Key Points
NSG-3GS mice support enhanced clonal outputs from human short-term repopulating cells (STRCs) without affecting their engrafting efficiency.
Increased human STRC clone sizes enable their more precise and efficient measurement by peripheral blood monitoring.
Abstract
Better methods to characterize normal human hematopoietic cells with short-term repopulating activity cells (STRCs) are needed to facilitate improving recovery rates in transplanted patients. We now show that 5-fold more human myeloid cells are produced in sublethally irradiated NOD/SCID-IL-2Receptor-γchain-null (NSG) mice engineered to constitutively produce human interleukin-3, granulocyte-macrophage colony-stimulating factor and Steel factor (NSG-3GS mice) than in regular NSG mice 3 weeks after an intravenous injection of CD34+ human cord blood cells. Importantly, the NSG-3GS mice also show a concomitant and matched increase in circulating mature human neutrophils. Imaging NSG-3GS recipients of lenti-luciferase-transduced cells showed that human cells being produced 3 weeks posttransplant were heterogeneously distributed, validating the blood as a more representative measure of transplanted STRC activity. Limiting dilution transplants further demonstrated that the early increase in human granulopoiesis in NSG-3GS mice reflects an expanded output of differentiated cells per STRC rather than an increase in STRC detection.
Introduction
Current evidence suggests that human hematopoiesis involves the production of sequential stages of phenotypically separable populations of cells with increasingly restricted lineage options and decreasing self-renewal abilities.1 These subsets are thus capable of different durations and spectra of differentiated cell outputs on transplantation into myelosuppressed recipients. Interestingly, the most primitive of these cell types engraft T cell– and B cell–depleted mice at high efficiency; whereas those with short-term repopulating activity cells (STRCs; ie, limited to a few weeks) do not unless the NK cells of the mice are also completely eliminated.2,3 Efficient engraftment of STRC with myeloid-restricted activity (STRC-Ms) is currently most effectively achieved in NOD/SCID-IL-2Rγc−/− (NSG) mice.4,5 This has heightened interest in the use of NSG mice to design specific and sensitive functional assays for these cells. Characterization of human STRCs and their expansion is of particular interest for clinical transplantation protocols that could benefit from improved methods to predict and accelerate neutrophil (and platelet) recovery in patients.5 However, this has remained a challenge because of the poor output of human neutrophils in NSG mice, which is insufficient for peripheral blood (PB) sampling to be used to assess transplanted STRC activity. We now show that this difficulty can be overcome using NSG mice bred to previously engineered transgenic mice6 to enable expression of 3 human growth factors that specifically stimulate human granulopoiesis; that is, interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and Steel factor (SF), hereafter referred to as NSG-3GS mice.
Methods
Cells
CD34+ cell–enriched (> 80% pure) suspensions of low-density human cord blood (CB) cells were isolated using the EasySep kit (StemCell Technologies) from previously pooled and cryopreserved anonymized samples (collected according to procedures approved by the University of British Columbia Research Ethics Board). Lineage marker-negative (Lin−) CD34+ cells were isolated as described.5
Transplantation procedure
Cells were injected intravenously along with 106 irradiated (15 Gy) human bone marrow (BM) cells into 8- to 10-week-old NSG or NSG-3GS mice (bred and maintained in the Animal Resource Center of the BC Cancer Research Center) within 24 hours of the mice being irradiated (315 cGy 137Cs γ-rays). The frequency of human myeloid (CD45+CD33/15+) cells present in BM aspirates and 50 to 100 μL of PB samples was determined by FACS analysis of cells labeled with appropriate antibodies as previously described.5 Absolute values for circulating human myeloid cells were derived using AccuCheck counting beads (Invitrogen). All animal studies were approved by the Animal Care Committee of the University of British Columbia.
Lentiviral transduction and bioluminescence imaging
CD34+ CB cells were prestimulated for 16 hours in a serum-free medium containing BIT (StemCell Technologies) and 100 ng/mL FLT3-L (Immunex), 100 ng/mL SF and 20 ng/mL G-CSF (StemCell Technologies), 20 ng/mL IL-3 (Novartis), and 20 ng/mL IL-6 (Cangene), then incubated for 6 hours in the same medium containing a MND-lentivirus encoding a firefly luciferase cDNA (MND-LUC) and then immediately washed and transplanted. To collect bioluminescent images, anaesthetized mice were injected intraperitoneally with 150 mg/kg D-luciferin (Caliper Life Sciences) and 10 minutes later placed in the supine position in a Xenogen IVIS Lumina system (f stop 1, binning factor 4, and 60 seconds) with Living Image Version 3.0 software (Caliper Life Sciences).
