Key Points
The impaired function of MSCs in proliferation ability and in inducing tolerogenic DCs may play a role in the pathogenesis of ITP.
The effect of THD in correcting dysfunctions of MSCs may suggests a therapeutic potential of THD in ITP patients.
Abstract
Thalidomide (THD) is an immunomodulatory agent used to treat immune-mediated diseases. Immune thrombocytopenia (ITP) is an autoimmune disorder in which impaired mesenchymal stem cells (MSCs) are potentially involved. We demonstrated that MSCs in ITP patients had reduced proliferative capacity and lost their immunosuppressive function, which could be corrected with THD treatment. According to the gene profile, the downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1, may be associated with THD modulation. Dendritic cells (DCs) played an important role in mediating the inhibitory activity of MSCs. To study the functional alteration of DCs elicited by MSCs, we sorted DCs after incubation with MSCs and performed T-lymphocyte reaction assays. The THD-modulated MSCs from ITP patients induced mature DCs to become tolerogenic DCs, whereas unmodulated MSCs had no effect. The induction of tolerogenicity in DCs by MSCs was dependent on the expression of TIEG1 in DCs. The study reveals the inability of MSCs from ITP patients to induce tolerogenic ability in DCs. THD could restore the regulatory effect of MSCs on DCs. These findings will help us understand the pathogenesis of ITP, and with appropriate safeguards, THD may benefit patients with ITP.
Introduction
Thalidomide (THD) was first introduced as a nonbarbiturate sedative-hypnotic drug in West Germany in the 1950s, but was withdrawn worldwide in 1961 due to its potential for teratogenesis. Presently, THD is used as an immunomodulatory agent1 to treat immune-mediated diseases.2-4 In particular, Falco et al successfully managed immune thrombocytopenia (ITP) with THD in a patient with multiple myeloma.5 ITP is an autoimmune hemorrhagic disorder in which both T and B cells are involved.6-9 Recently, Perez-Simon et al found impaired proliferative and functional capacity of mesenchymal stem cells (MSCs) in patients with ITP.10 Although a low dose of dexamethasone favors MSC expansion in vitro, it could impair immunosuppression of MSCs on peripheral blood mononuclear cells (PBMCs).11 It is not suitable for MSCs to be pretreated with dexamethasone when they are to be used to treat immunologic disease.11 Whether THD could correct the defects in MSCs remains unknown.
MSCs are stem cells with differentiation potential,12 and play a distinguished role in immune tolerance.13 MSCs can inhibit the activation and proliferation of T and B lymphocytes,14,15 and have inhibitory effects on dendritic cells (DCs).16-18 Particularly, Zhang et al reported that mouse MSCs induce DCs into a novel regulatory population.19 Previous studies suggested that the immunosuppression of MSCs was attributed to the disconnection between T cells and antigen-presenting cells (APCs)19 or that they act as veto cells themselves.20,21 Additional research reported that in the presence of MSCs, APCs could be transformed into regulatory APCs and inhibit T-cell activation.22 Several studies found that interleukin-10 (IL-10) and transforming growth factor–β1 (TGF-β1) were involved in the differentiation of the regulatory DC phenotype.23 Whether MSCs from ITP patients could induce APCs into regulatory APCs has not yet been determined.
Here we demonstrate that MSCs from ITP patients had a reduced proliferative capacity and had lost the ability to induce mature DCs (mDCs) into tolerogenic DCs, which could be corrected with THD treatment. The downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1 may explain the modulatory effects of THD on MSCs. The THD-modulated MSCs (THD-MSCs) could regulate mDCs by downregulating the expression of costimulating factors and IL-12 expression and by upregulating IL-10, TGF-β1, and indoleamine 2,3-dioxygenase (IDO) expression. MSCs induced mDCs into TIEG1-dependent tolerogenic DCs. The results provide a general evaluation of THD and THD-MSCs, and a potential therapeutic option for ITP patients.
Materials and methods
Patients and controls
Thirty-seven newly diagnosed primary ITP patients (24 females and 13 males; age range 16-77 years, median 45 years; platelet count range 0-23 × 109/L, median 6 × 109/L) were enrolled in this study (supplemental Table 1, available on the Blood website). The diagnosis of ITP patients was based on previously reported criteria.24 All patients were free from any ITP-specific therapy, including glucocorticosteroids or intravenous immunoglobulin G, within at least 3 months. Twelve samples of venous blood and 9 samples of bone marrow (BM) from healthy donors were used as controls (11 females and 10 males; age range 19-47 years, median 30 years; platelet count range 156-264 × 109/L, median 189 × 109/L). This study was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University, and was conducted in accordance with the Declaration of Helsinki.
Thalidomide
THD (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to form a stock solution of 20 mg/mL, which was kept for up to 1 week.
Cells
MSCs were isolated and cultured from BM of ITP patients (MSC [ITP]) and healthy controls (MSC [control]) as previously described,10 with minor modifications. The MSCs were maintained in MesenPRO RS medium (Invitrogen). Once the third passage was reached, the MSCs were frozen routinely in 10% DMSO and 90% fetal calf serum (FCS; Hyclone). The phenotypes of MSCs (CD34, CD45, CD73, CD90, and CD105) before and after modulation with THD (0.5 μg/mL) were determined as described previously,25,26 with minor modifications (supplemental Figure 1).
