Key Points
FLT3 N676K mutations without concurrent internal tandem duplication (ITD) are associated with core-binding factor leukemia.
N676K activates FLT3 and downstream signaling pathways.
Abstract
The t(8;21) and inv(16)/t(16;16) rearrangements affecting the core-binding factors RUNX1 and CBFB, respectively, are found in 15% to 20% of adult de novo acute myeloid leukemia (AML) cases and are associated with a favorable prognosis. Since the expression of the fusion genes CBFB/MYH11 or RUNX1/RUNX1T1 alone is not sufficient to cause leukemia, we performed exome sequencing of an AML sample with an inv(16) to identify mutations, which may collaborate with the CBFB/MYH11 fusion during leukemogenesis. We discovered an N676K mutation in the adenosine triphosphate (ATP)-binding domain (tyrosine kinase domain 1 [TKD1]) of the fms-related tyrosine kinase 3 (FLT3) gene. In a cohort of 84 de novo AML patients with a CBFB/MYH11 rearrangement and in 36 patients with a RUNX1/RUNX1T1 rearrangement, the FLT3 N676K mutation was identified in 5 and 1 patients, respectively (5 [6%] of 84; 1 [3%] of 36). The FLT3-N676K mutant alone leads to factor-independent growth in Ba/F3 cells and, together with a concurrent FLT3-ITD (internal tandem duplication), confers resistance to the FLT3 protein tyrosine kinase inhibitors (PTKIs) PKC412 and AC220. Gene expression analysis of AML patients with CBFB/MYH11 rearrangement and FLT3 N676K mutation showed a trend toward a specific expression profile. Ours is the first report of recurring FLT3 N676 mutations in core-binding factor (CBF) leukemias and suggests a defined subgroup of CBF leukemias. This trial was registered at www.clinicaltrials.gov as #NCT00266136.
Introduction
The inversion inv(16)(p13;q22), the translocation t(16;16)(p13;q22), and the translocation t(8;21)(q22;q22) are recurring rearrangements in acute myeloid leukemia (AML), which result in the fusion genes CBFB/MYH11 or RUNX1/RUNX1T1, respectively. These rearrangements are found in 15% to 20% of adult de novo AML cases and represent recognized World Health Organization entities that are associated with a favorable prognosis.1,2
CBFB and RUNX1 form the core-binding factor (CBF), a heterodimeric transcription factor essential for normal hematopoiesis. The CBFB/MYH11 and RUNX1/RUNX1T1 fusion proteins disrupt the physiologic activity of CBF, leading to the repression of CBF target genes and resulting in a block of differentiation and impaired hematopoiesis. Since knock-in mouse models have demonstrated that the expression of CBFB/MYH11 and RUNX1/RUNX1T1 by themselves is not sufficient to cause leukemia, it is highly likely that additional mutations are required for malignant transformation.1,3,4 Leukemogenesis is a multistep process. Mutations associated with myeloid malignancies have been found in genes involved in several functional classes: signaling pathways (eg, FLT3, KIT, RAS), transcription factors (eg, RUNX1/RUNX1T1, CBFB/MYH11), epigenetic regulators (eg, DNMT3A, IDH1, IDH2, TET2), tumor suppressors (eg, TP53, WT1), and splicing machinery (eg, SF3B1, SRSF2).5 In CBF leukemia, mutations in genes coding for signaling proteins (so-called proliferation drivers) are commonly found to collaborate with the CBF fusion genes.6 Mutations in KIT, FLT3, or NRAS/KRAS have frequently been detected in CBF leukemia.7-9 Up to 90% of AML patients with a CBFB/MYH11 fusion have either a mutation in a receptor tyrosine kinase (RTK) or in RAS.10,11 In general, these signaling pathway mutations are mutually exclusive.5 However, about 10% of AML patients with a CBFB/MYH11 fusion do not carry any of the currently known mutations.
To systematically identify additional collaborating mutations in CBFB/MYH11-positive AML patients, we performed exome sequencing of an AML with an inv(16) without any additional known genetic alterations. Using this approach, we identified an FLT3 N676K mutation. Screening a cohort of 120 CBF AML patients, we discovered the FLT3 N676K mutation to be present in 6 of these patients. Mutations affecting the ATP-binding pocket, in particular position N676, resulting in variable amino acid changes (N676D or N676S), were initially discovered in a screen for resistance to tyrosine kinase inhibitors (TKIs) in FLT3 internal tandem duplication (ITD)-expressing Ba/F3 cells.12,13 To the best of our knowledge, an FLT3 N676K point mutation has been reported just once before in a cytogenetically normal (CN) AML patient with an FLT3-ITD mutation who was screened to determine the cause of the acquired TKI resistance after PKC412 therapy.14 In this study, we report recurring FLT3 N676K mutations at first diagnosis of CBF AML without concurrent FLT3-ITD. Importantly, Ba/F3 cells expressing the FLT3 N676K mutation show factor-independent growth and sensitivity toward commonly used TKIs, suggesting that the presence of the FLT3 N676K mutation in CBF leukemia patients might open up new treatment options including TKI therapy.
Materials and methods
Patient samples
A diagnostic bone marrow sample was collected from an 18-year-old patient diagnosed with AML M4eo according to standard French-American-British and World Health Organization criteria in December 2003. The inv(16)(p13;q22) was detected by standard cytogenetics analysis (karyotype: 46,XY,inv(16)(p13;q22)[10]). The CBFB/MYH11 fusion transcript was confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR). No additional genetic alterations were detected at this time. The patient was enrolled in the AMLCG-1999 trial of the German AML Cooperative Group (NCT00266136), and written informed consent was obtained in accordance with the Declaration of Helsinki. The samples were obtained under AMLCG study protocols approved by the ethics committees of the participating centers. After induction chemotherapy and autologous peripheral blood stem cell transplantation, complete remission was achieved (<5% bone marrow blasts; CBFB/MYH11 transcripts no longer detectable by RT-PCR). A bone marrow sample at complete remission was used as normal control for exome sequencing.
