Key Points
Neutrophils of patients with FHL-5 with Munc18-2/STXBP2 mutations have impaired granule fusion and bacterial killing.
Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is caused by genetic defects in cytotoxic granule components or their fusion machinery, leading to impaired natural killer cell and/or T lymphocyte degranulation and/or cytotoxicity. This may accumulate into a life-threatening condition known as macrophage activation syndrome. STXBP2, also known as MUNC18-2, has recently been identified as the disease-causing gene in FHL type 5 (FHL-5). A role for STXBP2 in neutrophils, and for neutrophils in FHL in general, has not been documented thus far. Here, we report that FHL-5 neutrophils have a profound defect in granule mobilization, resulting in inadequate bacterial killing, in particular, of gram-negative Escherichia coli, but not of Staphylococcus aureus, which rather depends on intact reduced NAD phosphate oxidase activity. This impairment of bacterial killing may contribute to the apparent susceptibility to gastrointestinal tract inflammation in patients with FHL-5.
Introduction
Rare mutations (incidence: ∼1-4 cases/million depending on ethnicity) in 4 different genes, including PRF1 (encoding perforin-1) in familial hemophagocytic lymphohistiocytosis type 2 (FHL-2); UNC13D (encoding Munc13-4) in FHL type 3 (FHL-3); STX11 (encoding syntaxin 11) in FHL type 4 (FHL-4); and, more recently, also STXBP2 (also known as MUNC18-2), encoding the granule-associated docking protein syntaxin–binding protein 2 (STXBP2) in FHL type 5 (FHL-5), have been identified as disease-causing in patients with FHL.1-3 FHL-5 accounts for an estimated 10% of all FHL cases. STXBP2 is important for regulating intracellular granule trafficking and docking at the plasma membrane. Previous reports have documented the role of STXBP2 in natural killer (NK) cell, cell T lymphocyte, and platelet degranulation in patients with FHL-5.2-4 Some of the clinical features of FHL-5, such as the increased susceptibility to gastrointestinal tract bacterial infection,5 have remained unexplained. Therefore, it seems possible that STXBP2 plays a role in other leukocytes, such as neutrophils. Indeed, STXBP2 has been reported to localize to the different types of granules in human neutrophils.6,7 Neutrophil granules contain an array of antimicrobial constituents and proteases that will be secreted and/or released into the phagosome on mobilization, and this process contributes to microbial killing. Although some evidence exists for an involvement of STXBP2 in neutrophil granule function,7 compelling evidence for a direct role has not been documented. Here, we demonstrate that STXBP2 is involved in neutrophil granule mobilization and bacterial killing using neutrophils from 3 genetically defined patients with FHL-5.
Study design
Patients with FHL-5, control participants, and neutrophil isolation
Heparinized blood was collected, after informed consent and according to the Declaration of Helsinki 1964, from 3 unrelated patients with FHL-5 (supplemental Table 1) and healthy control participants, which included umbilical cord blood and blood from healthy adults. Granulocytes were isolated by density gradient centrifugation with isotonic Percoll (Pharmacia, Uppsala, Sweden) and erythrocyte lysis, as described previously.8 Granulocytes were washed and resuspended in N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES)–buffered saline solution (containing 132 mM of NaCl, 6.0 mM of KCl, 1.0 mM of CaCl2 1.0 mM of MgSO4, 1.2 mM of potassium phosphate, 20 mM of HEPES. 5.5 mM of glucose, and 0.5% (wt/vol) human serum albumin, pH 7.4). The purity of granulocytes always exceeded 95%. The study has been approved by our local institutional review board.