PBMCs were isolated from the venous blood by Ficoll density gradient separation. Either CD4+ T or CD14+ cells were isolated by positive selection with anti-CD4 or anti-CD14 micromagnetic beads (Miltenyi Biotec) from PBMCs. The purities of CD4+ and CD14+ were >98% by flow cytometry (data not shown). For carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich) labeled CD4+ T-cell generation, CD4+ T cells were labeled with CFSE at a concentration of 5 μM for 10 minutes at 37°C. The labeling reaction was quenched by adding cold FCS and incubating for 5 minutes on ice. The cells were washed 3 times with RPMI 1640 culture medium with 10% FCS prior to being added to cocultures. For mDC generation, CD14+ cells were plated in 6-well culture plates (Corning) in RPMI 1640 culture medium (Invitrogen) with 10% FCS, 1000 U/mL granulocyte-macrophage colony stimulating factor (R&D Systems) and 1000 U/mL IL-4 (R&D Systems) for 5 days. Cultures were incubated at 37°C in a 5% CO2 atmosphere. To activate DCs, lipopolysaccharides (1 μg/mL; Sigma-Aldrich) were added to the plates prior to culture for an additional 2 days. By day 7, >90% of the cells were CD14−CD11b+CD80+HLA-DR+ mDCs (supplemental Figure 2). For THD-MSC preparation, MSCs from ITP patients were preincubated for 3 days in RPMI 1640 culture medium containing 10% FCS plus 0.5 μg/mL THD in humidified air in 5% CO2 at 37°C. For tolerogenic DCs, mDCs were cocultured with either MSCs (control) or THD-MSCs (ITP), in a 1:1 (MSC:mDC) ratio, for 3 days in RPMI 1640 culture medium supplemented with 10% FCS. The cocultured cells were washed off with 0.1% trypsin. The tolerogenic DCs (MSC [control]–DCs and THD-MSC [ITP]–DCs) were purified using anti–HLA-DR micromagnetic beads (Miltenyi Biotec).
MSC proliferation assays
At passage 4, 1 × 103 MSCs per well were cultured in triplicate in a 96-well plate (Corning) and incubated in humidified air in 5% CO2 at 37°C. After adherence, these cells were treated with THD at concentrations of 0 (commensurable DMSO), 0.05, 0.1, 0.5, 1, 5, 10, and 50 μg/mL. The proliferation assay was assessed on day 3 using the Cell Counting Kit-8 (CCK8; Beyotime) according to the manufacturer’s recommendations.
Apoptosis and cell-cycle analysis
Briefly, 5 × 105 of MSCs (ITP, cultured in commensurable DMSO) or THD-MSCs (ITP, cultured in 0.5 μg/mL THD) were harvested and stained with Annexin V–fluorescein isothiocyanate/7-amino-actinomycin D (Annexin V/7-AAD; Beyotime) or with propidium iodide (Sigma-Aldrich) according to the manufacturer’s recommendations. Data acquisition was performed on a flow cytometer (FACSCalibur; Becton Dickinson; or Gallios; Beckman Coulter).
Microarray analysis
As described previously,27 microarray analysis was conducted on THD-MSCs (ITP, cultured in 0.5 μg/mL THD) and MSCs (ITP, cultured in commensurable DMSO) for 12 hours from 3 independent patients with ITP. The abundance of messenger RNA (mRNA) transcripts was measured using the 35k human Genome Array (CapitalBio Corp) representing 39 600 transcripts and ∼25 100 genes. The data were analyzed using the Molecule Annotation System (MAS 3.0, http://bioinfo.capitalbio.com/mas3/) (supplemental Methods).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) from MSCs (ITP/control) and THD-MSCs (ITP/control), cultured in commensurable DMSO or in 0.5 μg/mL THD for 12 hours. The method was described in detail in the supplemental Methods. The primer sets and genes included in quantitative polymerase chain reaction (qPCR) were listed in Table 1.
MSC inhibition assays
After labeling with CFSE, CD4+ T cells (1 × 105 per well) or phorbol myristate acetate (PMA) preactivated CD4+ T cells were cultured alone or cocultured with 1 × 104 per well MSCs (control), MSCs (ITP), or THD-MSCs (ITP), respectively, in the presence or absence of mDCs (1 × 104/well), in flat-bottom 96-well plates (Corning) containing 200 μL of RPMI 1640 culture medium with 10% FCS and 50 U/mL recombinant human IL-2 (rhIL-2; R&D Systems) in triplicate. The cell ratios were referred from previous studies.28 All cells were incubated in humidified air in 5% CO2 at 37°C for 3 days and then collected for fluorescence-activated cell sorter (FACS) analysis.
Tolerogenic DC inhibition assays
After coculture with MSCs (control), MSCs (ITP), or THD-MSCs (ITP), the MSC-DCs were sorted using anti–HLA-DR immunomagnetic beads (Miltenyi Biotec). CFSE-labeled allogeneic CD4+ T cells (1 × 105 per well) were cocultured with PMA, mDCs, MSC (control)–DCs, MSC (ITP)–DCs, THD-MSC (ITP)–DCs, MSC (control)–DCs + PMA, MSC (ITP)–DCs + PMA, THD-MSC (ITP)–DCs + PMA, MSC (control)–DCs + mDCs, MSC (ITP)–DCs + mDCs, or THD-MSC (ITP)–DCs + mDCs, respectively. All DCs (mDCs or MSC-DCs) were from the same person in each separate experiment. The cocultured cells were in a 10:1:1 ratio (T cell:mDC:MSC-DC). All cells were cultured in flat-bottom 96-well plates (Corning) containing 200 μL of RPMI 1640 culture medium with 10% FCS and 50 U/mL rhIL-2 (R&D Systems) in triplicate and incubated for 3 days in humidified air in 5% CO2 at 37°C. The cells were then collected for FACS analysis.
Cytokine production
CD4+ T cells (1 × 105 per well), mDCs (1 × 104 per well), and MSCs (1 × 104 per well) were cultured alone or in combination. The levels of IL-4, IFN-γ, IL-12, IDO, TGF-β, and IL-10 in cultural supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) (CUSABIO Biotech Co) after 3 days.
