Key Points
mRNA transfection is an effective tool to simultaneously engineer MSCs for enhanced homing and improved secretome.
MSCs can be systemically targeted to sites of inflammation to achieve therapeutically relevant concentrations of biological agents.
Abstract
Mesenchymal stem cells (MSCs) are promising candidates for cell-based therapy to treat several diseases and are compelling to consider as vehicles for delivery of biological agents. However, MSCs appear to act through a seemingly limited “hit-and-run” mode to quickly exert their therapeutic impact, mediated by several mechanisms, including a potent immunomodulatory secretome. Furthermore, MSC immunomodulatory properties are highly variable and the secretome composition following infusion is uncertain. To determine whether a transiently controlled antiinflammatory MSC secretome could be achieved at target sites of inflammation, we harnessed mRNA transfection to generate MSCs that simultaneously express functional rolling machinery (P-selectin glycoprotein ligand-1 [PSGL-1] and Sialyl-Lewisx [SLeX]) to rapidly target inflamed tissues and that express the potent immunosuppressive cytokine interleukin-10 (IL-10), which is not inherently produced by MSCs. Indeed, triple-transfected PSGL-1/SLeX/IL-10 MSCs transiently increased levels of IL-10 in the inflamed ear and showed a superior antiinflammatory effect in vivo, significantly reducing local inflammation following systemic administration. This was dependent on rapid localization of MSCs to the inflamed site. Overall, this study demonstrates that despite the rapid clearance of MSCs in vivo, engineered MSCs can be harnessed via a “hit-and-run” action for the targeted delivery of potent immunomodulatory factors to treat distant sites of inflammation.
Introduction
Mesenchymal stem cells or mesenchymal stromal cells (MSCs)1 are currently used in over 250 ongoing clinical trials (July 2013, clinicaltrials.gov) as a potential therapeutic to treat multiple inflammatory diseases.2-5 However, although results from several preclinical animal studies have been promising, clinical trials have produced mixed results.6-9 This may stem from heterogeneity of MSC populations, significant donor-to-donor variability, cell senescence, immunogenicity and negative effects of cryopreservation, and in vitro expansion on MSC phenotype.10 MSCs also exhibit minimal persistence following systemic administration and appear to act through a “hit-and-run” mechanism, exerting their therapeutic activity within 24 to 48 hours after transplantation.11-14 Furthermore, MSC immunomodulatory properties are highly variable, and its composition is uncertain after infusion. Although it is compelling to consider engineering strategy to improve control over MSCs by equipping them for targeted delivery of potent immunomodulatory factors, it is not clear how long this effect would last, if relevant concentrations could be achieved within discrete and distant inflammatory microenvironments and if this would be sufficient to achieve a therapeutic effect.
Systemically administrated culture-expanded MSCs target diseased or inflamed tissues with extremely low efficiency.11 Key ligands that are required for homing, such as adhesion ligands (eg, P-selectin glycoprotein ligand-1 [PSGL-1], Sialyl LewisX [SLeX]) or chemokine receptors, are minimally expressed by MSCs,15,16 or their expression is lost during culture expansion.17 Moreover, though cell phenotype can be accurately controlled within in vitro settings, it is challenging to control cell properties after transplantation because cells are at the mercy of the biological milieu. For instance, MSC immunosuppressive properties observed in vitro often do not correlate with in vivo function.6,18 We15 and others16,19-21 have previously shown that engineering MSCs with key homing ligands or chemokine receptors can specifically target cells to diseased sites.7 Other studies have attempted to use modified MSCs to express and deliver therapeutic factors.22-24 However, engineering strategies have largely focused on viral DNA-based genetic, enzymatic, or chemical engineering approaches; to date, simultaneously engineering MSCs with multiple different factors, which is necessary toward combining improved homing and induced expression of therapeutic agents, has been challenging.
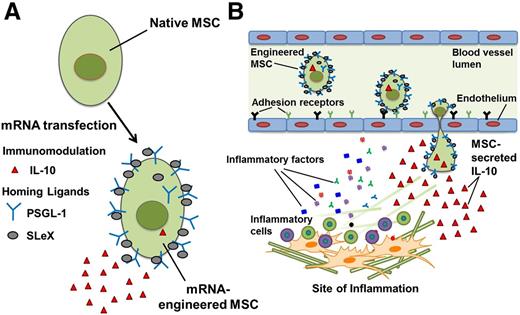
Considering the proposed “hit-and-run” MSC mode of action upon transplantation, we postulated that a combined approach might be beneficial in promoting rapid targeting of MSCs to disease sites to facilitate localized secretion of potent biological factors. To test this, we engineered MSCs, using messenger RNA (mRNA) transfection, to simultaneously express a combination of homing and therapeutic factors (Figure 1). To improve homing following systemic transplantation, we mRNA-transfected MSCs to express PSGL-1 and SLeX. PSGL-1 serves as a scaffold for the posttranslational SLeX modification (via α-(1,3)-fucosyltransferase [FUT7] activity), creating a functional ligand for P-selectin and E-selectin, resulting in cell tethering and rolling on the inflamed vascular endothelium, which is the first essential step for cell homing.25,26 Using mRNA transfection, we also generated MSCs producing the potent antiinflammatory cytokine, interleukin-10 (IL-10). Our results highlight mRNA transfection as a promising strategy to improve cell-based therapy via simultaneous control over different cell properties following transplantation, enabling targeted delivery of biologics to disease sites.
Improving MSC therapeutic potential via mRNA transfection with homing ligands and immunomodulatory factors. Illustration of (A) mRNA-engineered MSCs that express a combination of homing ligands (PSGL-1 and SLeX) and an immunomodulatory factor (IL-10), and (B) targeting mRNA-engineered MSCs to site of inflammation.
Improving MSC therapeutic potential via mRNA transfection with homing ligands and immunomodulatory factors. Illustration of (A) mRNA-engineered MSCs that express a combination of homing ligands (PSGL-1 and SLeX) and an immunomodulatory factor (IL-10), and (B) targeting mRNA-engineered MSCs to site of inflammation.
