To the editor:
Romiplostim and eltrombopag (a synthetic fusion peptibody and a nonpeptide molecule, respectively) are second-generation thrombopoietin mimetics/agonists that are structurally dissimilar to thrombopoietin and do not induce the formation of autoantibodies.1-3 They have been recently approved for the treatment of relapsed/resistant immune thrombocytopenia (ITP) and both are able to improve the production of new platelets, which, in this disease, are reduced by binding of antiplatelet antibodies to BM megakaryocytes.4 What is still not completely clear is whether these agents are cross-resistant. We describe herein 2 ITP patients who received eltrombopag as salvage therapy after they had become resistant to romiplostim.
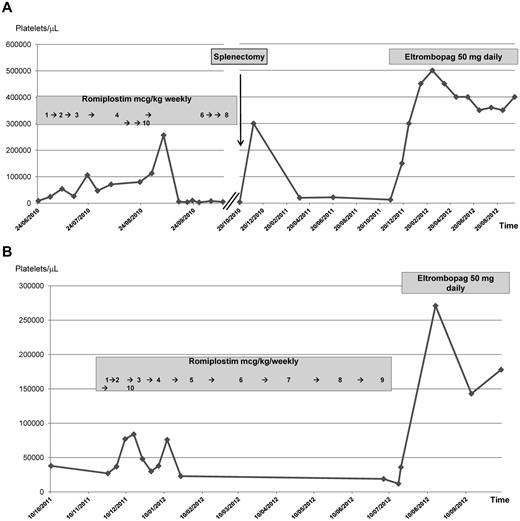
A 58-year-old man was diagnosed in November 2007 with recurrent ITP after several lines of treatment, including steroids, high-dose immunoglobulins, cyclosporine, and rituximab. All of these therapies had induced significant responses but these were of brief duration. In June 2010, the patient's platelet count dropped to 6000/μL and he was started on weekly subcutaneous administrations of romiplostim progressively increased from 1-4 μg/kg of body weight (Figure 1A). A complete response was achieved. However, 8 weeks later, a rapid decline of the platelet count occurred and further increasing romiplostim up to 10 μg/kg was not effective. After an episode of subdural bleeding, the patient underwent splenectomy, initially refused, in October 2010. A marked response was obtained, but 2 months later, the patient's platelet count was 3000/μL. Eltrombopag at a daily oral dose of 50 mg was started, with a prompt and sustained response that has been maintained to the present time.
Romiplostim and eltrombopag sequential administration and platelet count in 2 different patients (A and B) with chronic, pretreated ITP.
Romiplostim and eltrombopag sequential administration and platelet count in 2 different patients (A and B) with chronic, pretreated ITP.
A 49-year-old woman was diagnosed with ITP in September 2009. Because her platelet count was approximately 40 000/μL initially, no therapy was given until August 2011, when the platelet count dropped to 21 000/μL and cutaneous hemorrhagic manifestations appeared. High-dose dexamethasone induced a complete but transient platelet response. In December 2011, the patient's platelet count was 28 000/μL. The patient refused splenectomy and started romiplostim, which was increased weekly from 1-10 μg/kg of body weight, with a clinically significant but brief initial response (Figure 1B). In July 2012, the patient's platelet count was 14 000/μL. At that time, eltrombopag was begun orally at the daily dose of 50 mg/kg, achieving a complete and stable response that has been maintained to the present time.
These 2 cases suggest that, at the clinical level, there is probably no cross-resistance between romiplostim and eltrombopag. Both agents were well tolerated, but the quality and duration of response to eltrombopag were better than those previously obtained with romiplostim. A similar outcome was observed in a case that, to the best of our knowledge, is the only one reported in the literature so far, in which eltrombopag was administered before romiplostim.5 Our observations provide useful information for the management of ITP patients. These results are not completely unexpected if one takes into account that the 2 molecules bind the same receptor at different levels (romiplostim to the surface thrombopoietin receptor and eltrombopag to the thrombopoietin receptor's transmembrane domain). Confirmation in larger series of patients, including new potential indications currently being investigated (ie, in neoplastic disorders such as myelodysplastic syndromes) is required.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni D'Arena, MD, Department of Onco-Hematology, IRCCS Centro di Riferimento Oncologico della Basilicata, Via Padre Pio n1, 85028 Rionero in Vulture (Pz), Italy; e-mail: giovannidarena@libero.it.