FACS analysis
Cell staining was performed using fluorescein isothiocyanate– or phycoerythrin-conjugated anti-CD80 and anti-CD86 (BD Pharmingen) monoclonal antibodies. The cells were incubated with monoclonal antibodies for 30 minutes in the dark at room temperature. The isotype IgG of same species origin was used as a control. Data acquisition was performed on a flow cytometer (FACSCalibur; Becton Dickinson; or Gallios; Beckman Coulter).
Lentivirus interference
TIEG1 small hairpin RNA (shRNA) (h) lentiviral particles and control shRNA lentiviral particles were purchased from Santa Cruz Biotechnology. Prior to viral infection, the mDCs (1 ×105 cells per well) were cultured in a 24-well plate in optimal medium (Invitrogen) with 10% FCS without antibiotics, supplemented with 5 μg/mL polybrene. The mDCs were then infected with prepared virus (20 μL per well), and incubated in humidified air in 5% CO2 at 37°C overnight. The infected mDCs were maintained in 1 mL of RPMI 1640 culture medium containing 10% FCS (without Polybrene) until required for the mixed lymphocyte culture assays. The interference efficiency was >80% as analyzed by real-time PCR and western blot (data not shown).
Statistical analysis
Data were expressed as median with interquartile range. The statistical significance of differences observed between unmodulated, THD-modulated, and control groups was determined using the Mann-Whitney U test. Differences between MSCs (ITP) and THD-MSCs (ITP) or MSCs (control) and THD-MSCs (control) were determined using the Wilcoxon matched-pairs test. The minimal level of significance was P < .05.
Results
THD has potent ability to increase MSC proliferation in ITP patients
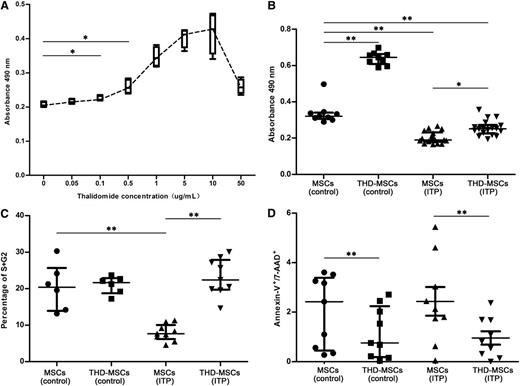
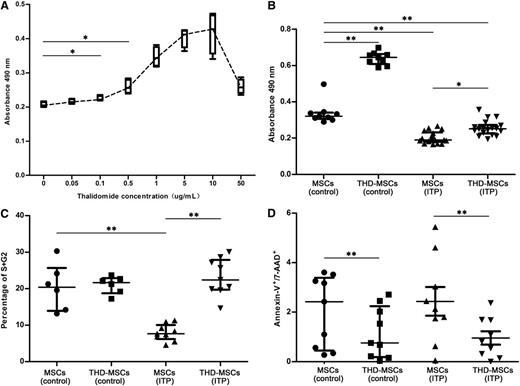
To investigate the effects of THD on proliferation of MSCs, we analyzed MSCs from ITP patients and healthy controls using CCK8 proliferative assays. As shown in Figure 1A, THD with concentration gradients from 0.1 μg/mL to 10 μg/mL could stimulate the proliferation of MSCs (ITP) in a dose-dependent manner. Yet for all patients, higher THD concentrations (>10 μg/mL) would lead to an opposite result. Based on our data, 0.1 μg/mL THD was the minimal effective concentration in ITP group. In clinics, an adult receiving an oral dose of 100 or 200 mg per day will attain a plasma concentration of 0.343 μg/mL (range, 0.05-1.45 μg/mL) or 0.875 μg/mL (range, 0.19-2.09 μg/mL), respectively.29 So, 0.5 μg/mL THD was selected in our research. In the remaining panels of Figure 1, the concentration of THD was 0.5 μg/mL. To further evaluate the mechanism we performed apoptosis and cell-cycle analysis. As shown in Figure 1B, the proliferation of MSCs from both ITP patients and healthy controls could be promoted after 0.5 μg/mL THD modulation. There were less cells entered into S + G2 phases in the MSC (ITP) group compared with those in MSCs (control). After THD modulation, the frequency of cells in S and G2 phases was increased in THD-MSCs (ITP) (Figure 1C). The apoptosis analysis of MSCs showed there was no significant difference of Annexin-V+/7-AAD+ cells between patients and healthy controls, and THD modulation could decrease the apoptosis rate both in patients and in controls (Figure 1D).
Effects of THD on proliferative responses of MSCs. The effects of THD on proliferative responses of MSCs were assayed using the Cell Counting Kit-8, cell-cycle, and apoptosis assays. The MSCs (control) represent MSCs from healthy controls. The THD-MSCs (control) represent the THD-modulated MSCs from controls. The MSCs (ITP) represent the MSCs from ITP patients without THD modulation. The THD-MSCs (ITP) represent the THD-modulated MSCs from ITP patients. (A) Administration of THD in concentration gradients between 0.1 μg/mL and 10 μg/mL stimulated the proliferation of MSCs (ITP) in a dose-dependent manner. Each box and whiskers denotes the optical density (OD) value in 1 independent experiment of 4 ITP patients. (B) THD corrected the lower proliferation of MSCs from ITP patients. Each dot denotes the OD value in 1 independent experiments. Statistical results represent median with interquartile range from 9 healthy controls and 18 ITP patients. (C) Cell cycle of MSCs (control), THD-MSCs (control), MSCs (ITP) and THD-MSCs (ITP). Each dot denotes the frequency of cells in S/G2 phase. Statistical results represent median with interquartile range from 6 healthy controls and 9 ITP patients. (D) Apoptosis of MSCs (control), THD-MSCs (control), MSCs (ITP), and THD-MSCs (ITP). Each dot denotes the frequency of Annexin V+/7-AAD+ cells. Statistical results represent median with interquartile range from 9 healthy controls and 9 ITP patients. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01.