In total, bone marrow or peripheral blood samples from 84 adult patients with newly diagnosed and untreated AML M4eo (CBFB/MYH11 fusion–positive, including the case analyzed by exome sequencing), from 36 patients with t(8;21) and from 90 patients with CN AML were used for targeted mutation screening.
Sample preparation and high-throughput sequencing
Genomic DNA was extracted from patients’ bone marrow or peripheral blood samples using QIAcube technology (Qiagen, Hilden, Germany). For exome sequencing of the index patient, 3 µg of genomic DNA was fragmented to an average size of 150 bp by using the Bioruptor sonicator (Diagenode, Liège, Belgium). Paired-end sequencing libraries were prepared using DNA sample prep reagent set 1 (NEBNext). Library preparation included end repair, adapter ligation, and PCR enrichment and was carried out as recommended by Illumina protocols. Exon-coding sequences were then captured by using SureSelect human all exon 50Mb kit version 3 (Agilent, Santa Clara, CA) according to the manufacturer’s instructions. Exome libraries were sequenced by performing 76-bp paired end reads on a Genome Analyzer IIx platform (Illumina, San Diego, CA). Sequence alignment and variant detection was performed as described previously.15
Sanger sequencing
The nonsynonymous somatic variant in the FLT3 gene (detected in the AML but not in the remission sample) was verified by sequencing both DNA strands using ABI 3100-Avant technology (Applied Biosystems) after PCR amplification of FLT3 exon 16. PCR and sequence analysis of genomic DNA was performed with forward primer 5′-TGCAGATTGACTCTGAGCTG-3′ and reverse primer 5′-CACTGTGACTGAGAAAAGACAAAG-3′, located in the 5′ and 3′ flanking introns, spanning the complete exon 16 and yielding a 327-bp PCR product corresponding to AA 649 to 685 of the human FLT3 protein (National Center for Biotechnology Information reference sequence NM_004119). The same assay was used on a total of 209 de novo AML patients (84 CBFB/MYH11- rearranged, 36 RUNX1/RUNX1T1-rearranged, and 90 CN-AML patients). Routine diagnostic tests included mutation analysis at defined positions of FLT3, KIT, KRAS, NRAS, NPM1, MLL and WT1 of all 84 AML M4eo samples (supplemental Table 3).
DNA constructs and vectors
The human FLT3-wild-type (WT) and the FLT3-ITD-NPOS constructs containing a 28 AA duplicated sequence (CSSDNEYFYVDFREYEYDLKWEFPRENL) inserted between AA 611/612 of human FLT3-WT were kindly provided by Gary Gilliland (Harvard Medical School, Boston, MA). The FLT3 constructs were subcloned into the MSCV-IRES-EYFP retroviral expression vector (kindly provided by R. K. Humphries, Terry Fox Laboratory, University of British Columbia, Vancouver, BC, Canada).
In vitro mutagenesis
The N676K mutation was introduced into the FLT3-WT and the FLT3-ITD-NPOS vectors by using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. The mutant FLT3 D835Y construct was generated by using the QuikChange Site-Directed Mutagenesis Kit.16 The correct sequence of all constructs was confirmed by sequencing.
Cell lines, reagents, and antibodies
Phoenix Eco cells were purchased from Orbigen (San Diego, CA) and cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin. Low-passage murine Ba/F3 cells and WEHI-3B cells were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany) and maintained in RPMI-1640 medium containing 10% fetal bovine serum, 0.5% penicillin/streptomycin, and 10% WEHI-3B conditioned medium as a source of interleukin-3 (IL-3). Recombinant human FLT3 ligand and recombinant murine IL-3 were obtained from Immunotools (Friesoythe, Germany). FLT3 inhibitor PKC412 was obtained from Novartis (Basel, Switzerland) and AC220 was obtained from SYNthesis Med Chem (Cambridge, United Kingdom).
The following antibodies were used: anti-AKT (9272), anti-pAKT (4060), anti-MAPK (9107), anti-pMAPK (9101), and anti-pSTAT5 (9351) (Cell Signaling Technology, Danvers, MA); anti-FLT3 (sc-480), anti-pTyr (sc-7020), anti-STAT5 (sc-835), and anti-GAPDH (sc-32233) from Santa Cruz Biotechnology (Santa Cruz, CA); and CD135-PE (IM2234U) and IgG1-PE isotype control (A07796) from Immunotech (Marseille, France). Stable transduction of Ba/F3 cells, western blot analysis, and detection of surface markers were performed as described previously.17,18
Proliferation and apoptotic cell death of Ba/F3 cells
Proliferation and apoptosis assays were carried out as described previously.17 For long-term proliferation assays, cells were seeded at a density of 2 × 105/mL in growth medium containing 0.1% WEHI-conditioned medium as source of murine IL-3 and as control in the presence of 10 ng/mL IL-3. After 72 hours, Ba/F3 cells were cleared from IL-3 by two centrifugation steps with phosphate-buffered saline and resuspended in medium without IL-3. Control cells were cultivated in the presence of 10 ng/mL IL-3. Viable cells were counted every day, and a cell density of 2.5 × 106 was not exceeded.
Gene expression profiling and microarray analyses
Pretreatment bone marrow samples from 33 patients (data deposited in GSE37642) were analyzed by using Affymetrix HG-U133 A/B oligonucleotide microarrays (Affymetrix, Santa Clara, CA) as described previously.19,20 For probes to probe set annotation, we used custom chip definition files based on GeneAnnot version 2.0, synchronized with GeneCards Version 3.04 (http://www.xlab.unimo.it/GA_CDF/).21 Normalization was carried out by the robust multichip average method as described by Irizarry et al.22 The Linear Models for Microarray Data (Limma) package was used to compute differentially regulated probe sets by comparing patients with CBFB/MYH11 rearrangement and mutations affecting FLT3 D835, NRAS, KRAS, KIT, or FLT3-ITD to patients with CBFB/MYH11 rearrangement and FLT3 N676K. Gene set enrichment analysis (GSEA) was performed with GSEA software (Broad Institute of Massachusetts Institute of Technology and Harvard) to assess significant changes in gene expression levels.23 The GSEA was run with 1000 permutations and compared with the “c2_kegg” collection from the Molecular Signatures Database (MsigDB 3.0) consisting of 186 gene sets. All statistical analyses were performed by using R 3.0.1 software and routines from the biostatistics software repository Bioconductor.
