production to environmental needs. The importance of exogenous versus endogenous TLR-ligands as well as cell-type dependent mechanisms are still to be determined, but the fact that antibiotic treatment reduces the efficiency of stem-cell mobilization by G-CSF in humans50 underscores the potential clinical importance of our findings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Kosgalwis, University of Ulm, for expert gnotobiotic animal breeding facilities.
H.G.K. was supported by grants from the German Research Foundation (SFB685, project A7) and Deutsche Krebshilfe (Max Eder Program, project 109833); M.R. is supported by the German Research Foundation KFO183 (Ra 988/4-2) and by the Federal Ministry of Education and Research (BMBF 01EO1003); and M.C.A. was supported by grants from the Deutsche Forschungsgemeinschaft (KFO 183/TP 4).
Authorship
Contribution: S.B. performed the majority of the experiments and contributed to the writing of the paper; S.W. designed some of the experiments, analyzed and interpreted data, and contributed to the writing of the paper; M.P.R., H.S., M.C.A., J.S.F., R.H., and H.G.R. provided mice; P.S., E.M., T.W., and M.M. contributed some of the experiments; M.R.M. and L.K. analyzed and interpreted data; and H.G.K. designed the project and experiments, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans-Georg Kopp, Dept Hematology/Oncology, Eberhard-Karls University, Otfried-Mueller-Str 10, D-72076 Tuebingen, Germany; e-mail: hans-georg.kopp@med.uni-tuebingen.de.
References
Author notes
S.B. and S.W. contributed equally to this work.

![Figure 2. Neutropenia-induced feedback in NSG mice. (A) NSG mice received 1A8, RB6-8C5, or PBS. Flow cytometric analyses of marrows are shown. Note the significant changes of LSK, GMP, and MEP. (B) Absolute cell numbers in both femora and tibiae of NSG mice (n = 5). RB6-8C5: p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) < 0.001; p(MEP) = 0.02; 1A8: p(LSK) < 0.001; p(GMP) = NS; p(MEP) = NS; p(CMP) = NS. (C) Plasma G-CSF and M-CSF levels in control and antibody-treated NSG mice. Note significant change of G-CSF [n(control) = 4; n(RB6-8C5) = 3; n(1A8) = 3]. G-CSF: p(RB6-8C5) = 0.003; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520002.jpeg?Expires=1769104170&Signature=ZTwDs8KQsXiBnkyzmBH5bds7HwmXuBo~nPgF98OUFR5lBe1LaScilpaTpAeZtbhJh-gdyDGPzfbeCTs-tdLdoOps9U5IMiuX49~ukmFb3g6MTwcU1IPdELD4~QtTQNwl8E61OPh5e-MOqfQvTo8ORY05cZxKcZmvdw8RpPToozXhWBjHIZCz7ZUzWzVHWfL8nkjj~FTbUsV-exupdXvwFsj0Owy2hnO7aEqOzHs0y-T-TsuLh~2pqT6VgbO6YDvy0MgvJoSiilcr~dFlxpQemswdPEIx8oiHm6ak6YtSv67qIFGfio2Yzf3tOUvBPxHH-1c2BxD73WD6glDGDOH6AA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Commensal germs and MyD88 are dispensable in G-CSF–mediated feedback. (A) Flow cytometry of peripheral blood from neutropenic GF C57BL/6 mice and controls (n = 3). (B) Absolute marrow cell numbers in both hind limbs of GF C57BL/6 mice. Note the similarity of changes in GF mice compared with SPF-kept C57BL/6 (n = 3). p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) = NS; p(MEP) = NS. (C) Plasma G-CSF levels in C57BL/6 and GF mice after neutrophil depletion with 1A8 versus controls. Note the significant increase of G-CSF in neutropenic mice and decreased baseline G-CSF levels in GF mice compared with animals maintained under SPF conditions [n(GF) = 3, n(C57BL/6) = 3; p(C57BL/6) = 0.04; p(GF C57BL/6) = 0.02].