Methods
Animals
All studies were in accordance with National Institutes of Health (NIH) guidelines for care and use of animals under approval of the Institutional Animal Care and Use Committees of Massachusetts General Hospital and Harvard Medical School. C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for all in vivo studies.
Cells
Primary human MSCs were purchased from Texas A&M Institute of Regenerative Medicine, where cells were isolated from healthy bone marrow (BM) (from consenting donors) and characterized.27 This proof-of-concept study focused on evaluating the short-term impact of mRNA-engineered MSCs on inflammation, and thus xenogenic graft rejection was not a significant concern. CD4+ T cells were isolated from fresh whole blood (Research Blood Components, Brighton, MA).
MSC culture
MSCs were expanded in α minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin-streptomycin. Cells were kept at 37°C with 5% CO2, and media were changed every 3 days. Cells were passaged using trypsin-EDTA. MSCs at passage 3-6 were used for all experiments.
mRNA synthesis
Templates for in vitro transcription of PSGL-1, FUT7, and IL-10 were constructed as previously described.28 All templates contained a T7 promoter at the 5′ end. The protein-coding sequence was flanked by the 5′- and 3′-untranslated regions (UTRs) from human β-globin (supplemental Figure 1A, found on the Blood Web site). An optimized Kozak sequence was included between the 5′-UTR and the start codon in the forward primers used to amplify the protein-coding sequences (supplemental Table 1). Capped, polyadenylated RNA was synthesized using the T7 mScript Standard mRNA Production System (CELLSCRIPT, Inc., Madison, Wisconsin). Temperature and duration of the in vitro transcription reaction were optimized as previously described.28 A modified nucleoside 5′-triphosphate solution was used, in which pseudouridine-5′-triphosphate (pseudo-UTP) and 5-methylcytidine-5′-triphosphate (5-methyl-CTP) were substituted for UTP and CTP, respectively.29 After transcription, uncapped RNA was purified using an RNeasy kit (Qiagen, Hilden, Germany) and a 5′ cap structure and 3′-poly(A) tail were added using the mScript kit. Transcripts were then repurified and quantified, and the success of the poly(A) tailing reaction was confirmed by agarose gel electrophoresis.
mRNA transfection
MSCs were plated at 50% to 70% confluency and incubated (37°C, 5% CO2) for 12 to 24 hours prior to transfection. mRNA of interest (1 μg per 10 cm2 surface area) was added to Opti-MEM solution (50 μL/μg mRNA). Lipofectamine RNAiMAX (4 μL/μg mRNA) was added to an equal amount of Opti-MEM solution. mRNA and lipofectamine solutions were mixed thoroughly, incubated for 15 minutes (room temperature), and then incubated (in serum-free media) with the cells for 4 hours.
Protein expression and secretion
Surface expression of the cell membrane markers, PSGL-1 and SLeX, on native and transfected MSCs was examined by flow cytometry using appropriate antibodies. For detection of IL-10 secretion, conditioned media were collected and analyzed via IL-10 enzyme-linked immunosorbent assay (ELISA) kit.
In vitro cell rolling
Cell rolling experiments were performed using Bioflux1000 (Fluxion Biosciences, San Francisco, CA). Also see the supplemental Methods.
Coculture of MSCs and CD4+ T cells
CD4+ T cells were isolated as described in the supplemental Methods. In 12-well plates, resting CD4+ T cells (1 × 105 cells/well) were plated on top of MSCs (5 × 104 per well), in the presence of CD3/CD28 Dynabeads (Life Technologies, Grand Island, NY) to induce T-cell activation.30 After 48 hours, 5-bromo-2′-deoxyuridine (BrdU) (100 mM) was added to each well for an additional 24 hours (in total, 72 hours of coculture). CD4+ T cells that incorporated BrdU were detected using a fluorescein isothiocyanate–conjugated antibody. Cells were also stained with the DNA-binding dye 7-aminoactinomycin D (7-AAD), and 7-AAD+/BrdU+ cells were defined as proliferating cells.
MSC homing to inflamed ear
Fluorescently labeled (with different dyes) native and PSGL-1/SLeX MSCs were co-injected (1:1 ratio) retroorbitally into C57BL/6 mice (1 × 105 cells per 20 g body weight; same procedure was performed for co-injection of double- and triple-transfected MSCs). Homing of native and transfected MSCs to mouse ear was imaged noninvasively (in real time) using a custom-built video-rate laser-scanning confocal microscope designed specifically for live animal imaging.31 Also see the supplemental Methods.
MSC homing to BM
Native and PSGL-1/SLeX MSCs were co-injected retroorbitally to nonirradiated and γ-irradiated mice; 24 hours later, mice were anesthetized, and BM images were acquired through the intact skull of a live mouse using a confocal/multiphoton microscope specifically designed for live animal imaging.15,16,32
In vivo cell rolling
Thirty-frames/second live videos were recorded to analyze velocities of rolling cells. For static images, 15 to 30 frames were averaged from live video to improve signal/noise ratio. We computed the average rolling velocity (ImageJ, NIH, Bethesda, MD) as the displacement of the centroid of the cell divided by the time interval between observations. Cell velocities and hemodynamic parameters were calculated as previously described.15,31,33
Ear thickness measurements
For baseline measurements, ear thickness of C57BL/6 mice was measured using a digital caliper, while ensuring minimal compression. Inflammation was then induced by injecting 30 µg lipopolysaccharide (LPS) (in 30 µL 0.9% saline) into the left ear and 30 µL 0.9% saline into the right ear. After 24 hours, each mouse was injected retroorbitally with 1 × 106 of native or transfected MSCs per 20 g body weight (n = 5 mice per treatment). To assess the antiinflammatory efficacy of the injected MSCs, we measured ear thickness again 24 hours after cell injection.
Measurement of IL-10 levels in mice ears
After MSC injection, mice were sacrificed using carbon dioxide exposure, and the ears were harvested. Ears were then ground in ice-cold extraction buffer (containing 10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100) using a homogenizer. Homogenates were centrifuged at 13 000× g for 10 minutes at 4°C, and the level of human IL-10 in the supernatant samples was quantified using an anti-human IL-10 ELISA kit.