Results
Exome sequencing of an AML M4eo patient
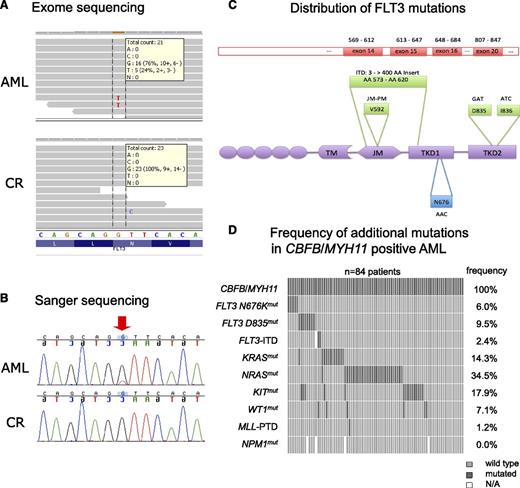
To systematically identify mutations that may collaborate with CBFB/MYH11 during leukemogenesis, we performed exome sequencing of an AML sample with inv(16). The sample was selected on the basis of sample availability and the absence of additional genetic alterations (FLT3-ITD, MLL-PTD [partial tandem duplication], FLT3-TKD, NPM1, NRAS, KRAS, KIT, and WT1 mutation negative). We sequenced the exome (protein coding regions) of the diagnostic sample and a remission sample from the same patient, generating at least 4 Gbp of sequence from each exome. This allowed us to cover more than 80% of RefSeq coding exon positions with a minimum read depth of 10 (supplemental Table 1). By comparing both exome sequences and excluding known polymorphisms, we were able to identify somatically acquired, leukemia-specific sequence variants. Nonsynonymous coding mutations were confirmed by using Sanger sequencing. We found a total of 2 somatic mutations, namely an N676K missense mutation in the ATP-binding domain (TKD1) of FLT3 (NM_004119.2:c.2028C>A; Figure 1A-C)24 and an A251V missense mutation in the CAT gene, which encodes the cytoplasmic enzyme catalase (supplemental Table 2).
FLT3 N676K mutations identified in CBFB/MYH11-rearranged AML. (A) Exome data sets of a CBFB/MYH11-positive AML sample (upper panels) and the corresponding follow-up sample from the same patient (lower panels) are displayed using the integrative genomics viewer.24 Horizontal gray bars symbolize the 76-bp reads aligned to the reference sequence. The frequency of 24% of the mutant nucleotide T in the diagnostic leukemia sample indicates a heterozygous point mutation causing an amino acid substitution (NM_004119.2:c.2028C>A; p.N676K), whereas in the follow-up sample, only the wild-type nucleotide G is detected at this position. Read depth and base count are indicated for the affected positions, respectively. (B) Sanger sequencing confirmed the FLT3 N676K mutation found initially by exome sequencing. Chromatograms are shown for both the diagnostic AML sample and the corresponding follow-up sample at complete remission (CR) from the same patient. (C) The structure of the human FLT3 protein includes the transmembrane domain (TM), the juxtamembrane domain (JM), and TKD1 and TKD2. Amino acid positions targeted by known recurrent mutations in AML are indicated in green above the corresponding domains. N676 is indicated in blue below the TKD1 domain. (D) Frequency distribution of additional genetic aberrations in 84 CBFB/MYH11-rearranged patients. Each column indicates one patient. Dark gray boxes indicate patients who are positive for the respective mutation; light gray boxes indicate wild-type status. Missing information is shown as a white space (N/A, not available). Gene names and types of mutations are indicated on the left. Mutation frequencies are indicated on the right. MLL-PTD, partial tandem duplications in the MLL gene.
FLT3 N676K mutations identified in CBFB/MYH11-rearranged AML. (A) Exome data sets of a CBFB/MYH11-positive AML sample (upper panels) and the corresponding follow-up sample from the same patient (lower panels) are displayed using the integrative genomics viewer.24 Horizontal gray bars symbolize the 76-bp reads aligned to the reference sequence. The frequency of 24% of the mutant nucleotide T in the diagnostic leukemia sample indicates a heterozygous point mutation causing an amino acid substitution (NM_004119.2:c.2028C>A; p.N676K), whereas in the follow-up sample, only the wild-type nucleotide G is detected at this position. Read depth and base count are indicated for the affected positions, respectively. (B) Sanger sequencing confirmed the FLT3 N676K mutation found initially by exome sequencing. Chromatograms are shown for both the diagnostic AML sample and the corresponding follow-up sample at complete remission (CR) from the same patient. (C) The structure of the human FLT3 protein includes the transmembrane domain (TM), the juxtamembrane domain (JM), and TKD1 and TKD2. Amino acid positions targeted by known recurrent mutations in AML are indicated in green above the corresponding domains. N676 is indicated in blue below the TKD1 domain. (D) Frequency distribution of additional genetic aberrations in 84 CBFB/MYH11-rearranged patients. Each column indicates one patient. Dark gray boxes indicate patients who are positive for the respective mutation; light gray boxes indicate wild-type status. Missing information is shown as a white space (N/A, not available). Gene names and types of mutations are indicated on the left. Mutation frequencies are indicated on the right. MLL-PTD, partial tandem duplications in the MLL gene.
