Key Points
Ectopic COP1 decreases C/EBPα and blocks granulocyte differentiation in 32D cells.
Trib1 binds to COP1 to enhance its ubiquitin ligase activity for C/EBPα. COP1 accelerates development of AML induced by Trib1.
Abstract
The ubiquitin ligase constitutively photomorphogenic 1 (COP1) is involved in many biological responses in mammalian cells, but its role in tumorigenesis remains unclear. Here we show that COP1 is a ubiquitin ligase for the tumor suppressor CCAAT/enhancer-binding protein (C/EBPα) and promotes its degradation in vivo, thereby blocking myeloid differentiation of hematopoietic cells for tumorigenesis. In this process, mammalian homolog of Tribbles, Trib1, which contains a COP1-binding motif, is essential for down-regulation of C/EBPα expression. Murine bone marrow transplantation experiments showed that coexpression of COP1 accelerates development of acute myeloid leukemia induced by Trib1, which pathologically resembles that of p42C/EBPα-deficient mice. Interestingly, coexpression of ligase activity-deficient COP1 mutant abrogated Trib1-induced leukemogenesis. These results indicate that COP1 and Trib1 act as an oncoprotein complex functioning upstream of C/EBPα, and its ligase activity is crucial for leukemogenesis.
Introduction
Constitutively photomorphogenic 1 (COP1) is an E3 ubiquitine ligase highly conserved from plants to mammals. COP1 was originally identified as a repressor of photomorphogenesis in plants, targeting transcription factors (HY5, HYH, HFR1, and LAF1) that promote development in response to light signals, for degradation in the dark.1 COP1 consists of 3 functional domains, a RING-finger domain, a coiled-coil domain, and a WD40 domain, and functions downstream of the COP9 signalosome complex. Although its role in light signaling is clear in plants, COP1 seems to function in many biological responses in mammals.2 Putative substrates of mammalian COP1 include c-Jun, ETV1, p53, acetyl-CoA carboxylase (ACC), TORC2, and FOXO1,3-8 suggesting that COP1 is involved in tumorigenesis, lipid metabolism, and gluconeogenesis. As for tumorigenesis, COP1 was first thought to act as an oncoprotein by suppressing p53,5 but recent studies with knockout mouse models suggest that COP1 may also function as a tumor suppressor in some tissues by antagonizing proto-oncogenic activity of c-Jun and ETV1.4,9 Thus, the physiological role of COP1 in tumorigenesis remains elusive.
Myeloid leukemia factor 1 (MLF1) was identified as a carboxyl-terminal component of the leukemic fusion protein NPM-MLF1 generated by the chromosomal translocation t(3;5) in acute myeloid leukemia (AML) patients.10 We have previously shown that, in response to ultraviolet light, MLF1 negatively regulates COP1 through physical interaction with the third component of the COP9 signalosome complex (CSN3) and consequently stabilizes the expression of p53, which inhibits cell cycle progression.11 However, little is known about why deregulation of a MLF1 pathway specifically causes AML and whether COP1, as a downstream component of MLF1-CSN3, is involved in leukemogenesis. It was recently found that mammalian homologs of Tribbles, Trib1, Trib2, and Trib3, have a consensus COP1-binding motif at the carboxyl-terminus,6 and Trib1 and Trib2 induce AML in mouse models,12,13 which implies that COP1 is directly connected to the leukemic pathway.
Tribbles, which is highly conserved from Drosophila to humans, was first discovered as a regulator of String/CDC25 for degradation to coordinate proliferation and morphogenesis during Drosophila development.14-16 Tribbles also facilitates proteasome-dependent degradation of slbo, the Drosophila homolog of the CCAAT/enhancer-binding protein (C/EBP) family, which is required for cell migration during oogenesis in flies.17 In mammals, C/EBPα is a critical transcription factor controlling myeloid differentiation and leukemogenesis. Mutations of C/EBPα are found in approximately 7% to 9% of patients with AML.18 In addition, C/EBPα is functionally suppressed at many levels in AML, such as transcriptional repression due to the leukemic fusion protein, AML1-ETO,19 post-translational modification by Flt3-internal tandem duplication,20 and protein degradation by Trib1 and Trib2. Genetically modified mice with a mutation specific to a p42 form in the C/EBPα locus developed AML with complete penetrance,21 indicating that C/EBPα is a key tumor suppressor functioning in the hematopoietic lineage. Trib1 was cloned as a factor up-regulated by retroviral integration in Hoxa9/Meis1-induced AML,13 and Trib2 was found as a downstream factor of Notch signaling in T-cell acute lymphoblastic leukemia cells.12 Ectopic expression of Trib1 and Trib2, but not Trib3, down-regulates C/EBPα in hematopoietic cells and induces a block of myeloid differentiation, leading to AML in mice,22 whereas mammalian Trib3 stimulates lipolysis during fasting and loss of insulin signaling by triggering the degradation of the enzyme promoting fatty acid synthesis (ACC) in adipose tissue.6 Although the COP1-binding site in Trib1/Trib2 sequences is required for the down-regulation of C/EBPα,23,24 conclusive evidence that COP1 is a bona fide E3 ligase for C/EBPα and involved in development of AML is missing.
In this study, we addressed the role of COP1 in myeloid leukemogenesis. We found that COP1 is an E3 ubiquitin ligase targeting C/EBPα for degradation through its adaptor protein Trib1 in vivo. COP1 exerted its activity of blocking myeloid differentiation and consequent development of AML in a manner highly dependent on Trib1 in myeloid-committed cells. We also showed that the ligase activity of COP1 was essential for degradation of C/EBPα and leukemogenesis.
Methods
Plasmid construction and retroviral production
All mice were bred and maintained in the Nara Institute of Science and Technology Animal Facility in accordance with Nara Institute of Science and Technology guidelines. We subcloned COP1 cDNA (wild type [WT] and variants) into the retroviral vectors plasmid murine stem cell virus (pMSCV)-puro-green fluorescent protein (GFP) (for expression of GFP-fused proteins11 ), and pMSCV–internal ribosome entry site (IRES)-GFP (a gift from Dr Owen Witte, for expression of untagged proteins). We used pMSCV-neo for expression of untagged Trib1. A vector for RNAi specific to mouse Trib1 was constructed based on the pSUPER RNAi system (pSUPER.retro.neo; OligoEngine). The siRNA sequence targeting Trib1 was 5′-CTCATCCAATGCAGAGTCT-3′. For viral production, the plasmid was cotransfected into 293T human embryonic kidney cells together with a plasmid encoding an ecotropic helper virus containing a defective virion-packaging (φ2) sequence. Culture supernatants containing retroviruses were harvested 48 to 72 hours after transfection and used for infection.
Cell culture and transfection
293T cells and NIH3T3 mouse fibroblasts (provided by Drs C. J. Sherr and M. F. Roussel) were cultured as described,11,25 and transfected with expression vectors via the calcium phosphate–DNA precipitation method.26 Transfected GFP and COP1 (after immunofluorescent staining25 ) were viewed under the fluorescence microscope as described.11,25
32Dcl3 cells were maintained in medium containing interleukin 3 (IL3) as described,27 infected by the spin infection procedure25 in the presence of polybrene (6 μg/mL), selected in puromycin (5 μg/mL) or G418 (1 mg/mL), and sorted for GFP-positive signals with a FACSAria flow cytometer (Becton Dickinson). Where indicated, cells were treated for 8 hours with 5 μM MG132 before harvest. For granulocytic differentiation, cells were transferred to medium containing 25 ng/mL human granulocyte-colony-stimulating factor (G-CSF) in lieu of IL3 and tested for expression of the cell surface marker Mac-1 by flow cytometry (see below) 2 to 4 days after the transfer to G-CSF. For several days after transfer to G-CSF, >95% of cells were viable and positive for GFP. Cell morphology was examined 14 days after culturing in G-CSF.