Results and discussion
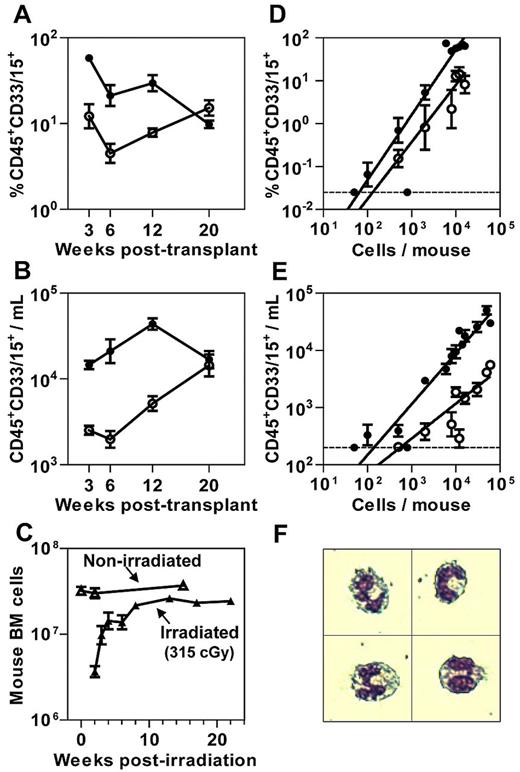
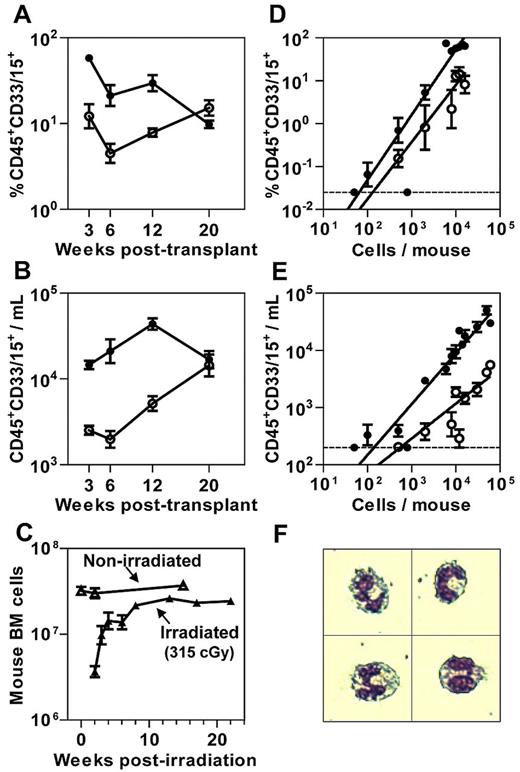
NSG-3GS mice were generated by crossing NSG mice with NS-3GS mice6 and selecting for F2 progeny homozygous for both the absence of the IL-2Rγc gene and the presence of the human 3GS cDNA transgene insert as carried out independently by the Mulloy laboratory.7 We then transplanted both genotypes of recipients with 104 lin−CD34+ CB cells to first compare the levels of human chimerism achieved in their BM (Figure 1A; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and PB (Figure 1B; supplemental Figure 1B) from 3 to 20 weeks later. This period was chosen because it was previously shown to encompass the sequential outputs of mature cells produced by different subsets of STRCs.8-10 The first of these are STRC-Ms which are predominantly CD34+CD38+8 and correlate with cells detected in vitro as primitive colony-forming cells.5 STRC numbers are inferred from their peak mature cell outputs seen at 3 weeks after transplant. STRC-M outputs are then followed by the progeny of CD34+CD38−CD90− cells that have lymphoid as well as myeloid potential (STRC-MLs), but because these have limited self-renewal ability, the production of mature progeny is not sustained beyond 16 to 20 weeks after transplant.8-10 The results of our time course comparison of cell outputs revealed a markedly elevated output of myeloid cells in NSG-3GS BM and PB during the first 12 weeks. Interestingly, this period also coincides with the time taken for the BM cellularity of nontransplanted but similarly irradiated NSG recipients to maximally recover (Figure 1C).