Recurring FLT3 N676K mutations in CBF AML
We sequenced FLT3 exon 16 (containing the codon of N676) in a cohort of 84 AML patients with CBFB/MYH11 rearrangement (71 patients with inv(16) and 13 patients with t(16;16)). Strikingly, we detected heterozygous missense mutations (N/K) at position 676 of FLT3 in 5 patients (6%) with inv(16) or t(16;16) (4 [6%] of 71 and 1 [8%] of 13, respectively). Thus, in AML with a CBFB/MYH11 fusion, FLT3 N676K mutations have a frequency similar to FLT3 D835 mutations (Figure 1D).
In 36 AML samples with a t(8;21)(q22;q22) and an RUNX1/RUNX1T1 fusion, 1 patient with an FLT3 N676K mutation could be identified (1 [3%] of 36). None of the CBF AML patients with an FLT3 N676K mutation had an additional FLT3-ITD or a D835 mutation. In contrast, in 90 AML patients with normal karyotype, we detected only a single patient with an FLT3 N676K mutation, and this patient had a concurrent FLT3-ITD similar to that of the patient described by Heidel et al.14 The incidence of FLT3 N676K without concurrent ITD in CBF AML (6/120) was compared with the incidence in CN-AML (0/90) by using a two-tailed Fisher’s exact test (P = .039). These results suggest a specific association between FLT3 N676K mutations and CBF leukemias.
To determine whether the FLT3 N676K mutations are somatically acquired, we sequenced remission samples where available. Paired diagnostic and remission material was available only from the N676K-positive patient with t(8;21) and from 1 patient with inv(16). In both patients, the N676K mutation could be detected at diagnosis but not in the remission sample (supplemental Figure 1). Deep amplicon sequencing of N676K-positive cases confirmed variable allele frequencies ranging from 11% to 44% indicating clonal heterogeneity (supplemental Table 5).
Additional mutations in CBFB/MYH11-rearranged AML
We analyzed mutational hotspots (see Materials and methods) of several commonly mutated genes (FLT3, KIT, KRAS, NRAS, NPM1, MLL, and WT1) in our 84 CBFB/MYH11-positive cohort. The mutation frequency of these genes in our cohort (Figure 1D) was similar to that in previous reports.7,8,25-27 We found KIT mutations in 18% of CBFB/MYH11-positive AMLs (14% exon 8 frameshift mutations, known to be frequent in inv(16) AML,7,9 and 4% D816 missense mutations). RAS missense mutations were present in 51% of the patients (14% KRAS, 37% NRAS). As expected, the FLT3-ITD mutation was rare in our cohort (2%), whereas the FLT3-TKD (D835) mutation had a frequency of 10%. Together with the 6% FLT3 N676K-mutated patients, a total of 18% (15/84) of the CBFB/MYH11-positive patients had an FLT3 mutation.
In addition to the common signaling pathway mutations, we found 7% of samples with WT1 mutations causing a frameshift in exon 7. MLL PTDs (1%) and NPM1 mutations (0%) were rare in our CBFB/MYH11-positive patients. In 18% of the patients, no mutation was detected in the mutational hotspots analyzed. In 12 patients (14%), more than one mutation was present. Seventy-nine percent of the patients (66/84) carried a mutation in FLT3, KRAS, NRAS, or KIT.
FLT3 N676K is strongly expressed on the cell surface of Ba/F3 cells
To analyze the transforming potential of the FLT3 N676K mutant, Ba/F3 cell lines stably expressing various FLT3 constructs were established. The expression of WT and mutant (mut) FLT3 receptors was confirmed by immunoblotting or flow cytometry (supplemental Figure 2). Like FLT3-WT, the FLT3 N676K receptor was highly expressed on the cell surface (mature receptor, 160 kDa) compared with FLT3-ITD and FLT3 D835Y. The FLT3-N676K-ITD double mutant showed the weakest cell surface expression. A weak cell surface expression was correlated with an enhanced expression of the immature receptor with a molecular weight of 130 kDa.28
The FLT3 N676K mutant receptor leads to cytokine-independent growth and resistance to apoptosis
Proliferation assays of Ba/F3 cells expressing FLT3 mutant receptors revealed a cytokine-independent growth. As described before, FLT3-ITD was able to fully transform Ba/F3 cells reaching 100% of IL-3–mediated growth. FLT3 D835Y-expressing cells reached 41%. The mutant FLT3 N676K receptor led to IL-3 and FLT3-ligand (FL) independent cell growth, and the Ba/F3 cells reached about 25% of the IL-3 reference proliferation rate at 72 hours of culture time (Figure 2A). This pro-proliferative phenotype increased over time and, eventually, FLT3 N676K-expressing cells reached a proliferation rate similar to that of FLT3 D835Y-expressing cells (Figure 2B). In addition to an enhanced proliferation, Ba/F3 cells expressing the various FLT3 mutants showed a strong resistance to apoptosis after cytokine deprivation (Figure 2C). This antiapoptotic phenotype was strongest in FLT3-ITD–expressing cells (only 4.5% apoptotic cells) followed by FLT3 D835Y (7%) and FLT3 N676K (10%)-expressing cells.
Transforming potential of FLT3 mutants in Ba/F3 cells. All experiments were performed in triplicates. Error bars represent standard deviation of the mean. (A) Ba/F3 cells expressing indicated FLT3 constructs were seeded at a density of 4 × 104 cells per mL in the presence or absence of 10 ng/mL IL-3 and 100 ng/mL FL. Viable cells were counted by trypan blue exclusion after 72 hours. (B) Ba/F3 cells transduced with the indicated FLT3 constructs were seeded at a density of 2 × 105 cells per mL in 0.1% WEHI-conditioned medium and cultured for 10 days. After 72 hours, cells were cleared from previous medium and resuspended in 0% WEHI-conditioned medium. Control cells were cultured in 10 ng/mL IL-3–supplemented medium. (C) Cells were cultured in the presence or absence of 10 ng/mL IL-3 for 72 hours and stained with Annexin V and 7-aminoactinomycin D. The percentage of apoptotic cells was determined by fluorescence-activated cell sorter analysis.