Statistics
For multiple pairwise comparisons, 1-way analysis of variance (ANOVA) was used with Tukey’s honestly significant difference (HSD) post hoc test. Statistical significance is denoted by P < .05. Statistical analysis for each figure is detailed in the figure legends. Also see supplemental Table 2 for detailed data ± standard deviation (SD) for relevant figures.
Results
mRNA synthesis and mesenchymal stem cell transfection
On the basis of previously established protocols,28,34-36 we synthesized mRNA through an in vitro transcription reaction templated by PCR and bacterial amplification of cDNA clones for all 3 genes of interest: PSGL-1, α-(1,3)-fucosyltransferase (FUT7), and interleukin-10 (IL-10). To increase the stability and the translation efficiency of the mRNA, a 5′ cap and polyA tail was incorporated along with human β-globin 5′ and 3′ UTRs. A Kozak sequence was included to enhance the initiation signal for translation of the proteins (supplemental Figure 1A). The successful synthesis of all these mRNAs was confirmed using agarose gel electrophoresis (supplemental Figure 1B).
Incorporation of modified nucleotides, such as the noncanonical pyrimidine derivatives, pseudouridine (Ψ), or 5-methylcytidine (5mC)—into in vitro–generated mRNA was reported to reduce immunogenicity and increase expression efficiency.28,29,34-36 It has been hypothesized that the incorporated modified nucleotides may disrupt the secondary structures of RNA, which impedes its interaction with RNA-binding proteins, resulting in decreased immunogenicity. Consistent with this hypothesis, we observed that unmodified, Ψ- or 5mC-incorporated mRNAs indeed adopt different secondary structures, as indicated by different migratory properties on agarose gel (supplemental Figure 1C).
In addition, these modifications can stabilize the delivered mRNAs and therefore improve protein yield. For instance, a recent study showed that incorporation of Ψ improved protein expression efficiency by reducing activation of RNA-activated protein kinase (PKR).37 Warren et al reported that complete substitution of cytidine with 5mC in Ψ-containing in vitro–transcribed RNA can further increase expression efficiency.35 Hence, we tested the impact of substituting uridine for Ψ, cytidine for 5mC, or both on protein expression efficiency in mRNA-transfected MSCs (supplemental Figure 1D-E). In contrast to Warren et al, we found that substitution of Ψ alone demonstrated the maximum expression efficiency, fivefold higher in comparison with unmodified mRNA and 3.5-fold higher than mRNA containing both Ψ and 5mC. Therefore, mRNA was incorporated with Ψ for all target proteins in subsequent experiments.
PSGL-1 and SLeX expression and stability following mRNA transfection
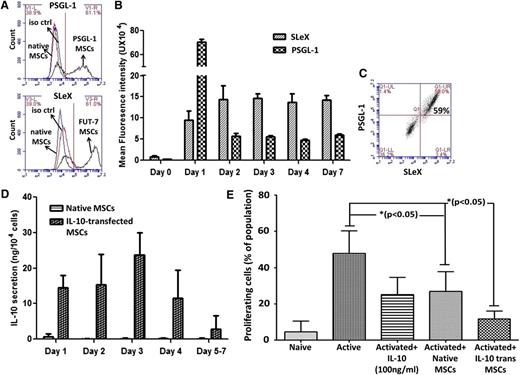
After MSC transfection with mRNA encoding PSGL-1 and FUT-7, we tested their expression via flow cytometry (Figure 2A-C). Because FUT7 is an intracellular enzyme, its expression was validated by the presence of its product, the tetra-saccharide SLeX, which serves a key role in mediating PSGL-1 interaction with its counter ligands, P- and E-selectin.25 As is shown in Figure 2A, although native MSCs lack expression of those surface markers, transfection with PSGL-1 or FUT-7 mRNA resulted in surface expression of PSGL-1 or SLeX, respectively. Further experiments demonstrated that maximal PSGL-1 expression is reached 1 day posttransfection, and SLeX expression peaks 2 days after transfection (Figure 2B). A significant advantage of using mRNA transfection is the ability to transfect cells with multiple factors simultaneously. For instance, when MSCs were transfected with both PSGL-1 and FUT7, 59.0% of cells expressed both PSGL-1 and SLeX (in comparison with a transfection efficiency of 61.1% or 61.0% upon transfection with only PSGL-1 or FUT7, respectively) and fewer than 5% of cells were transfected with only a single transcript (Figure 2C).
MSC expression of PSGL-1, SLeX, and IL-10 following mRNA transfection. (A) MSCs were transfected with either PSGL-1 (top) or FUT7 (bottom) and 24 hours later, flow cytometry analysis detected surface expression of PSGL-1 and SLeX, respectively. (B) After MSC transfection with PSGL-1 or FUT7, surface expression of PSGL-1 and SLeX, respectively, was confirmed for up to 7 days. Mean intensity of transfected cell population was normalized to respective isotype controls. Mean ± SD (n = 3). (C) Coexpression of PSGL-1 and SLeX from simultaneous transfection with PSGL-1 and FUT7 mRNA. (D) IL-10 secretion from native and IL-10 mRNA-transfected MSCs. Mean ± SD (n = 3). (E) IL-10 mRNA-transfected MSCs exhibit improved immunosuppressive effects in vitro. Resting human CD4+ T cells were cocultured with native or IL-10-transfected MSCs in the presence of CD3/CD28 Dynabeads, and CD4+ T cell proliferation was measured (7-AAD+/BrdU+ cells were defined as proliferating cells). *P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent SD (n = 3).
MSC expression of PSGL-1, SLeX, and IL-10 following mRNA transfection. (A) MSCs were transfected with either PSGL-1 (top) or FUT7 (bottom) and 24 hours later, flow cytometry analysis detected surface expression of PSGL-1 and SLeX, respectively. (B) After MSC transfection with PSGL-1 or FUT7, surface expression of PSGL-1 and SLeX, respectively, was confirmed for up to 7 days. Mean intensity of transfected cell population was normalized to respective isotype controls. Mean ± SD (n = 3). (C) Coexpression of PSGL-1 and SLeX from simultaneous transfection with PSGL-1 and FUT7 mRNA. (D) IL-10 secretion from native and IL-10 mRNA-transfected MSCs. Mean ± SD (n = 3). (E) IL-10 mRNA-transfected MSCs exhibit improved immunosuppressive effects in vitro. Resting human CD4+ T cells were cocultured with native or IL-10-transfected MSCs in the presence of CD3/CD28 Dynabeads, and CD4+ T cell proliferation was measured (7-AAD+/BrdU+ cells were defined as proliferating cells). *P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent SD (n = 3).