Degranulation assays
Neutrophil degranulation was examined as described previously.9 Briefly, neutrophils (2 × 106/mL) were incubated in HEPES-buffered saline solution at 37°C in a shaking water bath before adding the (priming) agents platelet-activating factor (PAF [1 μM]; Sigma, 5 minutes) or cytochalasin B (5 μg/mL, Sigma, 5 minutes) and were subsequently stimulated with formyl-methionyl-leucyl-phenylalanine (fMLP [μM]: Sigma, 15 minutes). After stimulation, cells were put on ice, washed with HEPES-buffer saline solution once, and subsequently stained with antibodies against neutrophil-granule markers: CD63-PE (IgG1, 435) and CD66b-FITC (IgG1, CLB-B13.9). Data are expressed as mean fluorescence intensities. The cells were analyzed on an LSRII flow cytometer equipped with FACSDiva software (BD). The release of elastase and lactoferrin was evaluated using enzyme-linked immunosorbent assay kits (HyCult Biotechnology) according to the manufacturer’s instructions.
Bacterial killing
Granulocyte bactericidal activity was determined using Escherichia coli (strain ML-35) and Staphylococcus aureus (strain 502A). Bacterial survival was measured by assaying bacterial colony formation as described previously.10
Statistics
When possible, statistical significance was determined using the Student t test or the Grubbs outlier test; P ≤ .05 was considered to be significant.
Results and discussion
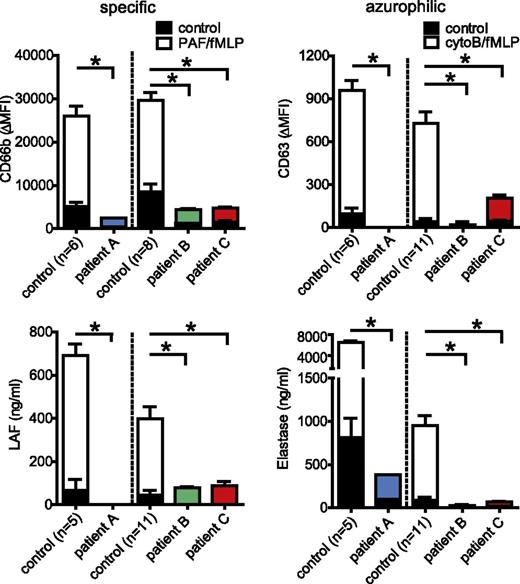
To characterize a role for STXBP2 in neutrophil function and a possible contribution of neutrophils to FHL-5, we analyzed 3 unrelated patients with defined mutations in STXBP-2 (supplemental Table 1). The absence of STXBP2 protein in the neutrophils of patients A and C was confirmed by western blot analysis, whereas patient B carrying a homozygous exon 15 splice site mutation, which is known to be associated with residual NK function and a milder clinical phenotype,11 did express substantial levels of a STXBP2 protein (supplemental Figure 1A). As reported previously,3 this splice site mutation yields a variant STXBP2 protein (supplemental Figure 1B), most likely with a partially impaired function. In line with this, and also with previous findings,11 a complete (patients A and C) or partial (patient B) absence of NK cell–mediated degranulation and cytotoxicity toward target cells was observed (supplemental Figure 2). The exocytosis of the different types of neutrophil granules,6 induced by either cytochalasin-B/fMLP or PAF/fMLP, was evaluated by monitoring secretion of the granule components elastase (azurophilic granules) or lactoferrin (specific granules) and the exposure of cell surface CD63 (azurophilic granules) or CD66b (secretory, tertiary, and specific granules) (Figure 1). This revealed a pronounced defect in the release of both types of vesicles in FHL-5 neutrophils of all 3 patients. Consistently, the cytoB/fMLP-stimulated release of extracellular proteolytic activity, analyzed by DQ-BSA proteolysis assay, which primarily measures neutrophil-derived serine proteases, appeared completely absent in FHL-5 neutrophils, although the total cellular content of the proteases was not different (supplemental Figure 3). Immuno-EM analysis of the tertiary, specific, and azurophilic granules, using gelatinase, lactoferrin, and myeloperoxidase as markers, respectively, showed normal granule appearances and frequencies (supplemental Figure 4), thereby excluding defects in granule biosynthesis. Measurement of intraphagosomal serine protease activity suggested that mobilization of neutrophil granules to phagosomes was also substantially impaired in FHL-5 neutrophils (supplemental Figure 5), although it was certainly not completely absent as also shown by the presence of both specific and azurophilic granule components in E coli–containing phagosomes (supplemental Figure 6). Collectively, these findings suggested, for the first time, that STXBP2 is required for neutrophil granule exocytosis and likewise also, at least in part, for granule mobilization to the phagosome.