Effects of THD on proliferative responses of MSCs. The effects of THD on proliferative responses of MSCs were assayed using the Cell Counting Kit-8, cell-cycle, and apoptosis assays. The MSCs (control) represent MSCs from healthy controls. The THD-MSCs (control) represent the THD-modulated MSCs from controls. The MSCs (ITP) represent the MSCs from ITP patients without THD modulation. The THD-MSCs (ITP) represent the THD-modulated MSCs from ITP patients. (A) Administration of THD in concentration gradients between 0.1 μg/mL and 10 μg/mL stimulated the proliferation of MSCs (ITP) in a dose-dependent manner. Each box and whiskers denotes the optical density (OD) value in 1 independent experiment of 4 ITP patients. (B) THD corrected the lower proliferation of MSCs from ITP patients. Each dot denotes the OD value in 1 independent experiments. Statistical results represent median with interquartile range from 9 healthy controls and 18 ITP patients. (C) Cell cycle of MSCs (control), THD-MSCs (control), MSCs (ITP) and THD-MSCs (ITP). Each dot denotes the frequency of cells in S/G2 phase. Statistical results represent median with interquartile range from 6 healthy controls and 9 ITP patients. (D) Apoptosis of MSCs (control), THD-MSCs (control), MSCs (ITP), and THD-MSCs (ITP). Each dot denotes the frequency of Annexin V+/7-AAD+ cells. Statistical results represent median with interquartile range from 9 healthy controls and 9 ITP patients. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01.
Analysis of global gene expression in MSCs before and after modulation with THD in vitro
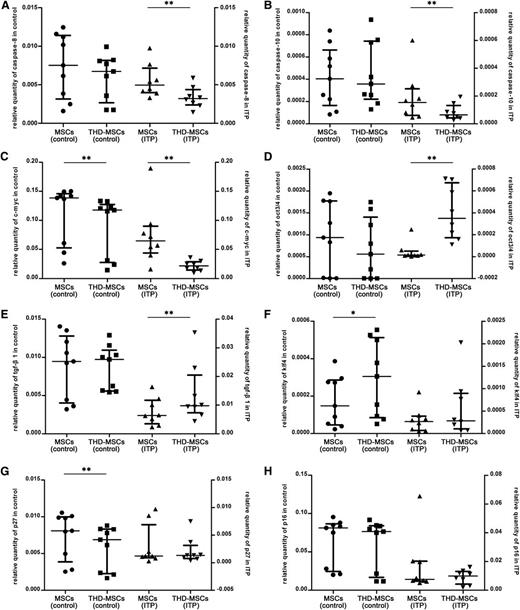
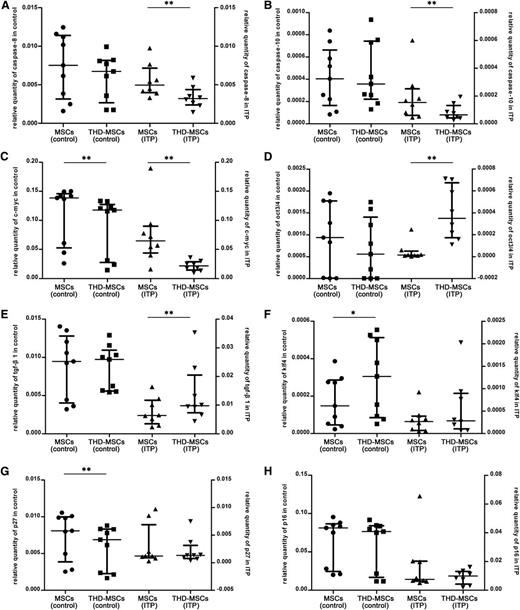
To further define the molecular changes in MSCs induced by THD, we performed microarray analysis. Differentially expressed genes indexed by Gene Ontology (Go) biological process of MSCs were shown in the Gene Expression Omnibus database under accession number GSE44948; enrichment for variants was associated with DNA-dependent regulation of transcription, cell cycle, mitosis, DNA replication, cell proliferation, apoptosis, regulation of cell growth, and regulation of apoptosis, etc. Combining with previous research,10,30-32 we selected several genes associated with cell proliferation, apoptosis and cell cycle, including p27, p16, caspase-8, caspase-10, klf4, c-myc, oct3/4, and tgf-β1 which may be associated with THD modulation. As shown in Figure 2A-B, the expression of caspase-8 and caspase-10 was downregulated in THD-MSCs (ITP), while there was no significant difference between MSCs (control) and THD-MSCs (control). The expression of c-myc was downregulated in both THD-MSCs (ITP) and THD-MSCs (control) (Figure 2C). The expression of oct3/4 and tgf-β1 was upregulated in THD-MSCs (ITP), while there was no obvious change between MSCs (control) and THD-MSCs (control) (Figure 2D-E). The expression of klf4 and p27 was upregulated or downregulated, respectively, while there was no obvious change between MSCs (ITP) and THD-MSCs (ITP) (Figure 2F-G). There was no obvious change of p16 either in MSCs (control) or in MSCs (ITP) before and after modulated with THD (Figure 2H). We confirmed the downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1, after THD modulation was specific for MSC (ITP) and explained the THD-induced increase in MSC proliferation, as well as their potentially ability to induce tolerogenic DCs.
The downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1 in MSCs (ITP) upon THD modulation. Expression levels of MSCs pre- or postexposed to 0.5 μg/mL THD for 12 hours from 8 ITP patients and 9 healthy controls. Each dot denotes the relative quantity of caspase-8, caspase-10, c-myc, oct3/4, klf4, tgf-β1, p27, and p16 (panels A-H, respectively). Statistical results represent median with interquartile range. Differences between MSCs (ITP) and THD-MSCs (ITP) or MSCs (control) and THD-MSCs (control) were determined using the Wilcoxon matched-pairs test. *P < .05; **P < .01.
The downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1 in MSCs (ITP) upon THD modulation. Expression levels of MSCs pre- or postexposed to 0.5 μg/mL THD for 12 hours from 8 ITP patients and 9 healthy controls. Each dot denotes the relative quantity of caspase-8, caspase-10, c-myc, oct3/4, klf4, tgf-β1, p27, and p16 (panels A-H, respectively). Statistical results represent median with interquartile range. Differences between MSCs (ITP) and THD-MSCs (ITP) or MSCs (control) and THD-MSCs (control) were determined using the Wilcoxon matched-pairs test. *P < .05; **P < .01.
THD-MSCs from ITP patients could induce mDCs into a tolerogenic DC population
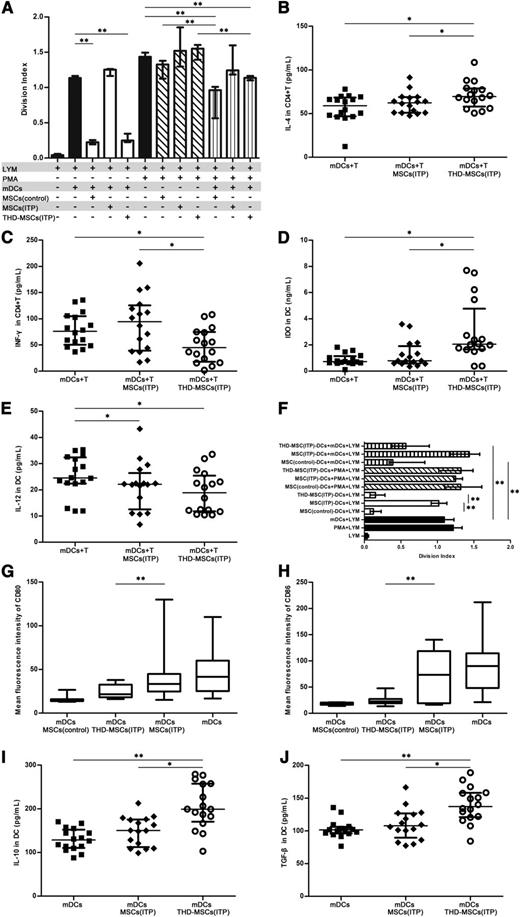
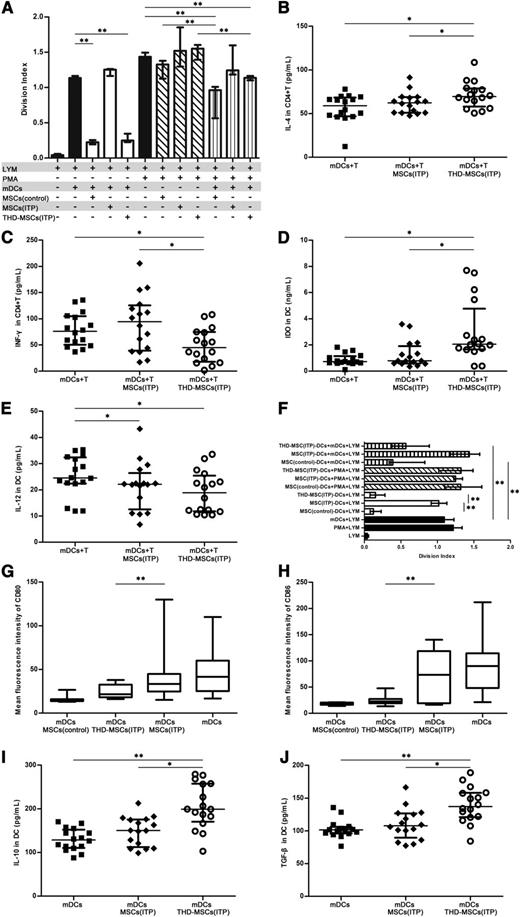
We postulated that MSCs in ITP patients may lose their immunosuppressive ability which could be increased after modulation with THD. To confirm the malfunction of MSCs (ITP), CFSE-labeled CD4+ T cells were stimulated by allogeneic mDCs or PMA in the presence or absence of MSCs (control), MSCs (ITP) or THD-MSCs (ITP). The data demonstrated that mDCs and PMA significantly stimulated T-cell proliferation (Figure 3A). When MSCs (control) or THD-MSCs (ITP) were added to mDC cocultures in the absence of PMA, they inhibited T-cell proliferation, whereas the addition of MSCs (ITP) had no inhibitory effect (Figure 3A). Notwithstanding, THD-MSCs (ITP) were unable to prevent T-cell proliferation activated in the presence of PMA. The data also showed that addition of mDCs to the coculture system alleviated T-cell proliferation. We also assayed the levels of IL-4, IFN-γ, IDO, and IL-12 in culture supernatants (Figure 3B-E). When CD4+ T cells and mDCs were cocultured with THD-MSCs (ITP), the level of IL-4 and IDO was higher while the IFN-γ level was lower than that cocultured with MSCs (ITP) or without MSCs. There was no significant difference in IL-12 level between cultures with THD-MSCs (ITP) or with MSCs (ITP), though in both cases it was lower than that without any MSCs. These data indicated that the MSCs from ITP patients had lost their regulatory capacity, which could be increased by THD modulation. After coculture with MSCs, the mDCs may have tolerogenic rather than stimulatory effects and play an important role in mediating the inhibitory activity of MSCs.