Bone marrow transplantation and analyses
Bone marrow (BM) cells were aseptically isolated from 8-week-old C57BL/6 mice (CLEA Japan) that had been injected with 5-fluorouracil (5-FU) (150 mg/kg of body weight; Kyowa Hakko Kogyo) intravenously 5 days before, incubated in BM medium (Dulbecco’s modified Eagle medium, 15% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 5% WEHI-3B conditioned medium, 6 ng/mL mouse IL3, 10 ng/mL human IL6, and 50 ng/mL mouse stem cell factor), and infected with a retroviral supernatant according to the spin infection procedure described previously in the presence of polybrene.25
Infected, unsorted BM cells (0.5-1 × 106 cells) were injected into the veins of 8- to 10-week-old C57BL/6 mice that had been lethally irradiated (10 Gy; M-150WE; SOFTEX) several hours before injection. All mice were maintained in the Nara Institute of Science and Technology Animal Facility in accordance with Nara Institute of Science and Technology guidelines. Blood samples were routinely analyzed with an automated cell counter (F-820 analyzer; Sysmex) and also by inspecting blood smears after May-Grunwald Giemsa staining.
BM cells isolated from leukemic mice were analyzed by flow cytometer and cultured in RPMI 1640 supplemented with 20% fetal bovine serum, 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin, and containing one of the following cytokines; mouse IL3 (2.5 ng/mL), mouse granulocyte macrophage-CSF (GM-CSF; 5 ng/mL), human CSF-1 (2000 U/mL), human G-CSF (25 ng/mL), and human erythropoietin (Epo) (1 U/mL). Recombinant cytokines were purchased from R&D. Cells were enumerated on the second and fourth days.
Flow cytometric analysis and cell sorting
PharM Lyse (BD Bioscience) was used to remove red blood cells. BM and spleen cells were analyzed and sorted with FACSAria (BD Biosciences) after staining with antibodies to CD3ε (145-2C11), B220 (RA3-6B2), TER-119 (TER-119), Mac-1 (CD11b, M1/70), Gr-1 (RB6-8C5), c-Kit (ACK2), Sca-1 (D7), and CD34 (RAM34) (all from eBioscience), and an anti-FcγRII/III (2.4G2; BD Bioscience), which were conjugated with phycoerythrin (PE), PE-Cy5, allophycocyanin, Pacific Blue, or biotin, and together with streptavidin-PE-Cy7. For the cell cycle analysis, cells were incubated in 10 μM bromodeoxyuridine for 15 minutes, and 5-bromo-2'-deoxyuridine (BrdU) incorporation and DNA content were determined according to the manufacturer’s instructions (allophycocyanin BrdU Flow Kit; BD Pharmingen).
Protein analyses
Cell lysis, immunoprecipitation, gel electrophoresis, and immunoblotting were performed as described.11 A rabbit polyclonal antibody to COP1 was generated using bacterially produced polypeptides in our laboratory.11 Mouse monoclonal antibodies to hemagglutinin (HA) peptide epitopes (12CA5) and γ-tubulin (GTU-88) were obtained from Sigma. A rabbit polyclonal antibody to an HA epitope (HA.11) and a mouse monoclonal antibody to a FLAG Immunology Ventures) epitope (M5) were purchased from BAbCO and Wastman Kodak Company, respectively. A rabbit polyclonal antibody to C/EBPα (sc-61) was obtained from Santa Cruz Biotechnology.
For the in vitro binding assay, COP1 cDNA was inserted into the vector pGEX (Pharmacia), expressed in bacteria, and purified as described.11 A crude cell extract containing HA-tagged Trib1 protein was prepared from 293T cells transfected with the HA-Trib1 expression vector. Binding assay was performed as described.11 Glutathione S-transferase (GST) proteins were quantitated by immunoblotting with antibody to GST.
For the in vivo ubiquitination assay, NIH3T3 cells were transfected with a combination of vectors encoding FLAG-C/EBPα, GFP-COP1, Trib1, and HA-Ub and harvested 4 days after transfection. Cells were lyzed in sodium dodecyl sulfate sample buffer (40 mM Tris-HCl, pH 6.8, 0.1 M dithiothreitol, 1% sodium dodecyl sulfate, 10% glycerol, and 0.05% bromophenol blue). After boiling, cell lysates were diluted with EBC buffer (50 mM Tris-HCl, pH 7.5, 120 mM NaCl, 0.5% NP-40, and 1 mM EDTA containing 5 mg/mL of aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM NaF, 0.1 mM NaVO4, and 1 mM dithiothreitol) and immunoprecipitated with an antibody to a FLAG epitope followed by immunoblotting with an antibody to an HA epitope.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated using the ISOGEN reagent (Nippon Gene) and reverse transcribed using RNase-free Superscript reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed within a linear range as described previously,28 and the data were normalized to the expression level of a control gene such as the β-actin gene for each sample. We confirmed that the reactions were quantitatively performed within a linear range by conducting several control experiments. The following oligonucleotide primers specific to mouse Trib1, Trib2, C/EBPα, COP1, and β-actin were used: Trib1, 5′-GGACTTTGGAGACATGCACTCCT-3′ (sense) and 5′-GACCAAAAGCGTATAGAGCATCACC-3′ (antisense); Trib2, 5′-GCAACATCAACCAAATCACG-3′ (sense) and 5′-GCGTCTTCCAAACTCTCCAG-3′ (antisense)29 ; C/EBPα, 5′-GAACAGCAACGAGTACCGGGTA-3′ (sense) and 5′-CCCATGGCCTTGACCAAGGAG-3′ (antisense)30 ; COP1, 5′-AGGTTTCAGTGGGACCTCTC-3′ (sense) and 5′-GACCTTTGACCTCTGTCCTG-3′ (antisense); β-actin, 5′-CTTCTACAATGAGCTGCGTGT-3′ (sense) and 5′-CAACGTCACACTTCATGATGG-3′ (antisense).
Results
COP1 induces degradation of C/EBPα and blocks myeloid differentiation
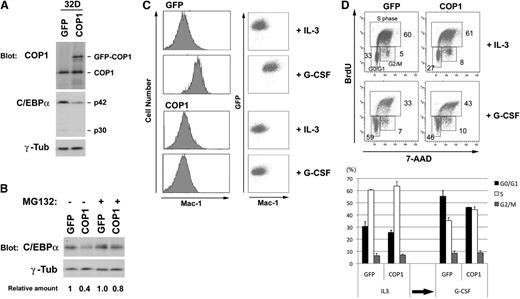
To test whether COP1 affects the level of C/EBPα and blocks myeloid differentiation essential for leukemic transformation, we introduced COP1 into murine 32Dcl3 immature myeloid cells: 32D cells proliferate in the presence of IL3 and differentiate into granulocytes in response to G-CSF treatment.27 We infected 32D cells with MSCV retroviruses expressing GFP alone and GFP-fused COP1 protein. After drug selection, we isolated the viable GFP-positive population by cell sorting. Western blot analysis showed that GFP-COP1 was expressed at a level equivalent to that of the endogenous protein in 32D cells, and ectopic expression of COP1 markedly reduced the endogenous level of C/EBPα (Figure 1A). Treatment with MG132, a specific chemical inhibitor of proteasomes, prevented the decrease of C/EBPα induced by ectopic COP1 (Figure 1B), indicating that down-regulation of C/EBPα expression was mediated by degradation through the ubiquitin-proteasome system.