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520004.jpeg?Expires=1769104170&Signature=l1KlnkQDx-31O8lss053FmG4Y2PXvrom~-ESx2EUGW~Wbms0XkYR93uxGDDhHIPDmWIakCzO7WhQgakGJlHnP-CWCSJXsGIhelk9v6FipoZG0EEDQbPJmNv8vEczZQzpVIPKO8uOB6eRemCPkfYnItgU3rd~7lNEAdrL9Vb~eWQHLofhnBmQVfiVcrbKh7xYlQZFR~Sj8uuFpW57XmYOeYmwUBq1yq4xGezDaS2pT9wxz939Wsa8cR1XBmZcZZ0q7DiuLrShOA2Ej3V1gpfvdfI5EMlm7RYBrAmxih2sfuVcqar2iCBtiLE5vDj-9JQVoek9WZiwhlw-bUmHj6G3Rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. TLR4 and TRIF are necessary for feedback granulopoiesis. (A) Plasma G-CSF levels in control and antibody treated C57BL/6 (n = 3, each), TLR4−/− (n = 3, each), MyD88−/− (n = 5, each), and TRIF−/− mice [n(1A8) = 5, n(PBS) = 4]. Note the significant increase of G-CSF in C57BL/6 and MyD88−/− and nonsignificant changes in the TLR4−/− and TRIF−/− mice. p(C57BL/6) = 0.04; p(TLR4−/−) = NS; p(MyD88−/−) < 0.001; p(TRIF−/−) = NS. (B) Absolute marrow cell numbers in TLR4−/− hind limbs after 8 days of 1A8-induced neutropenia. Note the insignificant changes of LSK, GMP, and MEP (n = 3). p(LSK, GMP, CMP, and MEP) = NS. (C) Absolute cell numbers in hind limbs of n = 5 MyD88−/− mice. LSK and GMP are significantly increased in neutropenia. p(LSK) = 0.03; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = 0.03. (D) Hind limb marrow cell numbers of n = 5 TRIF−/− mice after 8 days of 1A8-induced neutropenia versus PBS (n = 4). Although there is an increase of LSK, changes at progenitor level are nonsignificant. p(LSK) = 0.02; p(GMP) = NS; p(CMP) = NS; p(MEP) = NS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520006.jpeg?Expires=1769104170&Signature=y5uIJdA~iuc6R1n~fZ6jb9FQClRA1z57KHlLqtvFkSxi~USsRrgKUAmKN0KydXWxvE-k8dy7-ajS9NPRUfhy19IZom7scUiYAl7TV1wSxoKmmEXojzKV32w3A8DJ5DuGVwHMSV68TTzusKkKgf68-8ywNoX3YL7KN9AzSQGc4c2Eb9F1fwEjvj~vVz4CPUDvNwlP8s2UAx9nrrTxN6mSkwvCR73n30dMjC7uR91rw9tW9LOUy0qezJ2VXsc9Ba01v4SrQcx3Tkt56vPOtcjOqOJMLvURh7UZW47EF8Q9T~HOdiqjFtUbMAsd8tA~RESKod91WCuwPZYeZ66NZQCl8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Neutropenia-induced feedback in NSG mice. (A) NSG mice received 1A8, RB6-8C5, or PBS. Flow cytometric analyses of marrows are shown. Note the significant changes of LSK, GMP, and MEP. (B) Absolute cell numbers in both femora and tibiae of NSG mice (n = 5). RB6-8C5: p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) < 0.001; p(MEP) = 0.02; 1A8: p(LSK) < 0.001; p(GMP) = NS; p(MEP) = NS; p(CMP) = NS. (C) Plasma G-CSF and M-CSF levels in control and antibody-treated NSG mice. Note significant change of G-CSF [n(control) = 4; n(RB6-8C5) = 3; n(1A8) = 3]. G-CSF: p(RB6-8C5) = 0.003; p(1A8) < 0.001; M-CSF: p(1A8 and