Transforming potential of FLT3 mutants in Ba/F3 cells. All experiments were performed in triplicates. Error bars represent standard deviation of the mean. (A) Ba/F3 cells expressing indicated FLT3 constructs were seeded at a density of 4 × 104 cells per mL in the presence or absence of 10 ng/mL IL-3 and 100 ng/mL FL. Viable cells were counted by trypan blue exclusion after 72 hours. (B) Ba/F3 cells transduced with the indicated FLT3 constructs were seeded at a density of 2 × 105 cells per mL in 0.1% WEHI-conditioned medium and cultured for 10 days. After 72 hours, cells were cleared from previous medium and resuspended in 0% WEHI-conditioned medium. Control cells were cultured in 10 ng/mL IL-3–supplemented medium. (C) Cells were cultured in the presence or absence of 10 ng/mL IL-3 for 72 hours and stained with Annexin V and 7-aminoactinomycin D. The percentage of apoptotic cells was determined by fluorescence-activated cell sorter analysis.
Constitutive activation of FLT3 signaling in FLT3 N676K-expressing cells
To determine critical pathways for the transforming potential of the FLT3 mutants, we analyzed the activation of 3 key signaling molecules downstream of FLT3: the mitogen-activated protein kinase (MAPK), protein kinase B (AKT), and signal transducer and activator of transcription 5 (STAT5). Protein lysates of unstimulated and FL-stimulated Ba/F3 cells expressing FLT3 and the mutants were immunoblotted (Figure 3A). MAPK was strongly activated in all FL-stimulated FLT3 or FLT3 mutant-expressing cells. In contrast to FLT3-ITD–expressing cells, STAT5 was not phosphorylated in FLT3-WT or in the FLT3 N676K or the FLT3 D835Y mutant-expressing cells. MAPK was constitutively phosphorylated in unstimulated cells of the two TKD mutants N676K and D835Y compared with WT and ITD cells. To determine the activation of the FLT3 N676K mutant receptor, the protein was immunoprecipitated and analyzed for tyrosine phosphorylation by immunoblotting. The FLT3 N676K receptor showed a fivefold stronger constitutive phosphorylation as well as a twofold stronger phosphorylation after ligand stimulation compared with FLT3-WT, taking into account the total protein loaded on the gel (Figure 3B). In conclusion, FLT3 N676K mutant-expressing cells showed an enhanced signaling through the MAPK pathway but no aberrant activation of STAT5. Thus, the increased MAPK activation is most likely responsible for the mutant phenotypes.
Constitutive activation of FLT3 signaling by the FLT3 N676K mutant. Ba/F3 cells expressing indicated constructs were starved for 24 hours in media containing 0.3% fetal calf serum. Cells were left untreated or were stimulated with 100 ng/mL FL for 10 minutes. Crude cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by western blot for phosphorylation of signaling molecules. (A) STAT5, AKT, and MAPK activation was analyzed by using phospho-specific antibodies, and then stripped and reprobed with antibodies against total STAT5, AKT, and MAPK. Ba/F3 native cells were stimulated with 100 ng/mL IL-3 for 5 minutes; control and an antibody against GAPDH were used as loading control. (B) FLT3 receptor was immunoprecipitated with polyclonal FLT3 antibody, analyzed for tyrosine phosphorylation status by immunoblotting with a phospho-tyrosin antibody, stripped, and reprobed with FLT3 antibody.
Constitutive activation of FLT3 signaling by the FLT3 N676K mutant. Ba/F3 cells expressing indicated constructs were starved for 24 hours in media containing 0.3% fetal calf serum. Cells were left untreated or were stimulated with 100 ng/mL FL for 10 minutes. Crude cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by western blot for phosphorylation of signaling molecules. (A) STAT5, AKT, and MAPK activation was analyzed by using phospho-specific antibodies, and then stripped and reprobed with antibodies against total STAT5, AKT, and MAPK. Ba/F3 native cells were stimulated with 100 ng/mL IL-3 for 5 minutes; control and an antibody against GAPDH were used as loading control. (B) FLT3 receptor was immunoprecipitated with polyclonal FLT3 antibody, analyzed for tyrosine phosphorylation status by immunoblotting with a phospho-tyrosin antibody, stripped, and reprobed with FLT3 antibody.
FLT3 N676K-induced proliferation can be abrogated by selective PTK inhibition
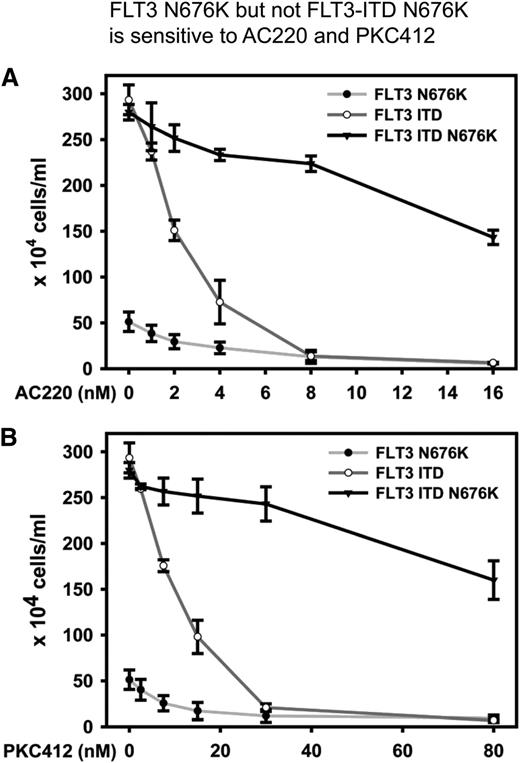
The FLT3 N676K mutation was previously described only in combination with an FLT3-ITD to mediate resistance to PTKIs.14 In previous studies it was not tested whether FLT3 N676K alone might be sufficient to confer protein tyrosine kinase inhibitor resistance. To address this question, we used PKC412 and AC220 as selective FLT3 inhibitors in increasing nontoxic concentrations (Figure 4). Nontoxicity of the inhibitors was confirmed in FLT3-WT–expressing Ba/F3 cells (supplemental Figure 5). Both compounds potently inhibited FLT3-ITD–expressing cells with a half maximal inhibitory concentration (IC50) of 13 nM for PKC412 and 2.5 nM for AC220, respectively. FLT3 N676K-expressing cells were also sensitive to FLT3 inhibitors with an IC50 of 7.5 nM for PKC412 and 3 nM for AC220. FLT3-ITD-N676K double mutants showed a strong resistance to both inhibitors (IC50 greater than 80 nM for PKC412 and greater than 16 nM for AC220). Taken together, the FLT3 N676K mutation with an ITD is very resistant to FLT3 inhibitors. However, cell proliferation driven by FLT3 N676K alone can be inhibited rather effectively.