Increased short-term myeloid cell output in NSG-3GS mouse BM and PB. Kinetics of myeloid cell reconstitution in the BM (A) and PB (B) of NSG (open circles) and NSG-3GS (closed circles) mice after their transplantation with 104 lin−CD34+ CB cells. Data are the results of 12 mice per group pooled from 2 independent comparison experiments. (C) Total mouse BM cells per 2 femurs plus 2 tibias at the times indicated after irradiation of mice with 315 cGy 137Cs but no transplanted cells (3-10 mice per time-point). (D) Linear relationship between the number of CD34+ CB cells transplanted and the extent of human myeloid cell chimerism produced in the BM of NSG and NSG-3GS mice 3 weeks after transplantation of 50 to 1.6 × 104 CD34+ CB cells (3-33 mice per cell dose). (E) Linear relationship between the number of CD34+ CB cells transplanted and the absolute number of circulating human myeloid cells in NSG and NSG-3GS mice 3 weeks later (3-33 mice per cell dose). (F) Wright-Giemsa–stained human CD45+CD33/15+ cells in the blood of NSG-3GS mice 3 weeks after their transplantation with human CD34+ CB cells, representing > 90% of the sorted CD45+CD33/15+ cells analyzed. All values shown are the mean ± SEM. The dotted lines in panels D and E indicate the limit of detection of human CD45+CD33/15+ cells in BM and PB samples, respectively.
Increased short-term myeloid cell output in NSG-3GS mouse BM and PB. Kinetics of myeloid cell reconstitution in the BM (A) and PB (B) of NSG (open circles) and NSG-3GS (closed circles) mice after their transplantation with 104 lin−CD34+ CB cells. Data are the results of 12 mice per group pooled from 2 independent comparison experiments. (C) Total mouse BM cells per 2 femurs plus 2 tibias at the times indicated after irradiation of mice with 315 cGy 137Cs but no transplanted cells (3-10 mice per time-point). (D) Linear relationship between the number of CD34+ CB cells transplanted and the extent of human myeloid cell chimerism produced in the BM of NSG and NSG-3GS mice 3 weeks after transplantation of 50 to 1.6 × 104 CD34+ CB cells (3-33 mice per cell dose). (E) Linear relationship between the number of CD34+ CB cells transplanted and the absolute number of circulating human myeloid cells in NSG and NSG-3GS mice 3 weeks later (3-33 mice per cell dose). (F) Wright-Giemsa–stained human CD45+CD33/15+ cells in the blood of NSG-3GS mice 3 weeks after their transplantation with human CD34+ CB cells, representing > 90% of the sorted CD45+CD33/15+ cells analyzed. All values shown are the mean ± SEM. The dotted lines in panels D and E indicate the limit of detection of human CD45+CD33/15+ cells in BM and PB samples, respectively.
These results suggest that the NSG-3GS host enhances mature myeloid cell outputs from STRCs at the stage of terminal myelopoiesis. To address this question we focused on a more detailed study of STRC-Ms. A cell dose–response analysis showed that GM outputs in both BM (Figure 1D) and PB (Figure 1E) were linearly related to the number of CD34+ cells injected over a wide range in both host genotypes (up to at least 104 CD34+ cells per NSG-3GS mouse and > 6 × 104 CD34+ cells per NSG mouse). These results also confirm the ∼ 5-fold higher output of human GM cells in the NSG-3GS mice and show that it is transplant cell dose–independent. As a result, human GM cells are readily detectable in the PB of NSG-3GS recipients even when small numbers of human CD34+ cells are transplanted in contrast to NSG mice where human GM cells are close to the limit of detection for transplant doses of < 104 CD34+ CB cells. Morphologic analyses confirmed that the human GM cells in the PB were human neutrophils (Figure 1F).
The established linearity of the cell dose–response experiments validated the use of limiting dilution analysis (LDA) to quantify the efficiency of STRC-M detection in the 2 host genotypes. The results showed that the frequency of STRC-Ms detected in both types of recipient was the same (1/870 and 1/600 CD34+ cells, P = .35; see supplemental Table 1 for details). Thus, the difference in 3-week human neutrophil outputs measured in the 2 strains is entirely attributable to a difference in the clonal outputs from similar numbers of STRCs.