Defective granule mobilization by FHL-5 neutrophils. Degranulation of neutrophils was examined in 1- day-old blood of healthy adults (n = 11) and fresh cord blood (n = 6) of healthy adults; and in fresh blood of FHL-5 patient A, 1-day-old blood in patient B, and 1-day-old blood in patient C. This was done by monitoring the release of (A) lactoferrin (specific granules) and surface exposure of (B) CD66b (secretory, tertiary, and specific granules) on stimulation with PAF/fMLF, and the release of (C) elastase (azurophilic granules) and surface exposure of (D) CD63 (azurophilic granules) on stimulation with cytoB/fMLF, respectively. Concentrations (ng/mL) of released factors, or the mean fluorescence intensities of surface markers for stimulated (white, blue, green, red) and unstimulated (black parts of the bars) cells are shown. The values shown are averages ± SEM from at least 2 independent experiments, each performed in triplicate, with the exception of patient A, who was evaluated once; *, P < .05; n.s., nonsignificant; Grubbs outlier test. Note that granule release is essentially absent in all 3 patients.
Defective granule mobilization by FHL-5 neutrophils. Degranulation of neutrophils was examined in 1- day-old blood of healthy adults (n = 11) and fresh cord blood (n = 6) of healthy adults; and in fresh blood of FHL-5 patient A, 1-day-old blood in patient B, and 1-day-old blood in patient C. This was done by monitoring the release of (A) lactoferrin (specific granules) and surface exposure of (B) CD66b (secretory, tertiary, and specific granules) on stimulation with PAF/fMLF, and the release of (C) elastase (azurophilic granules) and surface exposure of (D) CD63 (azurophilic granules) on stimulation with cytoB/fMLF, respectively. Concentrations (ng/mL) of released factors, or the mean fluorescence intensities of surface markers for stimulated (white, blue, green, red) and unstimulated (black parts of the bars) cells are shown. The values shown are averages ± SEM from at least 2 independent experiments, each performed in triplicate, with the exception of patient A, who was evaluated once; *, P < .05; n.s., nonsignificant; Grubbs outlier test. Note that granule release is essentially absent in all 3 patients.
Neutrophils are essential for controlling bacterial and fungal infections. There are 2 mechanisms that primarily contribute to neutrophil-mediated killing of microbes: (a) the reduced NAD phosphate (NADPH)3 oxidase, which on assembly and activation in the phagosomal membrane and/or plasma membrane produces toxic reactive oxygen species; and (b) proteolytic killing, which is assumed to require the fusion of the protease-loaded azurophilic granules with the phagosomal membrane and/or plasma membrane. Although gene-targeting experiments in the mouse have demonstrated that both mechanisms may act independently or in concert to achieve full destructive power, the relative contribution of each activity depends on the nature of the microbe as well.12-14
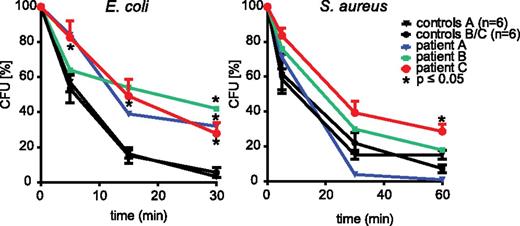
However, despite abundant information on neutrophil microbial killing in a human context regarding the role of the NADPH oxidase, an activity that is defective in chronic granulomatous disease, much less is known about the contribution of granule mobilization in human neutrophils. Clearly, the availability of FHL-5 neutrophils with a granule mobilization defect allowed us to explore this directly. Neutrophil-mediated killing of S aureus and E coli was evaluated. Figure 2 shows that the S aureus killing was virtually unaffected in FHL-5 neutrophils, but the killing of E coli was substantially impaired in cells from all patients investigated. Of relevance, the phagocytosis of the bacteria was apparently not significantly affected (supplemental Figure 7). In line with a complementary role for the NADPH oxidase in this context, S aureus killing was more or less abolished in