FLT3 N676K but not FLT3-ITD N676K is sensitive to AC220 and PKC412. Ba/F3 cells expressing indicated FLT3 variants were seeded at a density of 4 × 104 cells per mL and counted by trypan blue exclusion after 72 hours. All experiments were performed in triplicate. Error bars represent standard deviation of the mean. (A) Cells were treated with increasing nontoxic concentrations of selective TKI AC220. (B) Cells were treated with increasing nontoxic concentrations of TKI PKC412.
FLT3 N676K but not FLT3-ITD N676K is sensitive to AC220 and PKC412. Ba/F3 cells expressing indicated FLT3 variants were seeded at a density of 4 × 104 cells per mL and counted by trypan blue exclusion after 72 hours. All experiments were performed in triplicate. Error bars represent standard deviation of the mean. (A) Cells were treated with increasing nontoxic concentrations of selective TKI AC220. (B) Cells were treated with increasing nontoxic concentrations of TKI PKC412.
Differential gene expression in FLT3 N676K-mutated CBFB/MYH11-rearranged AML
To assess the impact of FLT3 N676K mutations on gene expression, we analyzed the gene expression profiles of 33 patients with CBFB/MYH11 rearranged AML. Four patients with FLT3 N676K mutations were compared with 29 patients with FLT3 D835 (n = 4), NRAS (n = 15), KRAS (n = 3), KIT (n = 3), WT1 (n = 1), and FLT3-ITD (n = 2) mutations or no mutations in any of these genes (n = 5). Some patients had no (n = 5) or more than 1 (n = 3) mutation. Finally, all unique probe sets with P < .005 and log fold-change >1.5 were selected for unsupervised clustering (n = 18). Interestingly, all cases with FLT3 N676K clustered together (supplemental Figure 3). Of these 18 genes, six were highly correlated with sex, since all cases with N676 mutation in our analysis were discovered in male patients. Interestingly, genes with high association and elevated levels in the N676K cluster were CCNA1 (cell cycle), PRG3 (immune response), and HLA-DQA1 (immune response). Genes with negative correlation to the N676K cluster were MEST (imprinting) and ARG1 (metabolism). To evaluate which pathways were associated with FLT3 N676K mutations, we compared the 4 patients with this mutation to 29 patients without this mutation. Nine gene sets were significantly enriched at a false discovery rate of <25% and P < .05 including metabolic, inflammation and degradation pathways (supplemental Table 4).
Structural mapping of the FLT3 N676K receptor mutation
Since we could demonstrate that a single point mutation in the ATP-binding domain is sufficient to constitutively activate the receptor and increase downstream signaling, we performed structural modeling of the FLT3 N676K mutant to gain further insights into the consequences of the mutation (Figure 5).
Structural mapping of N676K. Structure of the autoinhibited FLT3 kinase (Protein Data Bank accession number 1RJB) is shown as a ribbon model with highlighted secondary structure and color-coded domains. N676 forms hydrogen bonds to the backbone of H671, stabilizing a loop at the back of the substrate and inhibitor-binding pocket (asterisk). N676K will remove these hydrogen bonds, likely destabilizing the loop and the nearby substrate-binding pocket. This structural effect can explain resistance against TKIs, which target the nearby pocket. However, the mutation could also lift the autoinhibition of FLT3, providing a possible explanation for the observation that this mutation alone shows transforming potential.
Structural mapping of N676K. Structure of the autoinhibited FLT3 kinase (Protein Data Bank accession number 1RJB) is shown as a ribbon model with highlighted secondary structure and color-coded domains. N676 forms hydrogen bonds to the backbone of H671, stabilizing a loop at the back of the substrate and inhibitor-binding pocket (asterisk). N676K will remove these hydrogen bonds, likely destabilizing the loop and the nearby substrate-binding pocket. This structural effect can explain resistance against TKIs, which target the nearby pocket. However, the mutation could also lift the autoinhibition of FLT3, providing a possible explanation for the observation that this mutation alone shows transforming potential.
Mapping of the FLT3 N676K onto the crystal structure of FLT3 showed that this mutation destabilizes the fold of the kinase domain between the juxtamembrane domain (JMD) and a hydrophobic pocket that is the target of FLT3 inhibitors. The crystal structure of the inactive conformation of FLT3 showed that the JMD serves as a key autoinhibitory element regulating the kinase activity.29,30 N676K mutations might therefore interfere with the FLT3 autoinhibition by reducing the stability of the JMD, thus, suggesting a structural basis for the transforming activity observed in our experiments with Ba/F3 cells.
Clinical characteristics associated with FLT3 N676K mutations
Fifty-six of the 84 CBFB/MYH11-rearranged AML patients screened for mutations in this study were enrolled in the multicenter AMLCG-1999 trial of the German AML Cooperative Group (NCT00266136). Among these patients, five carried FLT3 N676K mutations. In this cohort, which was homogeneous with regard to both treatment and cytogenetics, the mutation was significantly associated with higher leukocyte counts (P = .02), elevated lactate dehydrogenase (P = .02), and male sex (P = .02) (Table 1). There was no significant difference in survival of patients with FLT3 N676K (n = 4) compared with patients with FLT3 N676 wt (n = 47) (supplemental Figure 4A). However, there was a trend toward reduced complete remission rates associated with FLT3 N676K mutations (Table 1).