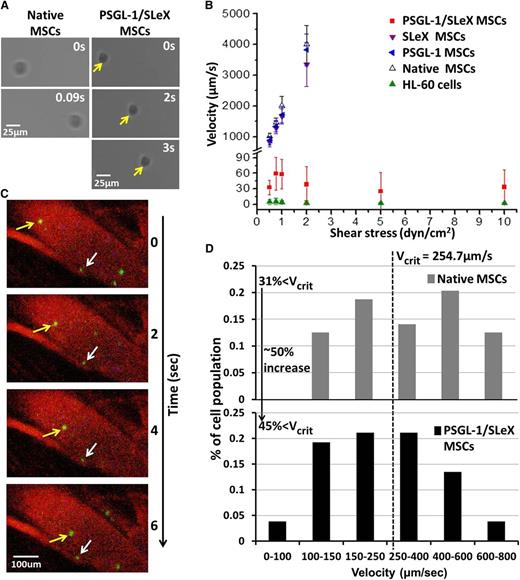
PSGL-1/SLeX MSCs exhibit robust rolling behavior on a P-selectin-coated surface
After validating the successful expression of the target proteins following mRNA transfection, we aimed to examine the ability of these newly expressed proteins to modulate MSC homing properties. PSGL-1, with a posttranslational SLeX modification (via FUT7 activity), is the functional ligand for P-selectin and E-selectin, which are upregulated on the blood vessel endothelium within inflamed tissues.25,26 The interaction between PSGL-1 and P-selectin induces cell tethering and rolling on the vascular endothelium, which is the first essential step during the homing cascade.25 To validate the ability of PSGL-1/FUT-7 mRNA cotransfection to induce MSC rolling, we introduced MSCs into a microfluidic channel that was coated with recombinant human P-selectin. Cells were then subjected to increasing shear flows, and their rolling properties on the P-selectin surface were assessed. Remarkably, PSGL-1/SLeX MSCs exhibited a robust cell rolling response (rolling velocity of ∼5-60 µm/s) under physiological shear stresses (2-10 dyn/cm2), comparable to that of HL-60 cells (promyelocytic leukemia cells), which are considered a gold standard for cell rolling studies (Figure 3A-B). Importantly, PSGL-1/SLeX/IL-10 MSCs (the product of “triple” mRNA cotransfection) also exhibited a robust rolling behavior, similar to that of PSGL-1/SLeX MSCs (supplemental Figure 2). On the contrary, native (untransfected) MSCs, or MSC transfected with only a single gene, ie, PSGL-1 or FUT7 alone, do not roll on a P-selectin surface (Figure 3B), demonstrating that both PSGL-1 and SLeX are required to generate a rolling response. Additional control groups, such as lipofectamine-treated MSCs, as well as MSCs transfected with scrambled RNA or IL-10 alone mRNA, did not display a rolling response on P-selectin substrates, thus behaving similarly to native MSCs (supplemental Figure 2).
PSGL-1/SLEX MSCs exhibit a robust rolling response on P-selectin-coated substrates in vitro and on inflamed endothelium in vivo. (A) Representative images showing PSGL-1/SLeX MSCs (yellow arrows) roll on a P-selectin surface in vitro at a substantially lower velocity than a native MSC. Shear stress in these images: 0.75 dyn/cm2. (B) Simultaneous expression of both PSGL-1 and SLeX is required to induce robust rolling of MSCs on P-selectin surface. Data are shown as mean velocity (calculated from 20 cells per group) ± SD. (C) Representative in vivo confocal microscopy images show a rolling (yellow arrows) and adhered (white arrows) PSGL-1/SLeX MSCs. (D) Histogram showing a representative velocity distribution of native MSCs and PSGL-1/SLeX MSCs on inflamed ear endothelium in vivo (representative analyzed population; velocity was calculated for at least 50 cells per group). Vcrit calculated as described in the “Methods” section.
PSGL-1/SLEX MSCs exhibit a robust rolling response on P-selectin-coated substrates in vitro and on inflamed endothelium in vivo. (A) Representative images showing PSGL-1/SLeX MSCs (yellow arrows) roll on a P-selectin surface in vitro at a substantially lower velocity than a native MSC. Shear stress in these images: 0.75 dyn/cm2. (B) Simultaneous expression of both PSGL-1 and SLeX is required to induce robust rolling of MSCs on P-selectin surface. Data are shown as mean velocity (calculated from 20 cells per group) ± SD. (C) Representative in vivo confocal microscopy images show a rolling (yellow arrows) and adhered (white arrows) PSGL-1/SLeX MSCs. (D) Histogram showing a representative velocity distribution of native MSCs and PSGL-1/SLeX MSCs on inflamed ear endothelium in vivo (representative analyzed population; velocity was calculated for at least 50 cells per group). Vcrit calculated as described in the “Methods” section.
PSGL-1/SLeX MSCs roll on inflamed endothelium in vivo
We next used a murine model to explore cell rolling in vivo, in which LPS was injected into the base of the right ear to induce local inflammation. This process is characterized by the upregulation of multiple adhesion ligands on the inflamed endothelium, including P- and E-selectin.15,26 Twenty-four hours later, native and PSGL-1/SLeX MSCs, stained with different vibrant membrane dyes, were co-injected retroorbitally and imaged in the inflamed ear vasculature using dynamic real-time intravital confocal microscopy. As shown in the representative time-lapsed images (Figure 3C), PSGL-1/SLeX MSCs exhibited a robust rolling behavior on LPS-induced inflamed endothelium in vivo. Transfected cells were observed rolling along the inflamed endothelium, whereas unmodified MSCs did not exhibit such substantial interactions with the endothelial vessel wall. As presented in Figure 3D, PSGL-1/SLeX MSCs rolled along the inflamed vessel wall with a reduced velocity in comparison with native MSCs, with 45% of the transfected cell population (in comparison with only 31% of the native MSC population) rolling at a velocity lower than the critical velocity. These data demonstrate that mRNA-mediated incorporation of functional rolling machinery (PSGL-1/SLeX) on the MSC surface is capable of triggering a specific rolling response of the modified cells on inflamed endothelium in vivo.
PSGL-1/SLeX MSCs exhibit enhanced homing to healthy and irradiated mouse BM
To examine whether improved rolling properties translate into enhanced MSC homing to inflamed tissues, we γ-irradiated C57BL/6 mice with a sublethal dose of 4.25 Gy, and 24 hours later, native and PSGL-1/SLeX MSCs were co-injected retroorbitally followed by calvarial BM imaging. As shown in Figure 4, the administered native MSCs were observed in the BM of both healthy (nonirradiated) and γ-irradiated mice (68.37 ± 13.43 and 60.54 ± 8.25 cells/mm2, respectively), confirming that native human MSCs are indeed capable of homing to the murine BM following systemic administration. Importantly, PSGL-1/SLeX MSCs demonstrated 48.5% increased homing vs native cells to healthy BM and 88.3% increased homing to irradiated BM (Figure 4B). These data strongly suggest that equipping MSCs with the homing ligands PSGL-1/SLeX via mRNA transfection significantly improves their homing to the BM, especially under inflammatory conditions.
PSGL-1/SLeX MSCs exhibit enhanced homing to healthy and γ-irradiated mouse BM. (A) Representative images of native MSCs (blue, DiD) and PSGL-1/SLeX MSCs (green, DiI) observed in both healthy and γ-irradiated BM (60× magnification; red, blood, rhodamine-dextran). (B) Quantitative analysis of MSC homing to nonirradiated or γ-irradiated BM. PSGL-1/SLeX MSCs exhibit enhanced homing to both healthy and γ-irradiated BM vs native MSCs (*P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent ± standard error of the mean [SEM] [n = 4 per group]).
PSGL-1/SLeX MSCs exhibit enhanced homing to healthy and γ-irradiated mouse BM. (A) Representative images of native MSCs (blue, DiD) and PSGL-1/SLeX MSCs (green, DiI) observed in both healthy and γ-irradiated BM (60× magnification; red, blood, rhodamine-dextran). (B) Quantitative analysis of MSC homing to nonirradiated or γ-irradiated BM. PSGL-1/SLeX MSCs exhibit enhanced homing to both healthy and γ-irradiated BM vs native MSCs (*P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent ± standard error of the mean [SEM] [n = 4 per group]).
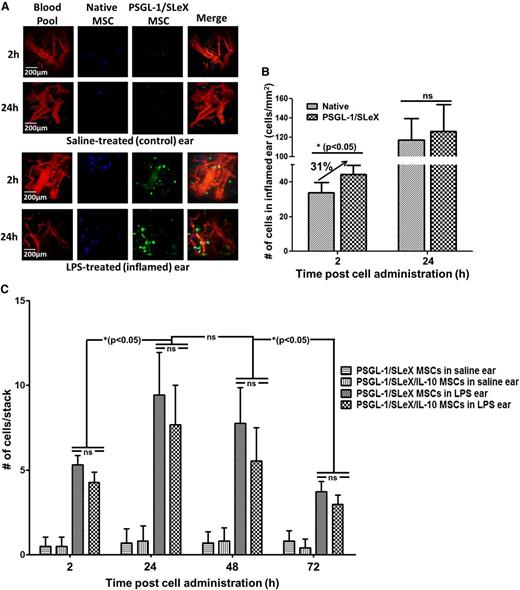
Systemically administered PSGL-1/SLeX MSCs efficiently home to inflamed ear
Next, we used the LPS-induced inflamed ear murine model to determine whether PSGL-1/SLeX MSCs display enhanced homing to other distant sites of inflammation following systemic administration. Two hours and 24 hours after cell injection, mouse ears were imaged using intravital confocal microscopy for the presence of injected cells (representative images shown in Figure 5A). Interestingly, the mRNA-transfected MSCs equipped with the homing ligands PSGL-1 and SLeX exhibited enhanced homing to inflamed ear 2 hours after injection, with a ∼30% increase in homing vs native MSCs (Figure 5A-B). Although higher numbers of both native and transfected MSCs were detected in the inflamed ear 24 hours postinjection, the advantageous homing of the transfected MSCs was abolished at that time point (Figure 5B). In contrast, only a negligible number of MSCs (native or modified, no statistical difference between the groups) were observed in the saline ear 2 hours and 24 hours postinjection (Figure 5A). A direct comparison between PSGL-1/SLEX and PSGL-1/SLeX/IL-10 MSCs (which were mRNA transfected to express PSGL-1/SLeX as well as IL-10; see below for further details) revealed similar homing capabilities of the 2 groups to inflamed ear pinna (Figure 5C; statistically insignificant difference between the groups in each time point). Moreover, MSC presence in the inflamed ear is transient, with a clear peak at 24 to 48 hours, followed by a decrease in the number of cells observed 72 hours postadministration (Figure 5C).
Incorporation of the PSGL-1/SLeX rolling machinery promotes rapid homing of MSCs to inflamed ear pinna. (A) Representative images of native MSCs (blue, DiD) and PSGL-1/SLeX MSCs (green, DiI) in healthy and inflamed mice ears (red, blood, rhodamine-dextran). (B) Quantitative analysis of MSC homing to the inflamed ear. Transfected MSCs exhibit statistically significant enhanced homing to the LPS-induced inflamed ear vs native MSCs at 2 hours postinjection (*P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent ± SEM) (n = 4 and n = 7 for 2 hours and 24 hours, respectively). (C) A direct comparison between PSGL/SLeX MSCs and PSGL-1/SLeX/IL-10 MSCs reveals similar homing to inflamed ear pinna (ns = no statistical difference observed between the 2 groups at each time point; 1-way ANOVA using Tukey’s HSD; error bars represent ± SD, n = 4). Rapid clearance of MSC is observed, with peak number of cells observed at 24 to 48 hours and a significant decrease observed at 72 hours after cell administration (stack dimensions are 474 × 488 microns; *P < .05, 1-way ANOVA using Tukey’s HSD).
Incorporation of the PSGL-1/SLeX rolling machinery promotes rapid homing of MSCs to inflamed ear pinna. (A) Representative images of native MSCs (blue, DiD) and PSGL-1/SLeX MSCs (green, DiI) in healthy and inflamed mice ears (red, blood, rhodamine-dextran). (B) Quantitative analysis of MSC homing to the inflamed ear. Transfected MSCs exhibit statistically significant enhanced homing to the LPS-induced inflamed ear vs native MSCs at 2 hours postinjection (*P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent ± SEM) (n = 4 and n = 7 for 2 hours and 24 hours, respectively). (C) A direct comparison between PSGL/SLeX MSCs and PSGL-1/SLeX/IL-10 MSCs reveals similar homing to inflamed ear pinna (ns = no statistical difference observed between the 2 groups at each time point; 1-way ANOVA using Tukey’s HSD; error bars represent ± SD, n = 4). Rapid clearance of MSC is observed, with peak number of cells observed at 24 to 48 hours and a significant decrease observed at 72 hours after cell administration (stack dimensions are 474 × 488 microns; *P < .05, 1-way ANOVA using Tukey’s HSD).
IL-10 MSCs display improved immunosuppressive properties in vitro
After the successful generation of MSCs with enhanced homing properties, we next aimed to explore whether MSC immunosuppressive properties can also be enhanced via mRNA transfection. For this, MSCs were transfected with mRNA encoding IL-10, an antiinflammatory cytokine that is not expressed by MSCs.38 MSCs transfected with IL-10 mRNA secreted IL-10 at significant levels (11.5-23.5 ng/day per 104 cells) for up to 4 days posttransfection (Figure 2D). We then aimed to assess IL-10 activity and test whether IL-10 mRNA transfection improved MSC immunosuppressive properties. For this purpose, we established a coculture system of CD4+ T cells and MSCs to assess the effect of our engineered MSCs on CD4+ T-cell proliferation. After 72 hours coculture of resting human CD4+ T cells with IL-10 mRNA-transfected or native MSCs (from unrelated donors) in the presence of CD3/CD28 Dynabeads to induce T-cell activation, BrdU incorporation by CD4+ T cells was evaluated by flow cytometery. Cells were also stained with the DNA-binding dye 7-AAD, and dually stained (7-AAD+/BrdU+) cells were defined as proliferating cells. As shown in Figure 2E, native MSCs suppress CD4+ T cell proliferation as efficient as IL-10 alone, decreasing the percentage of proliferating T cells from 48% in the absence of MSCs to 27% in the presence of native MSCs. Importantly, IL-10 mRNA transfection significantly enhanced the suppressive effect of MSCs on T-cell proliferation, further decreasing the percentage of proliferating T cells from 27% in the presence of native MSCs to only 11% in the presence of IL-10-expressing MSCs.
PSGL-1/SLeX/IL-10 MSCs exhibit improved antiinflammatory effect in vivo
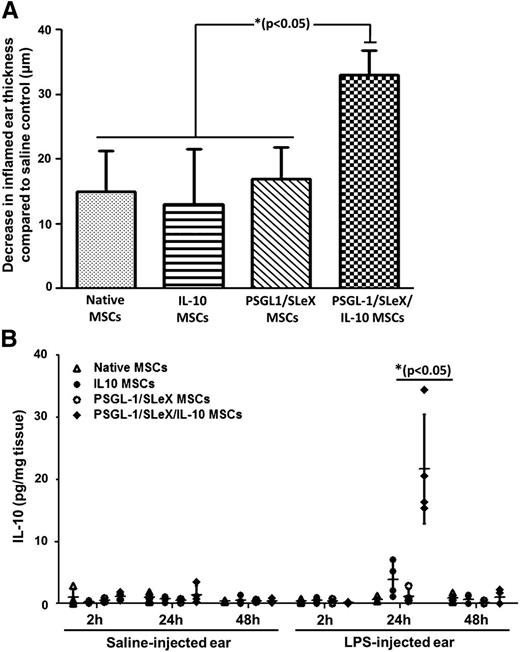
To explore whether mRNA-transfected MSCs exhibit improved antiinflammatory impact in vivo, we used the LPS-induced inflamed ear murine model. Twenty-four hours post-LPS injection, MSCs (either native or mRNA transfected with IL-10, PSGL-1/FUT7, or PSGL-1/FUT7/IL-10) were systemically injected, and after an additional 24 hours, ear thickness was used as a measure of the antiinflammatory effect. As shown in Figure 6A, all types of MSCs exhibited antiinflammatory effects, decreasing ear thickness in comparison with saline-injected control. The triple-transfected (PSGL-1/SLeX/IL-10) MSCs demonstrated significantly higher antiinflammatory impact than did all other MSC groups (33 μm decrease in ear thickness vs 15 μm for native MSC, 17 μm for PSGL-1/SLeX MSCs, and 13 μm for IL-10 MSCs). Of note, healthy ear thickness was 225 μm, and LPS injection resulted in a 70-μm increase when mice received only saline (control) as treatment (measured 48 hours post-LPS injection, which is 24 hours post-MSC treatment). Treatment with triple-transfected MSCs reduced the level of inflammation by nearly 50% (reducing ear thickness by 33 μm), twice the effect of the other cell treatment groups. Moreover, analysis of human IL-10 levels in mice ears revealed that only the triple-transfected MSCs significantly increased local IL-10 levels in the inflamed ear 24 hours after transplantation, subsequently dropping at 48 hours (Figure 6B). This corresponds to the reduced ear thickness also observed 24 hours after injection of PSGL-1/SLeX/IL-10 MSCs, implicating a direct involvement of the secreted IL-10 in the antiinflammatory process.
PSGL-1/SLeX/IL-10 MSCs display improved antiinflammatory impact in vivo via targeted delivery of IL-10 to the inflamed site. (A) Triple transfected PSGL-1/SLeX/IL-10 MSCs exhibit an antiinflammatory effect in vivo demonstrated via ear thickness measurements. Data are presented as decrease in inflamed ear thickness (after treatment with the different MSC groups) in comparison with control group (mice receiving saline treatment). *P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent SEM) (n = 5 per group). (B) Only triple-transfected MSCs deliver a significant amount of IL-10 to the inflamed ear. At the specified time points following MSC transplantation, mice were sacrificed; ears were harvested and analyzed for presence of human IL-10 using an ELISA assay. Each point in the graph represents data from a single mouse. *P < .05 vs all other groups at the same time point, 1-way ANOVA using Tukey’s HSD; error bars represent SD (n = 4 per group).
PSGL-1/SLeX/IL-10 MSCs display improved antiinflammatory impact in vivo via targeted delivery of IL-10 to the inflamed site. (A) Triple transfected PSGL-1/SLeX/IL-10 MSCs exhibit an antiinflammatory effect in vivo demonstrated via ear thickness measurements. Data are presented as decrease in inflamed ear thickness (after treatment with the different MSC groups) in comparison with control group (mice receiving saline treatment). *P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent SEM) (n = 5 per group). (B) Only triple-transfected MSCs deliver a significant amount of IL-10 to the inflamed ear. At the specified time points following MSC transplantation, mice were sacrificed; ears were harvested and analyzed for presence of human IL-10 using an ELISA assay. Each point in the graph represents data from a single mouse. *P < .05 vs all other groups at the same time point, 1-way ANOVA using Tukey’s HSD; error bars represent SD (n = 4 per group).
Discussion
One of the greatest challenges in exogenous cell therapy is to maximize the delivery of transplanted cells within specific sites in the body and to control the production of therapeutic factors.7,11 A variety of engineering approaches were previously used to control MSC phenotype following transplantation in an attempt to improve their therapeutic properties. Genetically modifying MSCs to overexpress chemokine receptors CXCR-4 or CCR-1 resulted in increased MSC engraftment to infarcted myocardium.20,21 In an attempt to improve MSC therapeutic properties, MSCs were also genetically engineered for Akt overexpression to improve survival39 and with the proangiogenic factor vascular endothelial growth factor40 to promote angiogenesis. Although stable DNA-based genetic manipulations yielded encouraging data in animal in vivo models, its translation into the clinic is challenging because of long-term safety concerns, and often a transient expression is preferred over stable expression approaches. In addition, achieving simultaneous expression of multiple factors is challenging, limiting the ability to concurrently control multiple cell properties.
We previously reported that chemical modification of MSC surface with SLeX increases MSC homing to sites of inflammation following systemic administration.15 It was also reported that enzymatic pretreatment of living cells, converting cell surface CD44 into HCELL, increases trafficking of infused MSCs to bone.16 Antibody coating and cytokine pretreatment of MSCs have also been used to increase MSC delivery to diseased tissues, such as inflamed colon and infarcted myocardium.19,41 Nevertheless, there still exists a significant need for a safe and robust strategy to simultaneously enhance MSC homing properties and tune the secretome to maximize therapeutic potential.
A major advantage of an mRNA approach, especially when compared with DNA-based transfection or enzymatic treatments, is the ability to simultaneously and transiently engineer cells with multiple factors. Approximately 60% of cells expressed both PSGL-1 and SLeX after dual simultaneous transfection, similar expression levels to when cells were transfected with only a single factor (Figure 2A,C). This is especially critical when considering the role of PSGL-1 and SLeX in the cellular rolling response. SLeX residues mediate specific interactions between PSGL-1 (expressed on leukocytes) and P/E-selectins (upregulated on the endothelial surface during inflammation), generating a rolling movement of leukocytes on inflamed blood vessels.26 Accordingly, only double-transfected MSCs, expressing both PSGL-1 and SLeX, exhibited a robust rolling response on P-selectin substrates under shear flow conditions (Figure 3A-B). According to the classical leukocyte homing cascade, the rolling step on inflamed endothelium is critical for slowing down the cells from the blood stream, followed by their firm adhesion to the blood vessel wall. We recently showed that MSC express relevant adhesive machinery to firmly adhere to activated endothelium, followed by spontaneous transmigration in the absence of exogenous chemokines.42 This was supported by our data here showing that enhanced rolling of PSGL-1/SLeX MSCs led to increased homing to the BM and inflamed ear following systemic injection. Interestingly, increased homing of PSGL-1/SLeX MSCs to BM was observed under both normal and γ irradiation-induced inflammatory conditions. The relatively high basal expression of adhesion ligands on BM blood vessels is likely sufficient to support the increased homing of modified MSCs (in comparison with unmodified MSCs) to healthy BM,43,44 whereas the additional upregulation in adhesion molecule levels following γ-irradiation45 further enhances homing of transfected cells (vs unmodified cells). Also, the high basal expression of adhesion ligands in the BM vasculature may account for the relatively small, statistically insignificant, difference observed in native and transfected MSC homing to irradiated BM vs healthy BM in our model. Interestingly, it was previously shown that local irradiation promoted MSC homing to exposed sites as well as their engraftment in multiple other organs.46 Testing the homing of mRNA-transfected MSCs in such irradiation-induced local inflammation models instead of a whole-body irradiation is also of interest and should be used in future studies. These results strongly suggest that mRNA transfection-mediated incorporation of a functional rolling mechanism is a useful generalized approach to promote cell homing to sites of inflammation following systemic administration.
Previously, cytokine pretreatment or genetic modifications of MSCs have been shown to enhance their engraftment within infarcted heart tissue and improve cardiac function.20,41 Similarly, others have reported positive results in animal models using genetically modified MSCs to express and deliver exogenous proteins, such as interferon-β and tumor necrosis factor–related apoptosis-inducing ligand to suppress tumor growth and IL-10 to attenuate collagen-induced arthritis and reduce the severity of graft-versus-host disease.22-24,47 Our ear inflammation model revealed a different trend. Although unmodified MSCs exhibited antiinflammatory effects, reducing inflamed ear thickness in comparison with the saline-treatment control group, this effect was limited and statistically insignificant for all the groups, except for the triple-transfected (PSGL-1/SLeX/IL-10) MSCs (Figure 6A), which abolished approximately 50% of the inflammation-induced ear swelling. IL-10 transfection alone or the double transfection with PSGL-1/FUT-7, which significantly increased MSC homing to the inflamed ear, did not exhibit any advantageous antiinflammatory effects over native MSCs, reducing ear thickness by a similar level. These data demonstrate that only a combined approach, equipping MSCs with homing ligands (PSGL-1/SLeX) and a potent antiinflammatory cytokine (IL-10), is sufficient to improve the antiinflammatory impact of systemically administered MSCs.