The THD-modulated MSCs induced mDCs into tolerogenic DCs in patients with ITP. (A) The inhibitory effect of MSCs on lymphocyte proliferation. CFSE-labeled allogeneic CD4+ T cells (1 × 105 per well) were stimulated with PMA (1 μg/mL) or mDCs (1 × 104 per well) and cocultured in the absence (−) or presence (+) of 1 × 104 per well MSCs (control, n = 9), MSCs (ITP, n = 9), or THD-MSCs (ITP, n = 9). After 3 days, the cells were collected and the division index was analyzed via FACS. Each bar denotes the division index of different conditions. Statistical results represent median with interquartile range. (B-E) The inhibitory effect of MSCs on cytokine secretion. CD4+ T cells (1 × 105 per well), mDCs (1 × 104 per well), and MSCs (1 × 104 per well) were cultured alone or in combination with MSCs (ITP, n = 16) or THD-MSCs (ITP, n = 16) as indicated. The levels of IL-4, IFN-γ, IDO, and IL-12 in the culture supernatant were determined using ELISA after 3 days’ culture. Each dot denotes the level of IL-4, IFN-γ, IDO, and IL-12. Statistical results represent median with interquartile range. (F) The tolerogenic effect of MSC-DCs on T-cell proliferation. After coculture with MSCs (control, n = 9), MSCs (ITP, n = 9), or MSCs (ITP, n = 9) modulated by THD, the MSC-DCs were sorted using anti-HLA-DR immunomagnetic beads. CFSE-labeled allogeneic CD4+ T cells (1 × 105 per well) were cocultured for 3 days with PMA (1 μg/mL) or mDCs (1 × 104 per well) in the presence of 1 × 104 per well MSC (control)–DCs, MSC (ITP)–DCs, or THD-MSC (ITP)–DCs, and analyzed via FACS. All DCs (mDC and MSC-DCs) in each experiment were from the same person. Each bar denotes the division index from different conditions. Statistical results represent median with interquartile range. (G-H) MSC-modulated mDC exhibit a unique pattern of activation markers. The mDCs were cultured in absence or presence of MSCs (control, n = 8), MSCs (ITP, n = 14), or THD-MSCs (ITP, n = 18) in a 1:1 ratio (mDC:MSC). After 3 days, the cells were collected to determine the MFI of CD80 and CD86 on mDCs by flow cytometry. Statistical results represent median with min to max of fluorescence intensity of CD80 and CD86. (I-J) The inhibitory effect of tolerogenic DCs was mediated by IL-10 and TGF-β. mDCs (1 × 104 per well) and MSCs (1 × 104 per well) were cultured alone or in combination for 3 days as indicated. The levels of IL-10 or TGF-β in the culture media were determined using ELISA. Data represent median with interquartile range. Each dot denotes the level of IL-10 or TGF-β. Statistical results represent median with interquartile range from 16 ITP patients. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01. MFI, mean fluorescence intensity.
The THD-modulated MSCs induced mDCs into tolerogenic DCs in patients with ITP. (A) The inhibitory effect of MSCs on lymphocyte proliferation. CFSE-labeled allogeneic CD4+ T cells (1 × 105 per well) were stimulated with PMA (1 μg/mL) or mDCs (1 × 104 per well) and cocultured in the absence (−) or presence (+) of 1 × 104 per well MSCs (control, n = 9), MSCs (ITP, n = 9), or THD-MSCs (ITP, n = 9). After 3 days, the cells were collected and the division index was analyzed via FACS. Each bar denotes the division index of different conditions. Statistical results represent median with interquartile range. (B-E) The inhibitory effect of MSCs on cytokine secretion. CD4+ T cells (1 × 105 per well), mDCs (1 × 104 per well), and MSCs (1 × 104 per well) were cultured alone or in combination with MSCs (ITP, n = 16) or THD-MSCs (ITP, n = 16) as indicated. The levels of IL-4, IFN-γ, IDO, and IL-12 in the culture supernatant were determined using ELISA after 3 days’ culture. Each dot denotes the level of IL-4, IFN-γ, IDO, and IL-12. Statistical results represent median with interquartile range. (F) The tolerogenic effect of MSC-DCs on T-cell proliferation. After coculture with MSCs (control, n = 9), MSCs (ITP, n = 9), or MSCs (ITP, n = 9) modulated by THD, the MSC-DCs were sorted using anti-HLA-DR immunomagnetic beads. CFSE-labeled allogeneic CD4+ T cells (1 × 105 per well) were cocultured for 3 days with PMA (1 μg/mL) or mDCs (1 × 104 per well) in the presence of 1 × 104 per well MSC (control)–DCs, MSC (ITP)–DCs, or THD-MSC (ITP)–DCs, and analyzed via FACS. All DCs (mDC and MSC-DCs) in each experiment were from the same person. Each bar denotes the division index from different conditions. Statistical results represent median with interquartile range. (G-H) MSC-modulated mDC exhibit a unique pattern of activation markers. The mDCs were cultured in absence or presence of MSCs (control, n = 8), MSCs (ITP, n = 14), or THD-MSCs (ITP, n = 18) in a 1:1 ratio (mDC:MSC). After 3 days, the cells were collected to determine the MFI of CD80 and CD86 on mDCs by flow cytometry. Statistical results represent median with min to max of fluorescence intensity of CD80 and CD86. (I-J) The inhibitory effect of tolerogenic DCs was mediated by IL-10 and TGF-β. mDCs (1 × 104 per well) and MSCs (1 × 104 per well) were cultured alone or in combination for 3 days as indicated. The levels of IL-10 or TGF-β in the culture media were determined using ELISA. Data represent median with interquartile range. Each dot denotes the level of IL-10 or TGF-β. Statistical results represent median with interquartile range from 16 ITP patients. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01. MFI, mean fluorescence intensity.