Characteristics of CBFB/MYH11-rearranged patients
| Variable . | FLT3 N676 wt . | FLT3 N676K mut . | P . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| No. of patients | 51 | 5 | |||
| Age, years | N/S | ||||
| Median | 42 | 54 | |||
| Range | 20-75 | 18-61 | |||
| Male sex | 22 | 43.1 | 5 | 100 | .02 |
| White blood cells × 109/L | .02 | ||||
| Median | 38.5 | 134 | |||
| Range | 1.3-316 | 54.1-259 | |||
| Hemoglobin, g/dL | N/S | ||||
| Median | 8.6 | 9.1 | |||
| Range | 4.4-14.6 | 8-14.1 | |||
| Platelets × 109/L | N/S | ||||
| Median | 32 | 44 | |||
| Range | 0.01-370 | 32-47 | |||
| LDH (U/L) | .02 | ||||
| Median | 666 | 1326 | |||
| Range | 143-1870 | 717-2508 | |||
| Bone marrow blasts (%) | N/S | ||||
| Median | 80 | 60 | |||
| Range | 25-95 | 10-90 | |||
| ECOG performance status ≥ 2 (%) | 14 | 29.2 | 1 | 20 | N/S |
| de novo AML | 46 | 90.2 | 3 | 75 | N/S |
| NPM1 mut | 0 | 0 | 0 | 0 | N/S |
| FLT3-ITD | 2 | 3.9 | 0 | 0 | N/S |
| FLT3-D835 | 5 | 9.8 | 0 | 0 | N/S |
| MLL-PTD | 1 | 2 | 0 | 0 | N/S |
| KRAS mut | 7 | 13.7 | 0 | 0 | N/S |
| NRAS mut | 21 | 41.2 | 0 | 0 | .15 |
| KIT mut | 10 | 19.6 | 0 | 0 | N/S |
| WT1 mut | 4 | 7.8 | 0 | 0 | N/S |
| Complete remission | 39 | 76 | 2 | 40 | .11 |
| Deceased | 19 | 37.2 | 2 | 40 | N/S |
| Variable . | FLT3 N676 wt . | FLT3 N676K mut . | P . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| No. of patients | 51 | 5 | |||
| Age, years | N/S | ||||
| Median | 42 | 54 | |||
| Range | 20-75 | 18-61 | |||
| Male sex | 22 | 43.1 | 5 | 100 | .02 |
| White blood cells × 109/L | .02 | ||||
| Median | 38.5 | 134 | |||
| Range | 1.3-316 | 54.1-259 | |||
| Hemoglobin, g/dL | N/S | ||||
| Median | 8.6 | 9.1 | |||
| Range | 4.4-14.6 | 8-14.1 | |||
| Platelets × 109/L | N/S | ||||
| Median | 32 | 44 | |||
| Range | 0.01-370 | 32-47 | |||
| LDH (U/L) | .02 | ||||
| Median | 666 | 1326 | |||
| Range | 143-1870 | 717-2508 | |||
| Bone marrow blasts (%) | N/S | ||||
| Median | 80 | 60 | |||
| Range | 25-95 | 10-90 | |||
| ECOG performance status ≥ 2 (%) | 14 | 29.2 | 1 | 20 | N/S |
| de novo AML | 46 | 90.2 | 3 | 75 | N/S |
| NPM1 mut | 0 | 0 | 0 | 0 | N/S |
| FLT3-ITD | 2 | 3.9 | 0 | 0 | N/S |
| FLT3-D835 | 5 | 9.8 | 0 | 0 | N/S |
| MLL-PTD | 1 | 2 | 0 | 0 | N/S |
| KRAS mut | 7 | 13.7 | 0 | 0 | N/S |
| NRAS mut | 21 | 41.2 | 0 | 0 | .15 |
| KIT mut | 10 | 19.6 | 0 | 0 | N/S |
| WT1 mut | 4 | 7.8 | 0 | 0 | N/S |
| Complete remission | 39 | 76 | 2 | 40 | .11 |
| Deceased | 19 | 37.2 | 2 | 40 | N/S |
All patients were enrolled in the AMLCG-99 trial and received intensive induction treatment. Categorical clinical variables of the FLT3 N676K-mutated (mut) and FLT3 N676 wild-type (wt) cohorts were compared by Fisher’s exact test. The continuous variables were compared by Mann-Whitney U test. P < .05 was considered significant. ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
We also compared CBFB/MYH11-rearranged AML patients with FLT3 point mutations affecting residues N676 or D835 (n = 9) to all other CBFB/MYH11-rearranged patients (n = 42) and did not observe a significant difference in survival (supplemental Figure 4B).
Discussion
Ours is the first report of recurring FLT3 N676K mutations in CBF leukemia. Despite the overall rather favorable prognosis associated with CBF rearrangements, almost one third of patients relapses within the first year after intensive chemotherapy and only 60% of CBF AML patients are still alive after 5 years. This heterogeneous clinical outcome of CBF AML patients may reflect the heterogeneity of additional genetic lesions in this subgroup and underscores the need for further investigation. Understanding the pathogenesis of AML is challenging because of the multitude of genetic events. By sequencing known mutational targets, we and others have demonstrated that between 80% and 90% of CBFB/MYH11-rearranged patients have mutations that activate either RAS signaling or RTK signaling (FLT3 and KIT), while other common AML-related gene mutations (eg, in NPM1, WT1, and MLL) are rarely found.7-11 The discovery of FLT3 N676K mutations adds another piece to the puzzle of CBF-related leukemogenesis, suggesting that the proportion of CBF leukemia with activating RTK mutations has been underestimated. Our observation of concurrent activating mutations in different genes (eg, KRAS and NRAS or NRAS and KIT; Figure 1D) suggests either clonal heterogeneity or multiple additive hits in synergistic pathways within CBFB/MYH11-rearranged AML.