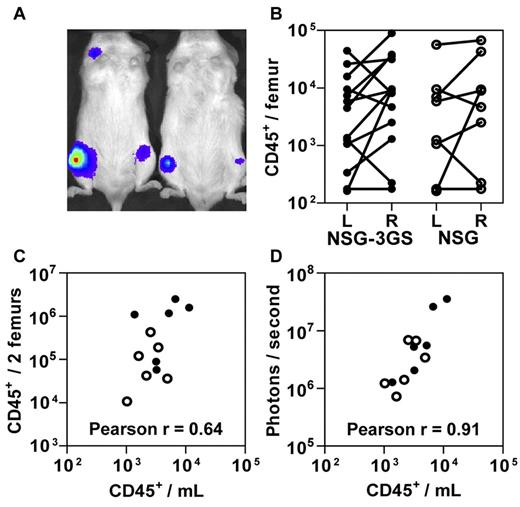
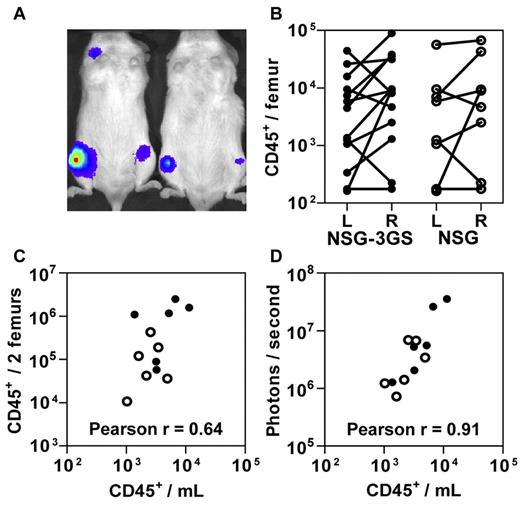
These findings suggested that NSG-3GS mice enable PB data to be used for LDA measurements of STRC-Ms and thereby circumvent some of the inter-mouse variability expected at early times posttransplant when the host BM is still recovering (Figure 1C). To investigate this possibility, we transplanted both genotypes of mice with 4 to 30 × 103 CD34+ CB cells that had been exposed to a lentivirus encoding the firefly luciferase cDNA. Assessment of their total body bioluminescence 3 weeks later revealed a highly heterogeneous distribution of labeled human cells (Figure 2A). A similar heterogeneity was mirrored in the level of human myeloid chimerism measured in paired aspirates of the right and left femurs of individual recipients of both strains (Figure 2B). Although a significant correlation between BM and PB values was achieved when the results for both femurs were combined (Figure 2C), the correlation between the bioluminescent end-point and absolute PB counts of human cells was even stronger (Figure 2D).
Circulating human cell and in vivo bioluminescence counts enable accurate assessment of total human cell engraftment. NSG-3GS (closed circles) and NSG (open circles) mice were transplanted with 0.4 to 3 × 104 luciferase-transduced CD34+ CB cells and 3 weeks later were assessed by bioluminescent imaging. (A) Representative image of transplanted mice (examples shown are NSG-3GS mice). (B) Total human cell counts in the left (L) and right (R) femurs in individually assessed NSG-3GS and NSG mice. Lines connect paired cell counts from individual mice which were significantly different (P < .05) for both strains. (C) Association between the absolute number of circulating human cells with the total number of human cells in both femurs (Pearson r = 0.64, P = .02). (D) Association between the absolute number of circulating human cells with photons emitted per second per mouse (Pearson r = 0.91, P < .001).
Circulating human cell and in vivo bioluminescence counts enable accurate assessment of total human cell engraftment. NSG-3GS (closed circles) and NSG (open circles) mice were transplanted with 0.4 to 3 × 104 luciferase-transduced CD34+ CB cells and 3 weeks later were assessed by bioluminescent imaging. (A) Representative image of transplanted mice (examples shown are NSG-3GS mice). (B) Total human cell counts in the left (L) and right (R) femurs in individually assessed NSG-3GS and NSG mice. Lines connect paired cell counts from individual mice which were significantly different (P < .05) for both strains. (C) Association between the absolute number of circulating human cells with the total number of human cells in both femurs (Pearson r = 0.64, P = .02). (D) Association between the absolute number of circulating human cells with photons emitted per second per mouse (Pearson r = 0.91, P < .001).
Together these findings demonstrate the ability of NSG-3GS mice to enable more precise and efficient determination of intravenously injected human STRCs using simple PB sampling and assessment methods. This assay will facilitate the characterization of factors that determine their properties and generation in vivo and in vitro, and thereby lead to improved adult transplant protocols where the STRC content of freshly isolated or cultured/genetically manipulated transplant harvests remains a serious limitation.11-15
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the British Columbia Women's Hospital and Health Center and the British Columbia Cancer Agency Stem Cell Assay Laboratory for assistance in procuring, processing, and cryopreserving CB samples; and Margaret Hale and Glenn Edin for excellent technical assistance.
This work was supported by grants from the Stem Cell Network (SCN) of Canada and the Cancer Stem Cell Research Consortium (CSCC).
Authorship
Contribution: P.H.M., R.K.H., and C.J.E. designed the experiments; P.H.M., A.M.S.C., P.A.B., D.J.H.F.K., K.D., G.R., and S.R. performed the experiments; and P.H.M. and C.J.E. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. J. Eaves, Terry Fox Laboratory, 675 West 10th Avenue, Vancouver, BC, V5Z 1L3, Canada; e-mail: ceaves@bccrc.ca.