To further assess whether the MSC-regulated mDCs (MSC-DCs) were functionally tolerogenic, the mDCs were sorted using immunomagnetic beads after incubation with MSCs. Allogeneic CD4+ T cells were then cocultured with the MSC-DCs and mDCs from the same individual at a 10:1:1 ratio. As shown in Figure 3F, the mDCs regulated by MSCs from healthy controls suppressed the proliferation of T lymphocytes, whereas the mDCs regulated by MSCs from ITP patients did not. However, the inhibitory capacity of mDCs was increased when they were incubated with THD-modulated ITP MSCs. We also investigated whether the inhibitory effect of these tolerogenic DCs was strong enough to silence the lymphocytes activated by PMA. The data demonstrated that mDCs regulated by MSCs from ITP patients, healthy controls, or THD-modulated MSCs were unable to inhibit T-cell proliferation. These results further confirmed that the MSCs from ITP patients had lost the ability to induce tolerogenicity in mDCs. Although the tolerogenic mDCs could not prevent T-cell proliferation activated by PMA, they did block the allogeneic mDC-activated T-cell proliferation.
Furthermore, we conducted phenotypic analysis of the mDCs cocultured with MSCs from healthy controls, from ITP patients or THD-modulated MSCs. As shown in Figure 3G-H, MSCs from ITP patients failed to downregulate CD80 and CD86 expression in mDCs, while MSCs from healthy controls significantly inhibited the expression of these costimulatory factors. Accordingly, THD-MSCs also downregulated the expression of CD80 and CD86 in mDCs. Since IL-10 and TGF-β1 are potent immunosuppressive cytokines secreted by APCs, we tested whether these cytokines play a role in tolerogenic DC-mediated inhibition. As shown in Figure 3I-J, the levels of IL-10 and TGF-β1 in the culture supernatants of THD-MSCs (ITP) and mDCs were higher than those of mDCs alone or mDCs plus MSCs (ITP). These results indicate that the MSC-elicited inhibitory property of DCs is related to DC maturation state and TGF-β1 or IL-10 cytokine production. Taken together, the study demonstrated that the regulatory ability of MSCs from ITP patients could be regained upon THD modulation. After coculture with MSCs, the mDCs differentiated into a novel tolerogenic DC population.
The induction of tolerogenicity in mDCs by THD-modulated MSCs was dependent on TIEG1
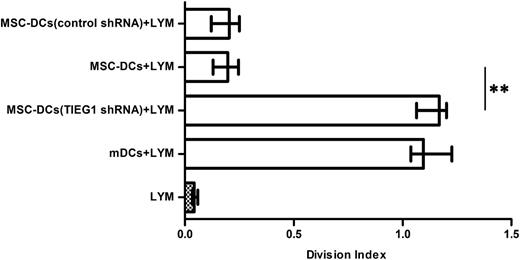
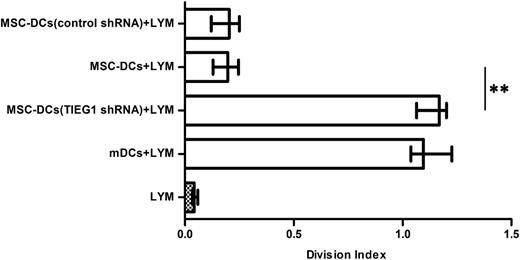
The mDCs infected with TIEG1 shRNA (h) lentivirus or control shRNA lentivirus were incubated with MSCs and sorted using immunomagnetic beads. The isolated mDCs were then cocultured with CFSE-labeled allogeneic CD4+ T cells and analyzed for T-cell proliferation. As shown in Figure 4, the mDCs infected with TIEG1 shRNA (h) lentiviral particles lost the ability to suppress T-cell proliferation. The results indicate that the acquisition of tolerance by mDCs upon incubation with MSCs depends on TIEG1.
The induction of tolerogenicity in mDCs by MSCs was dependent on TIEG1. The mDCs infected with TIEG1 shRNA (h) lentivirus or control shRNA lentivirus were incubated with MSCs for 3 days and sorted using immunomagnetic beads. The sorted DCs were then cocultured with CFSE-labeled allogeneic CD4+ T cells for 3 days, and analyzed for T-cell proliferation. Each bar represents the division index of lymphocyte proliferation. LYM, CFSE-labeled CD4+ T lymphocytes. mDCs + LYM, labeled T cells cocultured with mDCs. MSC-DCs (TIEG1 shRNA) + LYM, labeled T cells cocultured with lentivirus-infected mDCs after incubation with MSCs. MSC-DCs + LYM, labeled T cells cocultured with mDCs after incubation with MSCs. MSC-DCs (control shRNA) + LYM, labeled T cells cocultured with control lentivirus-infected mDCs after incubation with MSCs. Data represent median with interquartile range from 3 independent experiments. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01.
The induction of tolerogenicity in mDCs by MSCs was dependent on TIEG1. The mDCs infected with TIEG1 shRNA (h) lentivirus or control shRNA lentivirus were incubated with MSCs for 3 days and sorted using immunomagnetic beads. The sorted DCs were then cocultured with CFSE-labeled allogeneic CD4+ T cells for 3 days, and analyzed for T-cell proliferation. Each bar represents the division index of lymphocyte proliferation. LYM, CFSE-labeled CD4+ T lymphocytes. mDCs + LYM, labeled T cells cocultured with mDCs. MSC-DCs (TIEG1 shRNA) + LYM, labeled T cells cocultured with lentivirus-infected mDCs after incubation with MSCs. MSC-DCs + LYM, labeled T cells cocultured with mDCs after incubation with MSCs. MSC-DCs (control shRNA) + LYM, labeled T cells cocultured with control lentivirus-infected mDCs after incubation with MSCs. Data represent median with interquartile range from 3 independent experiments. Differences between 2 groups were compared using the Mann-Whitney U test. *P < .05; **P < .01.