RB6-8C5) = NS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520002.jpeg?Expires=1769104171&Signature=eRgDJ00s7296jJBnflhCH~-44QwaGRhU54894enojnBC4K88C4kyLenqDaYHPTkArODsU8Pc0orbaGbSLO0ltTEjl1XCGBPXHox--azKbfl7F1TOVWG2tXDBx12els~uHo2OoCJjTHGmiA639nkMD~zbPt~tMEGrvMpCOpSPQf5rpDkDK47rDiS8Wr-HDJdz-m9qCzlLgxu6GUMEAyZhoxpmfbNCFvhUGBC6ZC2m8g6~4syMe8rzNSAQtkEFjfl6Xok2njxOzATgXFeIXcnNiONkarEXIPnOYHb3C5Xq6GjYepd17Ubm1M5acwyFlZKquOFxW6UVG9vsm2iPRCJmSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Commensal germs and MyD88 are dispensable in G-CSF–mediated feedback. (A) Flow cytometry of peripheral blood from neutropenic GF C57BL/6 mice and controls (n = 3). (B) Absolute marrow cell numbers in both hind limbs of GF C57BL/6 mice. Note the similarity of changes in GF mice compared with SPF-kept C57BL/6 (n = 3). p(LSK) = 0.04; p(GMP) = 0.05; p(CMP) = NS; p(MEP) = NS. (C) Plasma G-CSF levels in C57BL/6 and GF mice after neutrophil depletion with 1A8 versus controls. Note the significant increase of G-CSF in neutropenic mice and decreased baseline G-CSF levels in GF mice compared with animals maintained under SPF conditions [n(GF) = 3, n(C57BL/6) = 3; p(C57BL/6) = 0.04; p(GF C57BL/6) = 0.02].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520004.jpeg?Expires=1769104171&Signature=RXya7hIQUPOWzFdYHPYSDynMBeUmzfQY6I8gnaoJmeKRn13mteAc1OQlc47n4szHS7V5F-u~1ggMe6TnrNJl2cDREbDXi0TrTjWZCpyfPaHj7m-FsUgKB9Ls1U1ReBc~p9v5MGo3BPOXxAp3xTFEFJdqhVehMkNHhosx2E6R-DLr4KxNdDdnB6bMHqZKauS8u53lZZNEjjr4WA5R-dhpUGX9XjE3nq4YtodOHZkhDH3ASEorQRhgD3ut4Yvfm~cJp~YTkzcPzBK5Ymf7xDmms2oKgLq6WAAsmcpLXYvXySZk2iPBc1tr3m1crSMDIc6elEXBb4BsNlZvRAEGPq1jpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. TLR4 and TRIF are necessary for feedback granulopoiesis. (A) Plasma G-CSF levels in control and antibody treated C57BL/6 (n = 3, each), TLR4−/− (n = 3, each), MyD88−/− (n = 5, each), and TRIF−/− mice [n(1A8) = 5, n(PBS) = 4]. Note the significant increase of G-CSF in C57BL/6 and MyD88−/− and nonsignificant changes in the TLR4−/− and TRIF−/− mice. p(C57BL/6) = 0.04; p(TLR4−/−) = NS; p(MyD88−/−) < 0.001; p(TRIF−/−) = NS. (B) Absolute marrow cell numbers in TLR4−/− hind limbs after 8 days of 1A8-induced neutropenia. Note the insignificant changes of LSK, GMP, and MEP (n = 3). p(LSK, GMP, CMP, and MEP) = NS. (C) Absolute cell numbers in hind limbs of n = 5 MyD88−/− mice. LSK and GMP are significantly increased in neutropenia. p(LSK) = 0.03; p(GMP) = 0.02; p(CMP) = NS; p(MEP) = 0.03. (D) Hind limb marrow cell numbers of n = 5 TRIF−/− mice after 8 days of 1A8-induced neutropenia versus PBS (n = 4). Although there is an increase of LSK, changes at progenitor level are nonsignificant. p(LSK) = 0.02; p(GMP) = NS; p(CMP) = NS; p(MEP) = NS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/5/10.1182_blood-2012-05-429589/4/m_zh89991301520006.jpeg?Expires=1769104171&Signature=THm5sZRJKjCLVc9yzW~h1TDxc3rI3e5QBEOvQ94pD5FFv7TGQVCS0wOhtrJOBvoWxPthX2ZHN4K6IvpNkGj~UpzpGUqfQhoqMGJ2Ug46W5tXtPqzGqxwPoyfLNE08hXABXwImE1HoPbkYxw6G3DA~2BzM-tmz-C48stQ-ATvVsx5pNKCmooIcBDgvF3pWI0CdRSslv-lLn97CAwQTXTrfBZioXDJGFZKL-qMjJ~rP19VbUSzGaew1K1CbWBWKIR29qR-KsqX2pjrNhJQa1kbotzxPB2L43ZmA0H15Ft90FMxKlg1ADlxl~ctRInqXEThAQNnyD7GxLlrb5IcQ5SgXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)