Ectopic expression of COP1 in 32D cells induces degradation of C/EBPα and blocks granulocytic differentiation in response to G-CSF. (A) 32D cells were infected with viruses containing GFP and GFP-COP1 and selected in the presence of puromycin. Lysates from GFP-positive cells were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (B) 32D transfectants (GFP and COP1) were incubated in the presence and absence of MG132 and analyzed by immunoblotting with antibodies to C/EBPα and γ-tubulin. The relative amounts of C/EBPα are presented as the ratio of C/EBPα and γ-tubulin, and calculated with the level of untreated 32D cells (GFP control) as 1.0. (C) 32D transfectants (GFP and COP1) prepared in A were cultured in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. To confirm the expression of GFP, results with the dot blot analysis are also shown. Results shown in A-C are representative of 2 independent experiments. (D) 32D transfectants (GFP and COP1) were cultured in IL3 and transferred to G-CSF for 4 days. BrdU incorporation and DNA content were analyzed by flow cytometer. Quantification of the data, average of 2 independent experiments, is also shown as means ± standard deviation (SD) at the bottom.
Ectopic expression of COP1 in 32D cells induces degradation of C/EBPα and blocks granulocytic differentiation in response to G-CSF. (A) 32D cells were infected with viruses containing GFP and GFP-COP1 and selected in the presence of puromycin. Lysates from GFP-positive cells were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (B) 32D transfectants (GFP and COP1) were incubated in the presence and absence of MG132 and analyzed by immunoblotting with antibodies to C/EBPα and γ-tubulin. The relative amounts of C/EBPα are presented as the ratio of C/EBPα and γ-tubulin, and calculated with the level of untreated 32D cells (GFP control) as 1.0. (C) 32D transfectants (GFP and COP1) prepared in A were cultured in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. To confirm the expression of GFP, results with the dot blot analysis are also shown. Results shown in A-C are representative of 2 independent experiments. (D) 32D transfectants (GFP and COP1) were cultured in IL3 and transferred to G-CSF for 4 days. BrdU incorporation and DNA content were analyzed by flow cytometer. Quantification of the data, average of 2 independent experiments, is also shown as means ± standard deviation (SD) at the bottom.
Transfer from IL3 to G-CSF induced granulocyte differentiation of 32D cells expressing GFP alone, as monitored by the up-regulation of Mac-1 expression on the cellular surface, but G-CSF–induced granulocyte differentiation was completely blocked in 32D cells expressing GFP-COP1 (Figure 1C). To look at the proliferation status, we examined BrdU incorporation and the cell cycle profile of these cells before and after transfer from IL3 to G-CSF (Figure 1D). In the presence of IL3, percentages of cells in the cycle were comparable between those expressing GFP and GFP-COP1 (S phase population, 60% and 61%, respectively). After transfer to G-CSF, a significant population of control cells expressing GFP alone entered the G0/G1 phase (59%), whereas more cells expressing COP1 remained in the S phase and failed to enter G0/G1 (46%) (Figure 1D). Although cells expressing COP1 were viable for several days in G-CSF (eg, little sub-G1 population in Figure 1D), they did not proliferate or survive in G-CSF for >1 week (counted by the dye exclusion method, data not shown). These results suggest that COP1 blocks myeloid differentiation by promoting degradation of C/EBPα in a proteasome-dependent manner.
E3 ubiquitin ligase activity of COP1 is required for degradation of C/EBPα and block of myeloid differentiation
To examine whether the E3 ubiquitin ligase activity of COP1 is essential for the degradation of C/EBPα and blocking of myeloid differentiation, we used 2 mutants of COP1, CS and ΔRING, as shown in Figure 2A. The CS mutant has 2 conserved cystine residues replaced with serine residues within the RING domain in the N terminus, and the ΔRING mutant lacks residues 138 to 175 corresponding to the whole RING-finger domain, resulting in complete inactivation of COP1’s ubiquitin ligase activity.3 We introduced these COP1 mutants fused with GFP into 32D cells and analyzed. As expected, the expression of the CS and ΔRING mutants failed to reduce the endogenous level of C/EBPα, and these cells lost COP1’s ability to block myeloid differentiation after G-CSF treatment (Figure 2B-C). Thus, the ligase activity of COP1 is required for the degradation of C/EBPα and resultant block of granulocyte differentiation.
Ligase activity-deficient COP1 mutants fail to induce degradation of C/EBPα and block granulocytic differentiation. (A) Schematic representation of ligase activity-deficient COP1 mutants. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (WT and mutants [CS and ΔRING]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants (GFP, COP1 WT and mutants) prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results shown in B and C are representative of 2 independent experiments. CS, an abbreviation of C138S/C141S.
Ligase activity-deficient COP1 mutants fail to induce degradation of C/EBPα and block granulocytic differentiation. (A) Schematic representation of ligase activity-deficient COP1 mutants. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (WT and mutants [CS and ΔRING]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants (GFP, COP1 WT and mutants) prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results shown in B and C are representative of 2 independent experiments. CS, an abbreviation of C138S/C141S.
Alternatively spliced form Δ4, but not Δ20 or Δ24, has the activity to induce degradation of CEBPα
COP1 has 4 forms due to alternative splicing (Figure 3A, upper), the WT (full length), Δ4 (lacking 4 amino acids coded in exon 4), Δ20 (lacking 20 amino acids coded in exon 7), and Δ24 (lacking both exons 4 and 7),3 some of which act in a dominant-negative fashion.31 WT COP1 and the Δ4 form were detected in both the cytoplasm and the nucleus with a higher level in the cytoplasm, whereas Δ20 and Δ24 were found in both the cytoplasm and the nucleus but predominantly in the nucleus (Figure 3A, lower). We assessed the ability of these forms to degrade C/EBPα and to block granulocyte differentiation using 32D cells. Despite a lower expression level, Δ4 was capable of inducing down-regulation of C/EBPα more efficiently than the WT, whereas both Δ20 and Δ24 failed to reduce C/EBPα (Figure 3B). Consistent with these results, Δ4 and the WT inhibited granulocyte differentiation of 32D cells in G-CSF, whereas Δ20 and Δ24 failed to do so (Figure 3C). Thus, deletion of exon 4 did not affect the activity of COP1, whereas the domain encoded in exon 7 seems to be essential for COP1’s function for degradation of C/EBPα and G-CSF–induced granulocytic differentiation. We determined which COP1 forms are dominantly expressed in 32D cells using unique restriction sites in exon 4.31 As shown in Figure 3D, 32D cells expressed only WT COP1.
Different ability of splicing variants of COP1 to induce degradation of C/EBPα and block granulocytic differentiation. (A) NIH3T3 cells were transfected with vectors containing GFP-fused full-length COP1 (WT) and splicing variants (Δ4, Δ20, and Δ24) shown in the schematic diagram (top). Cells were viewed under a fluorescence microscope. Cells transfected with untagged COP1 were stained with antibody to COP1 and visualized with an FITC-labeled secondary antibody. PC, phase contrast. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (full-length [WT] and splicing variants [Δ4, Δ20, and Δ24]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results are representative of 2 independent experiments. (D) RT-PCR products generated by use of template RNAs isolated from 32D cells and control NIH3T3 cells were digested with BstXI or DraIII. A lower panel shows the sizes of the digested fragments of WT COP1 and alternatively spliced variants.
Different ability of splicing variants of COP1 to induce degradation of C/EBPα and block granulocytic differentiation. (A) NIH3T3 cells were transfected with vectors containing GFP-fused full-length COP1 (WT) and splicing variants (Δ4, Δ20, and Δ24) shown in the schematic diagram (top). Cells were viewed under a fluorescence microscope. Cells transfected with untagged COP1 were stained with antibody to COP1 and visualized with an FITC-labeled secondary antibody. PC, phase contrast. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (full-length [WT] and splicing variants [Δ4, Δ20, and Δ24]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results are representative of 2 independent experiments. (D) RT-PCR products generated by use of template RNAs isolated from 32D cells and control NIH3T3 cells were digested with BstXI or DraIII. A lower panel shows the sizes of the digested fragments of WT COP1 and alternatively spliced variants.
COP1 requires Trib1 to block granulocytic differentiation and function as an E3 ubiquitin ligase for C/EBPα
Trib1 and Trib2 induce AML in mice by promoting degradation of C/EBPα12,24 and have a consensus COP1-binding motif. Therefore, we examined whether 32D cells express Trib1 and Trib2 by conducting a specific RT-PCR analysis, and found a significant amount of Trib1, but not Trib2 (Figure 4A). Knockdown of Trib1 in 32D cells abrogated COP1’s activity to block granulocytic differentiation in response to G-CSF (Figure 4B-C), indicating that COP1 requires Trib1 to function in the hematopoietic lineage.
Trib1 cooperates with COP1 to induce ubiquitination of C/EBPα and block granulocytic differentiation. (A) Total RNA was extracted from cells indicated on the top and analyzed by quantitative RT-PCR using a pair of primers specific to Trib1 and Trib2. (−), negative control. (B) 32D cells were coinfected with control and untagged COP1-containing viruses (the pMSCV-IRES-GFP retroviral vector [MigR1]) together with viruses containing control siRNA (siGL2) and siRNA specific to Trib1 (siTrib1). Cell lysates were subjected to immunoblotting with antibodies to COP1 and γ-tubulin. Total RNA was analyzed by quantitative RT-PCR using a pair of primers specific to Trib1. Quantitative data are shown. (C) 32D transfectants prepared as in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. (D) BM cells were fractionated into different lineages shown at the top, lineage marker (Gr-1, Mac-1, TER119, B220, and CD3ε) positive hematopoietic cells (Lin+), CD34−Lin−Sca-1+c-Kit+ (LSK) hematopoietic stem cells (HSCs), CD34+LSK multipotent progenitors (MPPs), FcγRII/IIIloCD34+ common myeloid progenitors (CMPs), FcγRII/IIIhiCD34+ GMPs, and FcγRII/IIIloCD34− megakaryocyte-erythroid progenitors. Control, positive control for C/EBPα and COP1. −, negative control. Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to C/EBPα, COP1, Trib1, Trib2, and β-actin. (E) BM cells were fractionated into different lineages by the surface markers shown at the top, Lin*negSca-1−Mac1lo/+c-Kit+ cells (Lin*, lineage marker without Mac-1 and Gr-1) containing committed myeloid progenitors, Lin*negSca-1−Mac1+/hic-Kitlo cells (differentiated immature myeloid cells), and differentiated population of cells positive for each lineage marker (granulocytes; Gr-1, macrophages; Mac-1, erythrocytes; TER119, B lymphocytes; B220, and T lymphocytes; CD3ε). Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to COP1, Trib1, Trib2, and β-actin. Control, positive control for COP1, Trib1, and Trib2. (−), negative control. (F) GST and GST-COP1 proteins produced in Escherichia coli were immobilized on glutathione beads and incubated with crude cell extracts containing HA-tagged Trib1 protein. The bound protein was detected by immunoblotting with antibody to an HA-epitope. GST-fused proteins were visualized by Coomassie brilliant blue staining. (G) NIH3T3 cells were transfected with a combination of vectors shown at the top. Cell lysates were analyzed by immunoprecipitation with an antibody to a FLAG epitope followed by immunoblotting with an antibody to a HA epitope, by immunoblotting with an antibody to COP1, and by quantitative RT-PCR using a pair of primers specific to Trib1 and β-actin. Results are representative of 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane (D-E and G).
Trib1 cooperates with COP1 to induce ubiquitination of C/EBPα and block granulocytic differentiation. (A) Total RNA was extracted from cells indicated on the top and analyzed by quantitative RT-PCR using a pair of primers specific to Trib1 and Trib2. (−), negative control. (B) 32D cells were coinfected with control and untagged COP1-containing viruses (the pMSCV-IRES-GFP retroviral vector [MigR1]) together with viruses containing control siRNA (siGL2) and siRNA specific to Trib1 (siTrib1). Cell lysates were subjected to immunoblotting with antibodies to COP1 and γ-tubulin. Total RNA was analyzed by quantitative RT-PCR using a pair of primers specific to Trib1. Quantitative data are shown. (C) 32D transfectants prepared as in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. (D) BM cells were fractionated into different lineages shown at the top, lineage marker (Gr-1, Mac-1, TER119, B220, and CD3ε) positive hematopoietic cells (Lin+), CD34−Lin−Sca-1+c-Kit+ (LSK) hematopoietic stem cells (HSCs), CD34+LSK multipotent progenitors (MPPs), FcγRII/IIIloCD34+ common myeloid progenitors (CMPs), FcγRII/IIIhiCD34+ GMPs, and FcγRII/IIIloCD34− megakaryocyte-erythroid progenitors. Control, positive control for C/EBPα and COP1. −, negative control. Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to C/EBPα, COP1, Trib1, Trib2, and β-actin. (E) BM cells were fractionated into different lineages by the surface markers shown at the top, Lin*negSca-1−Mac1lo/+c-Kit+ cells (Lin*, lineage marker without Mac-1 and Gr-1) containing committed myeloid progenitors, Lin*negSca-1−Mac1+/hic-Kitlo cells (differentiated immature myeloid cells), and differentiated population of cells positive for each lineage marker (granulocytes; Gr-1, macrophages; Mac-1, erythrocytes; TER119, B lymphocytes; B220, and T lymphocytes; CD3ε). Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to COP1, Trib1, Trib2, and β-actin. Control, positive control for COP1, Trib1, and Trib2. (−), negative control. (F) GST and GST-COP1 proteins produced in Escherichia coli were immobilized on glutathione beads and incubated with crude cell extracts containing HA-tagged Trib1 protein. The bound protein was detected by immunoblotting with antibody to an HA-epitope. GST-fused proteins were visualized by Coomassie brilliant blue staining. (G) NIH3T3 cells were transfected with a combination of vectors shown at the top. Cell lysates were analyzed by immunoprecipitation with an antibody to a FLAG epitope followed by immunoblotting with an antibody to a HA epitope, by immunoblotting with an antibody to COP1, and by quantitative RT-PCR using a pair of primers specific to Trib1 and β-actin. Results are representative of 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane (D-E and G).
In normal hematopoiesis, C/EBPα is required for granulocyte maturation and is most highly expressed in the granulocyte-macrophage progenitor (GMP) population.32 To examine whether the expression of COP1, Trib1, and Trib2 coincides with that of C/EBPα, we investigated the precise distribution of COP1, Trib1, and Trib2 at various stages of hematopoietic differentiation (Figure 4D-E). Both COP1 and Trib1 were expressed exclusively in the Lin+ population at the differential stage following the GMP population, in which C/EBPα was dominantly expressed. On the other hand, Trib2 was expressed in the megakaryocyte-erythroid progenitors with a low level of COP1 (Figure 4D).
We further pinpointed the population of Lin+ hematopoietic cells, in which COP1, Trib1, and Trib2 are expressed (Figure 4E), by separating Lin*negSca-1−Mac1lo/+c-Kit+ cells (Lin*; lineage marker without Mac-1 and Gr-1) containing committed myeloid progenitors, Lin*negSca-1−Mac1+/hic-Kitlo cells (differentiated immature myeloid cells21 ), and differentiated population of cells positive for each lineage marker (Gr-1, Mac-1, TER119, B220, and CD3ε). Committed myeloid progenitors expressed all COP1, Trib1, and Trib2, and their expression decreased as cells differentiated toward mature granulocytes and macrophages. In the erythroid lineage, expression of Trib2, but not Trib1, was sustained together with that of COP1 (TER119 population), whereas lymphoid cells expressed all 3. Thus, these distributions indicate that the expression of COP1 and Tribble proteins (Trib1 and Trib2) well coincided with each other among hematopoietic populations.
Tribbles have a consensus COP1-binding motif at the carboxyl terminus,6,22 and if COP1 and Trib1 function together as a ubiquitin ligase, COP1 should directly bind to Trib1. A GST-tagged recombinant COP1 protein was generated for an in vitro binding assay with a lysate prepared from 293T cells transfected with HA-tagged Trib1. In this GST-pulldown assay, we found direct interaction of COP1 with Trib1 in vitro (Figure 4F). We then examined whether COP1 and Trib1 induce ubiquitination of C/EBPα in the cell. Because overexpression of Trib1 alone induced down-regulation of C/EBPα, we used a minimum amount of Trib1 expression vector, which alone is not sufficient to ubiquitinate C/EBPα. C/EBPα coexpressed with COP1 and Trib1 in NIH3T3 fibroblasts was markedly polyubiquitinated, whereas the expression of COP1 and Trib1 alone failed to induce ubiquitination under this condition (Figure 4G). These results indicate that COP1 and Trib1 form a complex and function as a ubiquitin ligase for C/EBPα in the cell.
COP1 enhanced Trib1-induced inhibitory activity in myeloid differentiation in mice
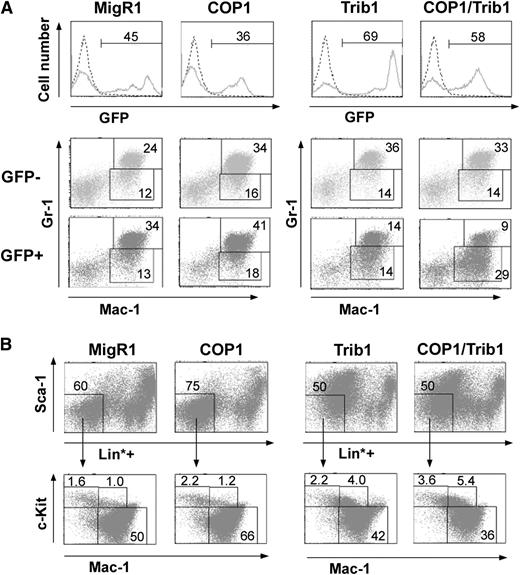
We next addressed whether ectopic COP1 expression has an effect on the proliferation and differentiation of mouse primary hematopoietic progenitor cells. We used the pMSCV-IRES-GFP retroviral vector (MigR1) to transduce the COP1 protein into BM cells and intravenously injected the cells into sublethally irradiated recipient C57BL/6 mice. Inconsistent with the results of the 32D cell experiments, COP1-transduced mice did not develop myeloid disorders 6 to 12 months after BM transplantation (BMT) (see below and Figure 6). We considered the possibility that a certain amount of Trib1 is required for COP1 to exert its inhibitory effect on myeloid differentiation in primary hematopoietic progenitors, because the Trib1 knockdown abrogated COP1’s inhibitory effect on G-CSF–induced granulocytic differentiation in 32D cells (Figure 4B-C) and murine hematopoietic myeloid progenitor cells expressed little endogenous Trib1 (Figure 4D). We then assessed whether coexpression of COP1 accelerates the effects of Trib1 on murine primary hematopoietic cells with transplanted mice. We used a pMSCV-neo-Trib1 retroviral vector cotransduced with a COP1 or MigR1 control vector. Six to 8 weeks after BMT, GFP-positive BM cells were analyzed by flow cytometry, as shown in Figure 5A. At this time point, the recipient mice looked healthy and had no anemia and normal counts of peripheral white blood cells (data not shown). However, in the GFP-positive BM population of Trib1-transduced mice, Mac-1+Gr-1lo immature granulocytic cells started to increase with a decrease of Mac-1+Gr-1hi normal granulocytes. Furthermore, in the GFP-positive population of COP1 cotransduced mice (COP1/Trib1-transduced mice), Mac-1+Gr-1lo cells markedly increased, with a smaller percentage of Mac1+Gr-1hi cells than that in Trib1-transduced mice, suggesting that COP1 coexpression enhances the effect induced by Trib1 in myelopoiesis. We further examined whether COP1 coexpression affects the distribution of the myeloid progenitors and immature myeloid cell populations in Trib1-transduced mice. We did not see any changes in the populations of hematopoietic stem cells and myeloid progenitors (CMP, GMP, and MEP) in Trib1-transduced and COP1/Trib1-transduced mice compared with the control and COP1-transduced mice (data not shown). However, in the populations of more committed myeloid progenitors,21 Trib1-transduced mice showed an increase of Lin*negSca-1−Mac1+c-Kit+ cells (immature myeloid cells containing committed myeloid progenitors) with a decrease of Lin*negSca-1−Mac1+c-Kitlo/− cells (differentiated myeloid cells), and COP1 cotransduction enhanced the phenotype of Trib1-transduced mice (Figure 5B).
Distribution of myeloid progenitor populations of transplanted BM cells at the early stage. BM cells were isolated from mice preinjected with 5-FU 5 days before, and infected with retroviral vectors containing control GFP (MigR1), COP1, and Trib1. Six to 8 weeks after BMT, mice were killed, and BM cells were analyzed by fluorescence-activated cell sorter. (A) The level of GFP signals in BM cells is shown in the upper panels (the percentage of GFP-positive cells). GFP-negative (GFP−, middle panels) and -positive (GFP+, lower panels) BM cells were stained with anti-Mac1 and anti–Gr-1 antibodies. The percentages of immature (Mac-1+Gr-1lo) and differentiated (Mac-1+Gr-1hi) granulocytes are shown. (B) GFP-positive BM cells were stained with a lineage cocktail without anti–Mac-1 and anti–Gr-1 (Lin*, upper panels), and the Lin*negSca-1− population was stained with anti–c-Kit and anti–Mac-1 (lower panels). The percentages of fractions containing CMP/GMP/MEP (Lin*negSca-1−Mac1−c-Kit+), committed myeloid progenitors (Lin*negSca-1−Mac1+c-Kit+), and differentiated myeloid cells (Lin*negSca-1−Mac1+c-Kitlo/−) are shown. Results are representative of 4 independent experiments.
Distribution of myeloid progenitor populations of transplanted BM cells at the early stage. BM cells were isolated from mice preinjected with 5-FU 5 days before, and infected with retroviral vectors containing control GFP (MigR1), COP1, and Trib1. Six to 8 weeks after BMT, mice were killed, and BM cells were analyzed by fluorescence-activated cell sorter. (A) The level of GFP signals in BM cells is shown in the upper panels (the percentage of GFP-positive cells). GFP-negative (GFP−, middle panels) and -positive (GFP+, lower panels) BM cells were stained with anti-Mac1 and anti–Gr-1 antibodies. The percentages of immature (Mac-1+Gr-1lo) and differentiated (Mac-1+Gr-1hi) granulocytes are shown. (B) GFP-positive BM cells were stained with a lineage cocktail without anti–Mac-1 and anti–Gr-1 (Lin*, upper panels), and the Lin*negSca-1− population was stained with anti–c-Kit and anti–Mac-1 (lower panels). The percentages of fractions containing CMP/GMP/MEP (Lin*negSca-1−Mac1−c-Kit+), committed myeloid progenitors (Lin*negSca-1−Mac1+c-Kit+), and differentiated myeloid cells (Lin*negSca-1−Mac1+c-Kitlo/−) are shown. Results are representative of 4 independent experiments.
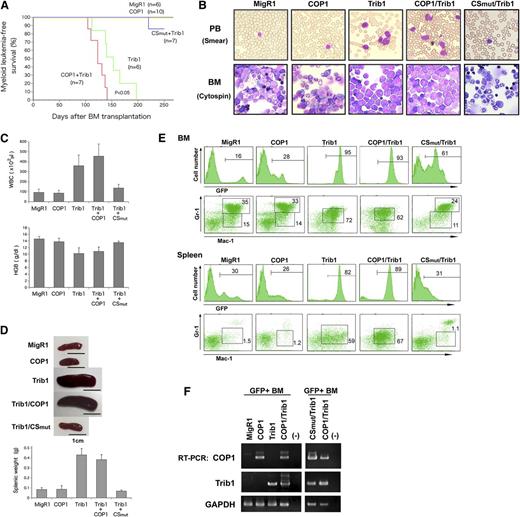
COP1 cooperates with Trib1 to induce ACL. Mice were transplanted with BM cells infected with retroviral vectors containing MigR1 (GFP+) control, COP1 (GFP+), CSmut (GFP+), and Trib1 and examined several months after BMT. (A) Myeloid leukemia-free survival curves of transplanted mice. Results are derived from 3 independent transfer experiments. The P value between Trib1 and COP1/Trib1 mice was calculated with log-rank test. P < .05. (B) May-Grunwald Giemsa–stained peripheral blood (PB) smears and cytospins of BM cells. (C) The counts of leukocytes (WBC) and hemoglobin (HGB). (D) Spleen weights of individual mice. Data presented in C and D are averages of 5 independent experiments shown as means ± SD. (E) Fluorescence-activated cell sorter analysis of BM and spleen cells for immature (Mac-1+Gr-1lo) and differentiated (Mac-1+Gr-1hi) granulocytes. The population of GFP-positive cells in BM and spleen is shown in the upper panels. (F) Total RNA extracted from GFP-positive BM cells was analyzed by quantitative RT-PCR using a pair of primers specific to COP1, Trib1, and GAPDH.
COP1 cooperates with Trib1 to induce ACL. Mice were transplanted with BM cells infected with retroviral vectors containing MigR1 (GFP+) control, COP1 (GFP+), CSmut (GFP+), and Trib1 and examined several months after BMT. (A) Myeloid leukemia-free survival curves of transplanted mice. Results are derived from 3 independent transfer experiments. The P value between Trib1 and COP1/Trib1 mice was calculated with log-rank test. P < .05. (B) May-Grunwald Giemsa–stained peripheral blood (PB) smears and cytospins of BM cells. (C) The counts of leukocytes (WBC) and hemoglobin (HGB). (D) Spleen weights of individual mice. Data presented in C and D are averages of 5 independent experiments shown as means ± SD. (E) Fluorescence-activated cell sorter analysis of BM and spleen cells for immature (Mac-1+Gr-1lo) and differentiated (Mac-1+Gr-1hi) granulocytes. The population of GFP-positive cells in BM and spleen is shown in the upper panels. (F) Total RNA extracted from GFP-positive BM cells was analyzed by quantitative RT-PCR using a pair of primers specific to COP1, Trib1, and GAPDH.
COP1 accelerates Trib1-induced AML development in mice
All Trib1-transduced mice developed AML and died at 120 to 200 days after BMT, which is consistent with previous reports.13,22 As expected from early phase analyses, coexpression of COP1 significantly accelerated the onset of Trib1-induced AML (P < .05; Figure 6A). All mice cotransduced with COP1 and Trib1 (COP1/Trib1 mice) eventually exhibited anemia and peripheral blood leukocytosis with markedly increased myeloid blasts in the BM (Figure 6B-C) and splenomegaly (Figure 6D). These pathological phenotypes were basically similar to those of Trib1-transduced mice (Trib1 mice). In Trib1 and COP1/Trib1 mice, GFP-positive Mac-1+Gr-1lo immature cells largely replaced normal BM cells with the absence of Mac-1+Gr-1hi mature granulocytes, and accumulated in spleen (Figure 6E). These results indicated that COP1 cotransduced mice developed AML with more rapid progression than Trib1 mice. To assess whether COP1 ligase activity is required for Trib1-induced AML, we used the activity-deficient CS mutant, shown in Figure 2A, in BMT experiments. Interestingly, all mice cotransduced with CS mutant and Trib1 (CSmut/Trib1 mice) did not develop AML at 220 days after BMT (Figure 6A-E), implying that COP1 ligase activity is essential for COP1/Trib1-induced leukemogenesis, and that the CS mutant acts as a dominant negative to suppress Trib1-mediated leukemogenesis. We confirmed the expression of COP1, CS mutant, and Trib1 in GFP-positive BM populations of each transduced mouse by RT-PCR (Figure 6F).
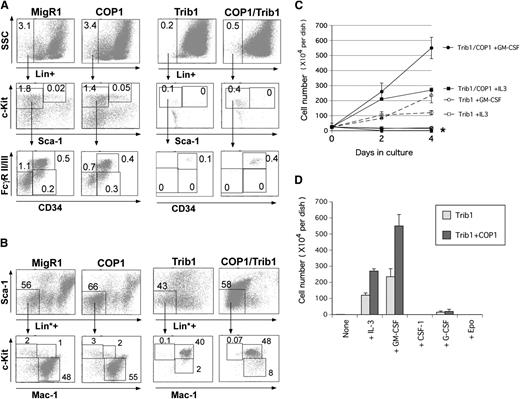
Although ectopic introduction of Trib1 and Trib2 induces C/EBPα degradation and AML,12,24 previous studies did not address the precise stage during myeloid differentiation, at which Trib1 and Trib2 overexpression is affected. We therefore examined leukemic cells from Trib1 and COP1/Trib1 mice by using multicolor cell surface markers. In leukemic Trib1 and COP1/Trib1 mice, lineage-negative populations including LSK and committed myeloid progenitors (CMP, GMP, and MEP) had almost disappeared from the BM (Figure 7A), and the granulocyte-macrophage–committed immature myelocyte population (Lin*negSca-1−Mac1+c-Kit+ cells) dominated (Figure 7B). This result was consistent with the phenotype of mice in which the p42 isoform of C/EBPα was specifically knocked out,21 implying that the COP1/Trib1 complex is the direct negative regulator of the C/EBPα protein.
COP1 cooperates with Trib1 to increase committed myeloid progenitors. (A) CMP (Lin−c-Kit+Sca-1−FcRloCD34+), GMP (Lin−c-Kit+Sca-1−FcRhiCD34+), and MEP (Lin−c-Kit+Sca-1−FcRloCD34−) in GFP-positive BM cells shown in Figure 6E were analyzed. (B) Several months after BMT, the populations containing CMP/GMP/MEP (Lin*negSca-1−Mac1−c-Kit+), committed myeloid progenitors (Lin*negSca-1−Mac1+c-Kit+), and differentiated myeloid cells (Lin*negSca-1−Mac1+c-Kitlo/−) in GFP-positive BM cells were analyzed as in Figure 5B. (C) BM cells isolated from mice transplanted with Trib1 BM cells and Trib1+COP1 BM cells were cultured in medium containing mouse IL3 (2.5 ng/mL), mouse GM-CSF (5 ng/mL), human CSF-1 (2000 U/mL), human G-CSF (25 ng/mL), and human Epo (1 U/mL). Cells were enumerated on the second and fourth days. *BM cells cultured with no cytokines, CSF-1, G-CSF, and Epo. (D) Summary of the data for the fourth day in C. Results shown in A and B are representative of 5 independent experiments. Data presented in C and D are averages of 3 independent experiments shown as means ± SD.
