The ability of the DNA methyltransferase inhibitors (DNMTi) to induce terminal differentiation in fibroblasts was first noted by Taylor and Jones in 19791 ; Silverman and Holland reported hematologic improvement in patients with myelodysplastic syndrome (MDS) in 1993.2 That azacitidine improves survival in patients with high-risk MDS and acute myeloid leukemia with MDS features compared with a combined comparator group of supportive care, low-dose cytarabine, and intensive cytarabine plus anthracycline, while inducing trilineage normalization in approximately 15% of patients3 makes the development of more potent, more specific drugs that behave like azacitidine imperative. The question is, how do the azanucleosides behave?
The incorporation of azacytosine nucleosides into DNA during S phase is followed by the formation of irreversible adducts with DNMT1,4,5 depleting the cell of active enzyme. Subsequent replication cycles in the absence of active DNMT1 leads to progressive reversal of cytosine methylation. In vitro, methylation reversal at CpG islands is often associated with re-expression of the associated gene.6 Thus, conventional wisdom assumes that cellular and clinical responses derive from a change in gene expression patterns resulting from promoter methylation reversal.
The problem is that such an association has been difficult to demonstrate. Several studies investigating changes in global methylation, methylation and/or expression of specific target genes, and genome-wide methylation and/or expression in patients receiving deoxyazacytidine (DAC) or 5-azacytidine (5AC), alone or in combination with other drugs, have failed to discriminate clinical responders from clinical nonresponders on the basis of baseline methylation/expression parameters or changes in these metrics (see figure).7,8 Measurable changes in methylation appear necessary for clinical response (no doubt as a marker of drug bio-availability and adequate concentration) but have not been shown to be sufficient or mechanistically linked. These studies may be critiqued on the basis of the methodologies used, the times at which the tumor was sampled, and the heterogeneity of the patients treated.
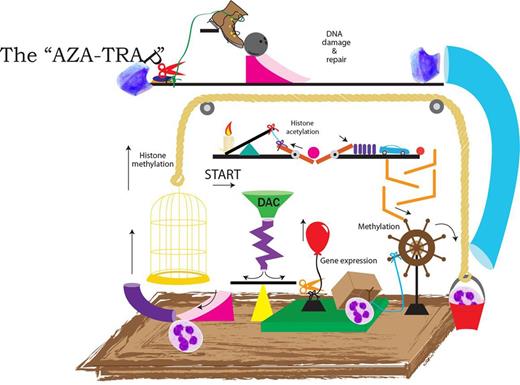
In this Rube Goldberg contraption, leukemic cells are entered at the “Start” arrow, treated with an azanuceloside analog, and emerge as normal neutrophils. Along the way, a variety of epigenetic targets and DNA damage and repair pathways are encountered. Klco et al illustrate that despite state-of-the-art genomic technology, the mechanisms accounting for the clinical activity of DNMT inhibitors in myeloid leukemias remain complex and unclear.
In this Rube Goldberg contraption, leukemic cells are entered at the “Start” arrow, treated with an azanuceloside analog, and emerge as normal neutrophils. Along the way, a variety of epigenetic targets and DNA damage and repair pathways are encountered. Klco et al illustrate that despite state-of-the-art genomic technology, the mechanisms accounting for the clinical activity of DNMT inhibitors in myeloid leukemias remain complex and unclear.
In this issue of Blood, Klco and colleagues treat primary acute myeloid leukemia bone marrow samples with DAC in a stromal co-culture system for 3 days before analyzing changes in the methylome and expression profiles.9 Methylation array data showed high methylation levels in some promoters, but also in gene bodies and 3′ untranslated regions. Furthermore, decitabine induced hypomethylation-favored areas with higher baseline methylation and extent of methylation reversal appeared to correlate with degree of initial methylation. Thus, methylation changes were frequently more extensive in gene bodies than in CpG islands, similar to a recent study by Yan et al.10 Post-mock and Post-DAC treatment samples of each leukemia cluster with themselves in unsupervised analysis, rather than with other treated samples, and correlation between changes in methylation and gene expression was “subtle,” and did not apply to CDH1 or CDKN2B, 2 frequently methylated tumor suppressor genes in myeloid neoplasms.
This well-done study by Klco et al parallels the clinical experience, demonstrating a lack of demonstrable direct connectivity between methylation reversal events in response to azanucleosides and canonical early changes in gene expression. It is therefore not surprising that connecting such changes to clinical responses that manifest several months later has been impossible. Building a better aza mousetrap is a laudable goal; if only we could figure out how the aza trap works.
Conflict-of-interest disclosure: S.D.G. receives research funding from the Celgene Corporation. The remaining author declares no competing financial interests. ■