Discussion
ITP is characterized by platelet phagocytosis within spleen33 and/or megakaryocyte destruction in BM.34 Autoantibodies promoted by enhanced T helper (Th) cells8 and T-cell–mediated cytotoxicity35 accelerated platelet destruction and suppressed platelet production. The imbalance between immune stimulation and regulation such as regulatory T cells36,37 and regulatory B cells38 was involved in the pathogenesis of ITP. MSCs possess an arsenal of immunosuppressive properties, which are mediated by the production of TGF-β1 and IL-10 as well as by the expression of IDO.39-41 MSCs from healthy individuals could suppress the secretion of IFN-γ by Th1 cells and increase production of IL-4 by Th2 cells.42 More importantly, these findings have provided the rationale for the use of healthy donor-derived MSCs in ITP patients43 and in the murine model.44
In this study, we demonstrated that MSCs from ITP patients had a lower proliferative capacity, which was consistent with previous reports.10 When treated with THD, their apoptosis decreased significantly and more cells entered into S + G2 phase. To further identify the underlying mechanisms involved in these changes, we first analyzed the gene-expression profile of MSCs modulated by THD using microarray. We confirmed that the downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1 after THD modulation was specific for MSC (ITP), which might explain the THD-induced increase in MSC proliferation.
Many kinds of regulatory DCs have been demonstrated to express low levels of HLA-DR, CD1a, CD80, and CD86 as well as decreased production of IL-12, but more IDO, TGF-β1, and IL-10.45-48 DCs are considered the primary target of the immunosuppressive activity of MSCs, which affect all major stages of the DC life cycle.16 We observed that after being cocultured with MSCs from healthy controls, the mDCs differentiated into a semimature population characterized by reduced expression of CD80 and CD86. In consistence, the stimulatory ability of MSC-treated mDCs on allogeneic T cells was impaired. Furthermore, we confirmed that these semimature DCs spontaneously secreted more IL-10, TGF-β, and IDO but less IL-12 (MSCs alone secret a small amount of TGF-β, IDO, and a trace amount of IL-10; data not shown). This profile might be involved in immune regulation. After modulation by THD, the MSCs from ITP patients affected the mDCs in a similar way, but unmodulated MSCs from patients had no effect. In addition, the overproduction of IL-4 and underproduction of IFN-γ in coculture supernatants containing MSCs from healthy controls or THD-modulated MSCs from ITP patients indicated that MSCs could balance T-lymphocyte cytokine profile by increasing IL-4 secretion and decreasing IFN-γ secretion. However, the MSCs from ITP patients combined with mDCs did not prevent T-lymphocyte activation, although this could be corrected partially by THD modulation. This led to the conclusion that the MSCs from ITP patients had lost their regulatory ability, but modulation by THD increased their regulatory capacity.
To determine the functional alteration of DCs upon regulation by MSCs, we sorted mDCs after incubation with different kinds of MSCs and conducted T-lymphocyte reaction assays. As expected, both the DCs induced by MSCs from healthy controls and those induced by THD-modulated MSCs from ITP patients suppressed T-cell proliferation activated by mDCs, whereas the mDCs induced by unmodulated MSCs from ITP patients did not. In line with previous observations,17,18 when mDCs were sorted after being cocultured with MSCs (control), MSCs (ITP) or THD-MSCs (ITP), these cells alone could not prevent PMA preactivated T-lymphocyte proliferation. Taken together, the complex interplay indicated that the suppressive effect of MSCs on DCs could be partly reversed and indicated that THD-modulated MSCs from ITP patients regain their ability to induce mDCs into tolerogenic DCs, which in turn could suppress allogeneic mDC-activated T-cell proliferation, thus demonstrating the therapeutic potential of THD to correct the impaired function of MSC in ITP.
TGF-β plays a central role in controlling cellular proliferation, differentiation, migration, and apoptosis. Wakkach et al found that TGF-β play a central role in eliciting the regulatory DC phenotype.23 TIEG1 is a member of the Krüppel-like factor (KLF) superfamily, which is a target gene of TGF-β.49 Overexpression of TIEG1 mimics the actions of TGF-β.50 Our study demonstrated that in the case of mDCs infected with TIEG1-interference lentivirus, the MSCs lost the ability to induce mDCs into tolerogenic DCs. These results indicated that the modulatory effects of MSCs on mDCs depend on the expression of TIEG1 in DCs.
Taken together, the downregulation of caspase-8 and caspase-10, and upregulation of oct3/4 and tgf-β1 may account for the modulatory effects of THD on MSCs. The impaired function of MSCs in inducing tolerogenic DCs may play a role in the pathogenesis of ITP, and could be partially remedied by THD. Our study suggests a therapeutic potential of THD in nonpregnant ITP patients.
The microarray data described in this article have been deposited in the Gene Expression Omnibus database (accession number GSE44948).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (no. 81125002), the National Natural Science Foundation of China (nos. 81070411, 81070396, 81070408, 81070407, 81170475, 81270577, 81270578), the 973 Program (nos. 2009CB521904, 2011CB503906), the Tai Shan Scholar Foundation, the State Program of the National Natural Science Foundation of China for Innovative Research Group 2011-2013 (no. 81021001), the Outstanding Young Scientist Research Award Foundation of Shandong Province (no. BS2010YY033), the National Key Vocational School About Clinical Specialty for Blood Disorders, Clinical Medicine Center Foundation of Shandong Province, and the Leading Medical Professionals Foundation of Shandong Province.
Authorship
Contribution: J.M., Y.-n.N., M.X., and J.P. designed and performed research, analyzed data, and wrote the paper; Y.H., N.W., X.-y.H., Y.-y.Y., H.L., and W.-d.H. performed research and analyzed data; L.-l.S., H.Z., Y.-n.M., and X.-g.L. evaluated the data and corrected the paper; Y.S., P.Q., C.-s.G., and M.H. reviewed the manuscript; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, 107 West Wenhua Rd, Jinan 250012, China; e-mail: junpeng88@sina.com.cn; and Ming Hou, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Jinan 250012, China; e-mail: houming@medmail.com.cn.
References
Author notes
J.M., Y.-n.N., and M.X. contributed equally to this work.