As shown in Figure 5B, the advantageous homing to inflamed ear of PSGL-1/FUT7 mRNA-transfected MSCs was transient; increased homing was observed 2 hours after injection (31% increase over control cells), yet after 24 hours, there was no significant difference observed. Examination of homing at additional time points would likely reveal a more significant advantage of PSGL-1/SLeX MSCs over native MSCs, and we aim to explore this in future studies. Importantly, this mRNA-induced temporary increase in cell homing, once combined with IL-10 transfection, was sufficient to significantly improve MSC antiinflammatory therapeutic impact (as demonstrated by ear thickness measurements). The presence of MSCs in the inflamed ear was transient, peaking at 24 to 48 hours, followed by a significant decrease 72 hours after cell administration (Figure 5C). In addition, triple-transfected MSCs yielded a transient increase in local levels of human IL-10 in the inflamed ear, with significant levels detected 24 hours after cell injection (Figure 6B), likely mediating the rapid decrease in ear thickness (Figure 6A). These data suggest that targeting MSCs to quickly deliver a therapeutic payload (eg, their native secretome strengthened with expression of biological agents such as IL-10) to diseased or damaged tissue may be a key step for improved MSC therapy. These data are consistent with the “hit-and-run” hypothesis recently suggested by Leblanc and colleagues to highlight a potential MSC mechanism of action following transplantation in patients.12 After tissue analysis of human patients injected with MSCs, and in accordance with previous studies, von Bahr et al concluded that long-term engraftment of MSCs is low and suggested that the observed therapeutic function of MSCs is via a rapid yet transient mechanism. Furthermore, although it was suggested that limited numbers of intravenously injected adipose tissue–derived MSCs could survive in immunocompromised mice for weeks (mostly in liver and lungs),48 most studies have demonstrated that the vast majority of BM-derived MSCs systemically administered into mice die and are cleared from the body within 48 to 72 hours postadministration,13,49 further supporting the hit-and-run concept. Accordingly, our findings suggest that optimal therapeutic efficacy will be achieved only if MSCs, engineered with improved immunomodulatory capabilities, are delivered rapidly to disease sites to exert maximal activity prior to their death and clearance. Although these data highlight the importance of MSC secretome in mediating its therapeutic effects, the potential role of other mechanisms, such as exosomes and mitochondrial transfer, should be further evaluated.50,51
The promising therapeutic potential of MSCs is not yet fully translated into the clinic. Given the narrow window of opportunity to achieve their therapeutic effects, strategies enabling rapid delivery of “upgraded” MSCs to disease sites appear necessary. This approach should help alleviate issues relating to MSC heterogeneity, loss of phenotype during culture expansion, or donor-to-donor variations. In this study, we have used mRNA transfection to simultaneously engineer MSCs to improve homing and to achieve targeted delivery of potent biological agents to locally treat inflamed tissue. Aligned with the MSC hit-and-run mode of action, mRNA transfection emerges as a promising platform for improved cell-based therapy, and this strategy should be useful as a generalized approach to achieve targeted delivery of biologics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH, Heart, Lung and Blood Institute grant HL095722 to J.M.K, and by the American Heart Association grant 0970178N to J.M.K. This work was also supported by a Prostate Cancer Foundation Challenge Award to J.M.K.
Authorship
Contribution: O.L., W.Z., M.F.Y., C.P.L., and J.M.K. designed experiments and interpreted data; O.L., W.Z., L.J.M., S.L., K.T., M.F., J.A.P., V.S., P.A., J.N., C.H.C., P.E., and M.A. performed the experiments and analyzed data; and O.L., W.Z., M.F.Y., and J.M.K. cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for W.Z. is Department of Pharmaceutical Sciences, University of California, Irvine, CA.
Correspondence: Jeffrey M. Karp, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139; e-mail: jeffkarp.bwh@gmail.com; and Mehmet Fatih Yanik, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139; e-mail: yanik@mit.edu.
References
Author notes
O.L. and W.Z. contributed equally to this study.

![Figure 4. PSGL-1/SLeX MSCs exhibit enhanced homing to healthy and γ-irradiated mouse BM. (A) Representative images of native MSCs (blue, DiD) and PSGL-1/SLeX MSCs (green, DiI) observed in both healthy and γ-irradiated BM (60× magnification; red, blood, rhodamine-dextran). (B) Quantitative analysis of MSC homing to nonirradiated or γ-irradiated BM. PSGL-1/SLeX MSCs exhibit enhanced homing to both healthy and γ-irradiated BM vs native MSCs (*P < .05, 1-way ANOVA using Tukey’s HSD; error bars represent ± standard error of the mean [SEM] [n = 4 per group]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/14/10.1182_blood-2013-04-495119/4/m_e23f4.jpeg?Expires=1769984016&Signature=lnFOFQESg~jwA1NMPN2~kCMe4L1CvZ6YzQzs1N0JpDuT6SbFBF-k1l1R2DjBOkBHJ87lBPjN8ZtUvyiAufG0v4N5Yt-XMASSpwDhSCz67ms9j2d9WTafj-uOH~THigEIZsmHcI~iuclOLLNVEogUo4pFA~aOd61m6sxI-JNvBAQKw7rRO5lruYSqPOUCzPD9YyUWW6phg0EonWokqMXC1tQRTobdvGZbSCoiS893qAOncnTaVjeulLnMIdt1xZihRgpwZ-2E82vWHmhJ6DumRmZ92Wobnw1r0RBLCAK-FZGG1SYQtHmTwNJGjIsgSHl-MofGwzlXIa4AeFn2PZiyhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)