COP1 cooperates with Trib1 to increase committed myeloid progenitors. (A) CMP (Lin−c-Kit+Sca-1−FcRloCD34+), GMP (Lin−c-Kit+Sca-1−FcRhiCD34+), and MEP (Lin−c-Kit+Sca-1−FcRloCD34−) in GFP-positive BM cells shown in Figure 6E were analyzed. (B) Several months after BMT, the populations containing CMP/GMP/MEP (Lin*negSca-1−Mac1−c-Kit+), committed myeloid progenitors (Lin*negSca-1−Mac1+c-Kit+), and differentiated myeloid cells (Lin*negSca-1−Mac1+c-Kitlo/−) in GFP-positive BM cells were analyzed as in Figure 5B. (C) BM cells isolated from mice transplanted with Trib1 BM cells and Trib1+COP1 BM cells were cultured in medium containing mouse IL3 (2.5 ng/mL), mouse GM-CSF (5 ng/mL), human CSF-1 (2000 U/mL), human G-CSF (25 ng/mL), and human Epo (1 U/mL). Cells were enumerated on the second and fourth days. *BM cells cultured with no cytokines, CSF-1, G-CSF, and Epo. (D) Summary of the data for the fourth day in C. Results shown in A and B are representative of 5 independent experiments. Data presented in C and D are averages of 3 independent experiments shown as means ± SD.
We further examined the growth ability and dependency on growth factors of these primary Mac1+c-Kit+ leukemic cells in a liquid culture in vitro. Leukemic cells derived from Trib1 and COP1/Trib1 mice survived and proliferated in the presence of IL3 and GM-CSF, but underwent apoptosis in the culture without cytokines or with CSF-1, G-CSF, and Epo (Figure 7C). COP1/Trib1 leukemic cells proliferated ∼2 times faster than Trib1 leukemic cells in culture in the presence of IL3 and GM-CSF (Figure 7D), indicating that COP1 coexpression enhanced growth ability induced by Trib1.
Discussion
We showed here that COP1 together with Trib1 functions as an E3 ubiquitin ligase for C/EBPα and consequently causes AML in mice, in which the ligase activity of COP1 is essential. We first tested the effects of COP1 on the differentiation of 32D immature myeloid cells and found that ectopic expression of COP1 in 32D cells induced proteasome-dependent degradation of C/EBPα and inhibited G-CSF-induced granulocytic differentiation, which led us to the possibility that COP1 causes AML by targeting C/EBPα in vivo. We then examined its effects on primary murine BM cells. COP1-transduced BM transfer experiments revealed that COP1 itself did not induce AML but did promote AML development in collaboration with Trib1 in mice. This result seems to be inconsistent with that of the experiment with 32D cells. However, we found that the expression of endogenous Trib1 was required for the COP1 activity because 32D cells expressed endogenous Trib1 and its knockdown abrogated the effects of COP1. In normal myelopoiesis, the distribution of COP1 is well correlated with that of Trib1 and is most abundant in the lineage positive population next to the differentiation stage of GMP myeloid committed progenitors, in which C/EBPα is dominantly expressed. These results imply that COP1 requires Trib1 to exert its activity on C/EBPα in myelopoiesis. Indeed, our in vitro ubiquitination assay showed that COP1 markedly promotes polyubiquitination of C/EBPα only in the presence of Trib1.
Despite that Trib1 and Trib2 promote degradation of C/EBPα and cause AML in mice,12,24 it has not been tested which population at the differentiation stage in hematopoiesis is targeted in Tribbles-induced AML. We showed, for the first time, the phenotype of COP1- and Tribbles-associated leukemic cells using a precise flow cytometric analysis with multiple surface markers in mouse models. We found that both Trib1- and COP1/Trib1-induced leukemic blasts were detected mostly in a compartment of Mac1+c-Kit+ committed myeloid progenitors. The phenotype was consistent with that of the leukemic blasts developed in p42C/EBPα-deficient mice.21 These results support the finding that COP1 is a negative regulator upstream of C/EBPα and that the COP1-Trib1-C/EBPα pathway plays an important role in normal myelopoiesis.
Mammalian COP1 exerts its ubiquitin ligase activity in various biological responses, and, in contrast to the well-known activity of COP1 in plants,1 its function, especially, in cell proliferation and tumorigenesis, remains ambiguous.2 Initially, COP1 was placed upstream of the tumor suppressor p535,11 and thought to function as an oncoprotein. In fact, overexpression of COP1 was detected in breast cancer.33 Furthermore, along with previous studies from 2 other groups,23,24 our study showed that COP1 bound to Tribbles (Trib1 and Trib2) down-regulated C/EBPα to induce acute myelogenous leukemia in mice. These observations were all consistent with the idea that COP1 acts as an oncoprotein. However, recent reports on genetically engineered mice suggest that COP1 behaves as a tumor suppressor rather than as an oncoprotein.4,9 COP1 deficiency in embryos did not induce the accumulation of p53, and its embryonic lethality was not rescued by the p53 null background, indicating that COP1 behaves differently from MDM2, an E3 ligase specific to p53. COP1 hypomorphic mice were T-cell lymphoma prone in association with high levels of phosphorylated c-Jun.9 In addition, it was recently reported that COP1 causes degradation of the proto-oncoproteins (E26 transformation-specific [ETS] variant) (ETV)1, ETV4, and ETV5, the ETS family of transcription factors, which are often truncated in human prostate cancer. COP1 deficiency in murine prostates induced low-grade intraepithelial neoplasia.4 These COP1-deficient mouse models support the idea that COP1 is a tumor suppressor. These studies seem to be contradictory to our findings using COP1-introduced BMT mice. However, many E3 ligases exhibit multiple functions by targeting several different substrates in a manner dependent on the tissue and differentiation stage of cells. Indeed, COP1 is also involved in signaling pathways of lipid metabolism and gluconeogenesis,6,7 which are not associated with tumorigenesis. It may not be appropriate to categorize COP1 as only an oncogene or a tumor suppressor.
We showed the importance of adaptor proteins to the functional multiplicity of COP1. COP1 failed to ubiquitinate C/EBPα in the absence of Trib1. Similarly, others reported that COP1 does not ubiquitinate ACC in the cells expressing a Trib3 mutant lacking a consensus COP1-binding motif6 and does not ubiquitinate c-Jun without the coexpression of DET1.3 These facts strengthen the notion that adaptor proteins such as the Tribbles family and DET1 determine the differentiation stage- and tissue-specific activities of COP1. In addition, we showed that 3 forms of COP1 generated by alternative splicing, Δ4, Δ20, and Δ24, contribute to the regulation of COP1’s activity. Δ4 behaved similarly to the WT in terms of blocking myeloid differentiation, but Δ20 and Δ24 lacked this activity. In Δ20 and Δ24, exon 7 is missing, which contains the C terminus of the coiled-coil domain and a putative nuclear export signal sequence (our unpublished observation), resulting in an inability to interact with COP1-binding proteins and exert activity. Our observations support the report that Δ20 (also called COP1D) functions in a dominant negative manner.31 Thus, it is feasible that COP1 activity and substrate specificity are regulated in multiple ways, depending on its adaptor proteins, and by the balance between the WT and alternatively spliced forms.
Recent studies regarding COP1 raise several critical questions: is COP1 an oncogene or a tumor suppressor; and how can COP1 specifically target many substrates for degradation in various tissues? Our findings may help to answer these questions and to develop an appropriate strategy for cancer therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs C. J. Sherr and M. F. Roussel for the NIH3T3 cell line.