The specific occurrence of recurring FLT3 N676K mutations in the CBFB/MYH11-rearranged AML subgroup, which accounts for only 6% of AML, might explain why FLT3 N676K mutations have remained undetected in previous full-length FLT3 mutation screens of unselected AML patients.31 Other large studies limited FLT3 mutational screening to FLT3-ITD mutations (exon 14/15) and TKD2 mutations (exon 20; eg, D835) and thus would have missed FLT3 N676K mutations (exon 16).7,26
Even though FLT3 N676K in combination with FLT3-ITD had previously been shown to lead to PTKI resistance,12,14 we show in this report that the FLT3 N676K mutant on its own exhibits gain-of-function properties. Notably, FLT3 N676K alone has direct transforming potential in Ba/F3 cells through increased downstream signaling similar to that of the FLT3 D835 mutant but weaker than FLT3-ITD (Figures 3 and 4). The power of the gene expression analysis of the FLT3 N676K-mutated patients is limited by the small sample size. However, FLT3 N676K-mutated cases clustered together after unsupervised clustering analysis of gene expression in CBFB/MYH11-rearranged AML with different mutations affecting FLT3, NRAS, KRAS, KIT, and WT1 (supplemental Figure 3). These findings suggest a distinct biologic subgroup within CBFB/MYH11-rearranged AML characterized by FLT3 N676K mutations.
On the basis of the crystal structure of FLT3, Cools et al12 proposed that mutation of N676 destabilizes the conformation of the hinge segment, which makes H-bonds with the lactam ring of the PTKI PKC412. We suggest that a mutation at position N676 may also activate FLT3 by disturbing its autoinhibition capacity (Figure 5). Taken together, mutations at position N676 most probably have two consequences: activating FLT3 and, together with a concurrent FLT3-ITD, conferring PTKI resistance. The additional N676K mutation on the ITD background might change the conformation of the FLT3-ITD protein in a way that the binding site of the PTKIs is masked, since the ITD results in an extension of the JMD, which leads to a conformational change of the kinase domain. The fact that the FLT3 N676K alone (without concurrent ITD) does not confer PTKI resistance, might also be related to its localization on the cell surface, in contrast to the mostly intracellular localization of the ITD-N676K double mutant. Hence, N676K-mutated FLT3 might be exposed to higher inhibitor concentrations at the cell surface, possibly allowing efficient inhibition of the TKDs directly beneath the cell membrane. It was shown by others that there is higher intracellular accumulation of PTKIs in the more sensitive AML cells lines than in the less sensitive ones.32
In contrast to the initial report of an FLT3 N676K mutation as a late arising, disease-modifying event, detected at the time of clinical relapse while on PKC412 monotherapy,14 all of our N676K mutations were detectable at initial diagnosis. Since cell proliferation driven by FLT3 N676K alone could be greatly reduced by FLT3 inhibitors, N676K-positive patients without concurrent ITD may actually benefit from treatment with FLT3 inhibitors.
Our clinical data did not show a significant impact of FLT3 N676K mutations on survival within CBFB/MYH11-rearranged AML patients, but there was a trend toward reduced complete remission rates (Table 1). The significant association of FLT3 N676K mutations with higher leukocyte counts, elevated lactate dehydrogenase levels, and male sex (Table 1), suggests a distinct biology of these leukemias. Given the small number of FLT3 N676K–positive patients (n = 4) in our patient cohort, the prognostic significance of the FLT3 N676K mutation needs to be investigated in larger patient cohorts.
The varying allele frequencies of the FLT3 N676K mutation ranging from 14% to 44% in those cases in which the presence of the CBFB/MYH11 rearrangement was detected by fluorescence in situ hybridization in the majority of the bone marrow cells (supplemental Table 5) indicate that the FLT3 N676K mutation did not always represent the dominant leukemic clone at diagnosis. It would be interesting to study the clonal evolution in those cases by assessing the FLT3 N676 status at relapse; unfortunately, no relapse samples from our patients were available.
Although FLT3 has been known for more than a decade to be mutated in about one third of AML patients, it appears that the spectrum of FLT3 mutations is still not fully understood. In particular, defined genetic subgroups of AML might harbor specific FLT3 mutations. Unbiased mutation screening using exome sequencing allows the detection of novel sequence variations even in extensively studied genes such as FLT3.
Presented as an oral presentation at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the participating centers of the German Acute Myeloid Leukemia Cooperative Group 1999 (AMLCG-1999) trial.
This work was supported by grant 109031 from the German Cancer Aid (P.A.G and S.K.B.); by National Genome Research Network Plus grant PKL-01-GS0876-6 from the Federal Ministry of Education and Research (BMBF) (S.K.B.); and by the Collaborative Research Center 684 Molecular Mechanisms of Normal and Malignant Hematopoiesis, projects A3, A6, A12 and knock-on funding 2011 from the German Research Foundation (DFG) (K.-P.H., S.K.B., K.S., and P.A.G.).
Authorship
Contribution: S.O., H.P., K.S., S.K.B., and. P.A.G. conceived and designed the experiments; S.O., H.P., N.P.K., B.K., E.Z., and S.K. performed experiments; S.O., H.P., and T.H. analyzed data; K.-P.H. performed structural modeling; S.V. and A.G. provided bioinformatics support; H.B. managed the Genome Analyzer IIx platform; S.O., B.K., A.D., E.Z., P.M.K., S.S., J.B., S.K.B., and K.S. characterized patient samples; M.C.S., J.B., W.E.B., T.B., B.J.W., and W.H. coordinated the German Acute Myeloid Leukemia Cooperative Group clinical trial; P.A.G., K.S., and S.K.B. supervised the project; and S.O., H.P., T.H., S.K.B., and P.A.G. wrote the manuscript.
Conflict-of-interest disclosure: P.A.G. and S.K. received honoraria from Illumina. The remaining authors declare no competing financial interests.
Correspondence: Philipp A. Greif, Marchioninistrasse 25, 81377 München, Germany; e-mail: pgreif@med.uni-muenchen.de.
References
Author notes
S.O. and H.P. contributed equally to this work




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal