To the editor:
In autoimmune hemolytic anemia (AIHA), autoantibody-mediated complement activation results in C3 deposition on RBCs and possibly the formation of the membrane attack complex cumulating in intravascular hemolysis.1-4 In case of acute signs of tissue hypoxia, life-saving transfusion with RBC units is required. However, recovery of RBC transfusions is inadequate because autoantibodies react with recipient and donor cells.1 We hypothesized that being it is a plasma-derived inhibitor of the classic complement pathway, C1-esterase-inhibitor (C1-INH) may improve the recovery of RBC transfusion in AIHA patients.5 In the present study, we investigated whether C1-INH can enhance the efficacy of RBC transfusions.
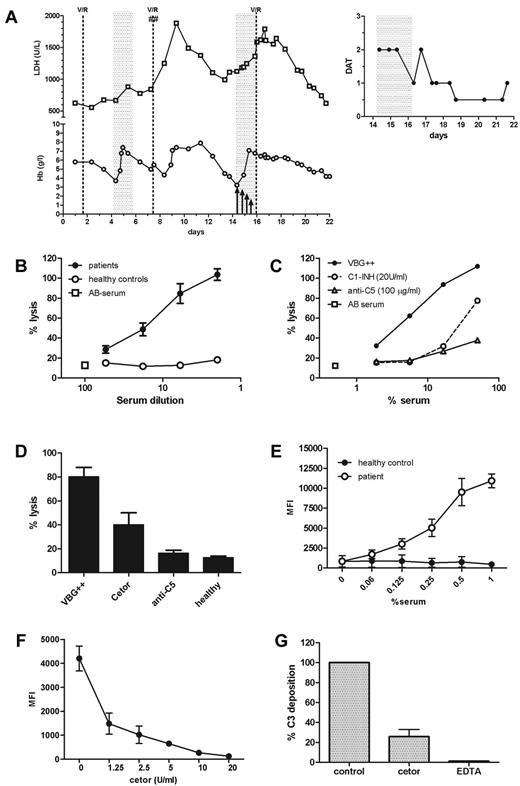
A 64-year-old female patient suffering from a diffuse large B-cell non-Hodgkin lymphoma was admitted with AIHA with intravascular hemolysis because of warm IgM autoantibodies. Direct antiglobulin test (DAT) for complement C3d was strongly positive. Despite treatment (Figure 1A), the patient's anemia worsened and she became symptomatic at a hemoglobin level of 3.7 g/L. Three RBC units were transfused. However, recovery of RBC transfusion was modest, resulting in accelerated hemolysis reaching a hemoglobin level of 4.4 g/L 3.5 days after the transfusion. Hemoglobin levels increased and even transiently stabilized, but then quickly dropped to a nadir of 3.3 g/L after short-term high-dose methylprednisolone therapy. Because of progressive hypoxia-related confusion, transfusion of 3 RBC units was started. Knowing that plasma-purified C1-INH (Cetor) has an excellent safety profile,5,6 we decided to coadminister C1-INH to enhance the efficacy of the RBC transfusion in this patient after a regime that turned out to be efficient in sepsis patients.6,7 Before transfusion, 6000 U of C1-INH were administered intravenously. Three additional infusions of 4000, 2000, and 1000 U were administered 22, 38, and 50 hours, respectively, after the first C1-INH dose. As evidenced by the DAT for C3d (Figure 1A inset) and lactate dehydrogenase levels, C1-INH administration indeed attenuated both complement deposition on RBCs and hemolysis. Moreover, recovery of RBC transfusion was much better compared with the first transfusion. In the further clinical course, there were no signs of hemolysis and the patient's hemoglobin levels stabilized.
Inhibition of complement deposition and destruction of RBC by C1-inhibitor in AIHA. (A) C1-INH was administered at a dose of 6000 U before transfusion of 3 RBC concentrates. After transfusion, another 4000 U of C1-INH was administered, followed by 2 doses of 2000 U and 1000 U every 12 hours (as indicated by arrows). The inset at the top represents the DAT for complement C3d during and after treatment with C1-INH. The DAT for C3d is indicated as very strong (3+), strong (2+), moderate (1+), weak (0.5+), or negative (0). V/R indicates vincristine/rituximab; ##, methylprednisolone; and gray shaded areas, transfusion of 3 RBC concentrates. (B) Hemolysis of bromelain-treated human O-typed erythrocytes (2% hematocrit, 90 minutes of incubation at 37°C) after incubation with either patient serum (n = 5) or healthy control serum (n = 4) in the presence of 25% normal human AB serum. Percentage of lysis was compared with a 100% lysis control that was determined by incubating the erythrocytes in distilled water. Incubation with only AB serum served as a negative control. Results are shown as means and SEM (error bars). (C) Hemolysis of bromelain-treated human O-typed erythrocytes (2% hematocrit) after incubation with patient serum was inhibited by C1-INH (20 U/mL) and mAb anti-C5 (100 μg/mL). Representative graph of one patient serum sample is shown. (D) Twenty U/mL of C1-INH significantly (P < .05) inhibited the hemolysis induced by 16% patient serum. Means and SEM (error bars) of 5 selected patients are shown. (E) Representative graph of C3 deposition on human erythrocytes after incubation with either patient serum or healthy control serum in the presence of 25% normal human AB serum. Data are expressed as means and SEM (error bars) of triplicate measurements. MFI indicates mean fluorescence intensity. (F) C1-inhibitor dose dependently inhibited C3 deposition after incubation of human O-typed erythrocytes (1% hematocrit) with 0.1% patient serum (serum 1). Data are expressed as means and SEM (error bars) of triplicate measurements. (G) Twenty U/mL of C1-INH inhibited C3 deposition induced by 0.1% patient serum. Incubation in the presence of 20mM EDTA served as a control. Results are depicted as a percentage of control, which was not inhibited and was set to 100%. Means and SEM (error bars) of 5 selected patients are shown.
Inhibition of complement deposition and destruction of RBC by C1-inhibitor in AIHA. (A) C1-INH was administered at a dose of 6000 U before transfusion of 3 RBC concentrates. After transfusion, another 4000 U of C1-INH was administered, followed by 2 doses of 2000 U and 1000 U every 12 hours (as indicated by arrows). The inset at the top represents the DAT for complement C3d during and after treatment with C1-INH. The DAT for C3d is indicated as very strong (3+), strong (2+), moderate (1+), weak (0.5+), or negative (0). V/R indicates vincristine/rituximab; ##, methylprednisolone; and gray shaded areas, transfusion of 3 RBC concentrates. (B) Hemolysis of bromelain-treated human O-typed erythrocytes (2% hematocrit, 90 minutes of incubation at 37°C) after incubation with either patient serum (n = 5) or healthy control serum (n = 4) in the presence of 25% normal human AB serum. Percentage of lysis was compared with a 100% lysis control that was determined by incubating the erythrocytes in distilled water. Incubation with only AB serum served as a negative control. Results are shown as means and SEM (error bars). (C) Hemolysis of bromelain-treated human O-typed erythrocytes (2% hematocrit) after incubation with patient serum was inhibited by C1-INH (20 U/mL) and mAb anti-C5 (100 μg/mL). Representative graph of one patient serum sample is shown. (D) Twenty U/mL of C1-INH significantly (P < .05) inhibited the hemolysis induced by 16% patient serum. Means and SEM (error bars) of 5 selected patients are shown. (E) Representative graph of C3 deposition on human erythrocytes after incubation with either patient serum or healthy control serum in the presence of 25% normal human AB serum. Data are expressed as means and SEM (error bars) of triplicate measurements. MFI indicates mean fluorescence intensity. (F) C1-inhibitor dose dependently inhibited C3 deposition after incubation of human O-typed erythrocytes (1% hematocrit) with 0.1% patient serum (serum 1). Data are expressed as means and SEM (error bars) of triplicate measurements. (G) Twenty U/mL of C1-INH inhibited C3 deposition induced by 0.1% patient serum. Incubation in the presence of 20mM EDTA served as a control. Results are depicted as a percentage of control, which was not inhibited and was set to 100%. Means and SEM (error bars) of 5 selected patients are shown.
To support the observed in vivo effects of C1-INH, we performed in vitro experiments with 5 selected patient sera samples with a positive DAT for complement C3d and patient serum-induced hemolysis of bromelain-treated RBCs. Hemolysis of human O-typed RBCs was induced by incubation with these patient sera (Figure 1B). As expected, monoclonal anti-C5 completely abrogated antibody-induced hemolysis. A fixed high concentration of C1-INH (20 U/mL) also inhibited a substantial part of the lysis (Figure 1C-D). To analyze the effect of C1-INH on C3 deposition on the RBC surface, flow cytometry was used after incubation of human RBCs with patient sera. To ensure that sufficient intact RBCs were left for analysis, incubations were performed in the presence of excess amounts of monoclonal anti-C5, completely abrogating lysis while leaving C3 deposition unaffected. Incubation of RBCs with patient sera resulted in significant C3 deposition, which could be significantly and dose dependently inhibited by C1-INH to background levels (Figure 1E-G). In conclusion, our results show that C1-INH has potential as an effective and safe therapy to control complement-induced RBC destruction in AIHA patients.
Authorship
Conflict-of-interest disclosure: D.W. and S.Z. receive an unrestricted grant from Viropharma Inc. The remaining authors declare no competing financial interests.
Correspondence: Sacha Zeerleder, PhD, MD, Department of Immunopathology, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: s.zeerleder@sanquin.nl.