This work was supported by Grants-in-Aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, and Culture of Japan.
Authorship
Contribution: N.Y.-K. designed the experiments; A.Y., J.-y.K., I.N., and N.Y.-K. performed experiments and analyzed the data; and J.-y.K. and N.Y.-K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Noriko Yoneda-Kato, Graduate School of Biological Sciences, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0101, Japan; e-mail: noriko-k@bs.naist.jp.

![Figure 2. Ligase activity-deficient COP1 mutants fail to induce degradation of C/EBPα and block granulocytic differentiation. (A) Schematic representation of ligase activity-deficient COP1 mutants. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (WT and mutants [CS and ΔRING]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants (GFP, COP1 WT and mutants) prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results shown in B and C are representative of 2 independent experiments. CS, an abbreviation of C138S/C141S.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/10/10.1182_blood-2012-12-476101/4/m_1750f2.jpeg?Expires=1769196264&Signature=2TNriulmlRA60uYCnm2513PxwWP0LWHVqG6N0CVtBwy1WU6iK9RA7LmizXyEhAOciicbLKVwg1CpMUNE3ttDsoMBKhDppD0a-c0taocfM9EsoWQvYW6BTo0ObUaaKpS-vdPaEf9INcoArR5nOrNirXke-tt3Ula6hI9rBsAWsp~prjfU8UYPUlQdVoDmiBeuhfNYjxOTARLXg8WiRJMYUMp~wbk3GPzcivYq1mLVlR5SUtHbSIiJDaFMJy~iQ5Q~R8~DIXR-E-XizvFc9Y3EFmJek-~L58RBqVqtSUA5LW8SVS43RN4M5IIgwnTwUrnL6csMl8qlAX7Eok~Upm~m0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Different ability of splicing variants of COP1 to induce degradation of C/EBPα and block granulocytic differentiation. (A) NIH3T3 cells were transfected with vectors containing GFP-fused full-length COP1 (WT) and splicing variants (Δ4, Δ20, and Δ24) shown in the schematic diagram (top). Cells were viewed under a fluorescence microscope. Cells transfected with untagged COP1 were stained with antibody to COP1 and visualized with an FITC-labeled secondary antibody. PC, phase contrast. (B) Lysates from GFP-positive 32D cells infected with viruses containing GFP, GFP-COP1 (full-length [WT] and splicing variants [Δ4, Δ20, and Δ24]) were analyzed by western blotting using antibodies against COP1, C/EBPα, and γ-tubulin. (C) 32D transfectants prepared in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. Results are representative of 2 independent experiments. (D) RT-PCR products generated by use of template RNAs isolated from 32D cells and control NIH3T3 cells were digested with BstXI or DraIII. A lower panel shows the sizes of the digested fragments of WT COP1 and alternatively spliced variants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/10/10.1182_blood-2012-12-476101/4/m_1750f3.jpeg?Expires=1769196264&Signature=FMeMAKJ8vHVPDOEnSt3MJ0tonG3YX401hD4KuXVxaIa58dU1qDCRDMdBxxwAI3Xtfd9To9t50htTYBFPS7BKLlklqtDWAmwCl7whL-aIZMcJEB~EcX-0RjXyG5FK8GHLa-o~IfH1eSDvoNCQ4xGBhPZcOeOG4xieQ0-1fwhd9imW6EZB0PDJ36FkNIom8sDMY1paYg55WFeAeLKwZ8a21L6WkC79JDT4XHGxRHuPPNUPuphP7-znJRDBIVXmhrj4xMFJnLK5lKeAMC-ouNw3yjXWvQuQft5GadLHhGVm8KjXJzZfGwHz3apRoaUu78LJxssD9nH8LQxbTOIBr~QBtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Trib1 cooperates with COP1 to induce ubiquitination of C/EBPα and block granulocytic differentiation. (A) Total RNA was extracted from cells indicated on the top and analyzed by quantitative RT-PCR using a pair of primers specific to Trib1 and Trib2. (−), negative control. (B) 32D cells were coinfected with control and untagged COP1-containing viruses (the pMSCV-IRES-GFP retroviral vector [MigR1]) together with viruses containing control siRNA (siGL2) and siRNA specific to Trib1 (siTrib1). Cell lysates were subjected to immunoblotting with antibodies to COP1 and γ-tubulin. Total RNA was analyzed by quantitative RT-PCR using a pair of primers specific to Trib1. Quantitative data are shown. (C) 32D transfectants prepared as in B were maintained in IL3, transferred to G-CSF for 4 days, and analyzed for expression of Mac-1 by flow cytometer. (D) BM cells were fractionated into different lineages shown at the top, lineage marker (Gr-1, Mac-1, TER119, B220, and CD3ε) positive hematopoietic cells (Lin+), CD34−Lin−Sca-1+c-Kit+ (LSK) hematopoietic stem cells (HSCs), CD34+LSK multipotent progenitors (MPPs), FcγRII/IIIloCD34+ common myeloid progenitors (CMPs), FcγRII/IIIhiCD34+ GMPs, and FcγRII/IIIloCD34− megakaryocyte-erythroid progenitors. Control, positive control for C/EBPα and COP1. −, negative control. Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to C/EBPα, COP1, Trib1, Trib2, and β-actin. (E) BM cells were fractionated into different lineages by the surface markers shown at the top, Lin*negSca-1−Mac1lo/+c-Kit+ cells (Lin*, lineage marker without Mac-1 and Gr-1) containing committed myeloid progenitors, Lin*negSca-1−Mac1+/hic-Kitlo cells (differentiated immature myeloid cells), and differentiated population of cells positive for each lineage marker (granulocytes; Gr-1, macrophages; Mac-1, erythrocytes; TER119, B lymphocytes; B220, and T lymphocytes; CD3ε). Total RNA extracted from each cell was analyzed by quantitative RT-PCR using a pair of primers specific to COP1, Trib1, Trib2, and β-actin. Control, positive control for COP1, Trib1, and Trib2. (−), negative control. (F) GST and GST-COP1 proteins produced in Escherichia coli were immobilized on glutathione beads and incubated with crude cell extracts containing HA-tagged Trib1 protein. The bound protein was detected by immunoblotting with antibody to an HA-epitope. GST-fused proteins were visualized by Coomassie brilliant blue staining. (G) NIH3T3 cells were transfected with a combination of vectors shown at the top. Cell lysates were analyzed by immunoprecipitation with an antibody to a FLAG epitope followed by immunoblotting with an antibody to a HA epitope, by immunoblotting with an antibody to COP1, and by quantitative RT-PCR using a pair of primers specific to Trib1 and β-actin. Results are representative of 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane (D-E and G).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/10/10.1182_blood-2012-12-476101/4/m_1750f4.jpeg?Expires=1769196264&Signature=ncQ7VdHhnKT7rcov7LHsCARo64ZBkiXTUcAj2k6CRjm6vwFyWy2c2L1S7vxKHESNNlL7nvRWAeoGDm22qPlauPksbOk~byPX9EPpvQ1B5pXnUuLq7Qy6pNUp968OmfkUR-zk~MB~7ZBfSl6Qz1X7W8KAUby8PZA99atlyx32ukirOQLHCkTVsaFdWE2fWw2aWoAsu6~BUT3hRlF3D1QQ02~Z8Updasa7ap2DzYvjnrNXArovG~18f7oZmX7~~ygxignp1ccCdzr5QOs1wP3QjSvh94T61nGbPc0yJ96R-C464PbScyrPt-9-~mt3PR~hSqh-ajDP4uGQXocBG1Olsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)