cells treated with the NADPH oxidase inhibitor diphenylene iodonium, whereas the killing of E coli was only partially impaired (supplemental Figure 8). Similar abnormalities in killing have been observed with neutrophils from patients with chronic granulomatous disease15 (data not shown). It should be noted that the production of reactive oxygen species by the NADPH oxidase in response to particulate stimuli, such as unopsonized or opsonized zymosan (supplemental Figure 9A), or soluble stimuli, such as PAF/fMLP (not shown), was not impaired in FHL-5 neutrophils. However, the intraphagosomal oxidation of DHR-coated E coli appeared slightly delayed in FHL-5 neutrophils (supplemental Figure 9B). Because DHR oxidation requires myeloperoxidase activity16 this is consistent with the impaired mobilization of granules to phagosomes noted above. Collectively, these findings suggest that, although S aureus killing depends primarily on the NADPH oxidase, both the oxidase as well as granule mobilization–dependent mechanisms, either to the plasma membrane and/or to the phagosomal membrane, play a role in E coli killing. Finally, chemotaxis of FHL-5 neutrophils was apparently normal (supplemental Figure 10).
Impaired bacterial killing by FHL-5 neutrophils. Killing of S aureus and E coli was assessed in cord blood (control A; n = 6) of healthy (control B/C; n = 10) control participants and in patients with FHL-5 as described in the Study design section. Remaining viable bacteria were quantified as colony-forming units (CFU) and were expressed as % of CFU at t = 0. For the control group, the values shown are averages ± SEM, and for the patients the average of 2 measurements from 2 (patient A/B) or 3 (patient C) independent experiments are shown; *, P < .05; Grubbs outlier test (patient A/B) or Student t test (patient C). Note that the killing of S aureus is virtually normal, whereas E coli killing is significantly impaired for all 3 patients.
Impaired bacterial killing by FHL-5 neutrophils. Killing of S aureus and E coli was assessed in cord blood (control A; n = 6) of healthy (control B/C; n = 10) control participants and in patients with FHL-5 as described in the Study design section. Remaining viable bacteria were quantified as colony-forming units (CFU) and were expressed as % of CFU at t = 0. For the control group, the values shown are averages ± SEM, and for the patients the average of 2 measurements from 2 (patient A/B) or 3 (patient C) independent experiments are shown; *, P < .05; Grubbs outlier test (patient A/B) or Student t test (patient C). Note that the killing of S aureus is virtually normal, whereas E coli killing is significantly impaired for all 3 patients.
Taken together, to our knowledge these findings provide the first direct demonstration of a requirement for STXBP2 in particular, and granule mobilization in general, in the killing of bacteria by human neutrophils. This defect in bacterial killing may also explain some of the unexplained symptoms, such as increased susceptibility to gastrointestinal tract inflammation, which has previously been reported in patients with FHL-5.5
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Prof Dr Dirk Roos for useful discussions and his advice on the manuscript, and to Prof Dr Stephan Ehl for his help with mutation analysis.
Authorship
Contribution: X.W.Z., R.G., A.D., M.v.H., J.L.v.H., A.T.J.T., H.J., A.B.M., L.v.d.C., and K.T. performed experiments and analyzed results; D.G.R., J.J.B., I.K., and J.V.D.W.T.B. provided reagents, patient material, and/or advice; T.W.K. and T.K.v.d.B. designed experiments; and T.K.v.d.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timo K. van den Berg, Sanquin Research and Landsteiner Laboratory, Academic Medical Center, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: t.k.vandenberg@sanquin.nl.
References
Author notes
X.W.Z., R.G., and A.D. contributed equally to this study.