In this issue of Blood, Walker et al investigate the preclinical potential of KPT-330, an exportin-1 (XPO1, also known as chromosome maintenance protein 1 [CRM1]) inhibitor, against both accelerated phase (AP) and blast crisis chronic myeloid leukemia (CML-BC) and against Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL), all of which are diseases of significant unmet clinical need.1 The authors provide encouraging data from both a leukemic mouse model and a single CML-AP patient, corroborating mechanistic studies suggesting that KPT-330 efficacy relies on targeting abundantly expressed XPO1, followed by the reactivation of protein phosphatase 2A (PP2A).
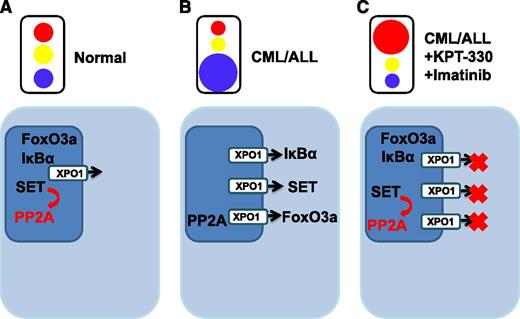
Normal cells maintain homeostasis by tightly regulating intracellular trafficking of ions, small molecules and proteins. (A) Proteins located predominantly in the nucleus are able to function normally (eg, SET is able to activate its target PP2A, a tumor suppressor). (B) Leukemic transformation abrogates the trafficking of cargo with inappropriate diversions provided by upregulated karyopherins, such as XPO1, providing the “green light” to export specific proteins out of the nucleus. Protein activity is altered (eg, PP2A is no longer appropriately activated). (C) KPT-300 with imatinib treatment introduces a “red light” to halt subverted proteins from being diverted from the nucleus, and nuclear proteins are able to function within their appropriate context.
Normal cells maintain homeostasis by tightly regulating intracellular trafficking of ions, small molecules and proteins. (A) Proteins located predominantly in the nucleus are able to function normally (eg, SET is able to activate its target PP2A, a tumor suppressor). (B) Leukemic transformation abrogates the trafficking of cargo with inappropriate diversions provided by upregulated karyopherins, such as XPO1, providing the “green light” to export specific proteins out of the nucleus. Protein activity is altered (eg, PP2A is no longer appropriately activated). (C) KPT-300 with imatinib treatment introduces a “red light” to halt subverted proteins from being diverted from the nucleus, and nuclear proteins are able to function within their appropriate context.
Normal cellular homeostasis depends on the cell’s ability to compartmentalize proteins within specific subcellular compartments. Intracellular transport of proteins to their correct locations is accomplished, in part, by signaling sequences encoded within the proteins themselves. In addition, the nuclear envelope aids cellular organization by forming a barrier restricting passage between the nucleus and cytoplasm. However during carcinogenesis, these normal processes are deregulated, and as a result, nuclear export is subverted. Normal trafficking of critical proteins that maintain regulated cell growth is rerouted, skewing normal flow and creating cellular mayhem (see figure).
At this time, tyrosine kinase inhibitors represent a critical component in first-line therapy for advanced-phase CML and Ph+ ALL, but unfortunately, the majority of patients experience suboptimal responses and a marginal prolongation of life. Under therapy, minimal residual disease persists and evolves to become fully drug-resistant as the result of clonal heterogeneity, genomic instability, Bcr-abl kinase mutations, and failure to eradicate leukemia-initiating cells, fueling disease relapse. There is therefore an urgent need for improved treatment of these forms of leukemia.
KPT-330 is an oral drug currently undergoing phase 1 studies in patients with advanced, relapsed, and refractory solid tumors (clinicaltrials.gov, no. NCT01607905); hematological malignancies (clinicaltrials.gov, no. NCT01607892); and sarcoma (clinicaltrials.gov, no. NCT01896505). KPT-330 has a novel mechanism of action by inhibiting nuclear-cytoplasmic transport (a key target being XPO1) within cells, triggering significant cellular death and showing promise in multiple cancers.2-4
Ions, small molecules, and proteins less than 40 to 65 kDa cross the nuclear membrane in a passive manner; however larger proteins require the assistance of transport molecules called karyopherins. XPO1, a subclass of karyopherins, is able to transport both RNA and proteins mediated by Ran GTPase activating protein. XPO1 possesses the ability to shuttle more than 200 different proteins, including several tumor-suppressor proteins such as retinoblastoma, adenomatous polyposis coli, p53, p21, breast cancer 1, and forkhead box (FoxO), in addition to the oncogene Bcr-abl. In particular cancers, increased XPO1 activity is thought to lead to relocalization of nuclear factors, excluding them from their normal sites of activity and thus favoring cancer initiation, progression, and eventual drug resistance. XPO1 is overexpressed in several cancers, including ovarian, myeloma, pancreatic, osteosarcoma, glioma, and cervical cancer, in which XPO1 expression is negatively correlated with progression-free survival,5 making KPT-330 a potentially viable therapeutic for these cancers.
Importantly, as shown by Walker et al, XPO1 expression is augmented in CML-AP and CML-BC and in B-ALL (Ph+/−). The authors have capitalized on this characteristic to investigate the potential for the clinically relevant karyopherin inhibitor, KPT-330. After complete inhibition of Bcr-abl kinase activity with imatinib, XPO1 was only partially inhibited, suggesting its enhanced expression was both kinase-dependent and kinase-independent in Ph+ cells. This adds to a growing list of proteins whose aberrant expression is at least partially independent of Bcr-abl kinase, suggesting the need for agents that target alternative mechanisms of aberrant signaling. KPT-330 treatment, at concentrations that have been achieved in clinical trials, induced significant apoptosis (observed to be independent of Bcr-abl inhibition) and impaired the colony-forming ability of CML progenitors, with a concurrent decrease in the activity and transcription of XPO1. The effect of KPT-330 was relatively selective, with a 3-fold difference in EC50 for normal CD34+ cells. The authors also demonstrated that induction of XPO1 transcription may be independent of Bcr-abl and, likely, through PI-3K, Akt, or protein kinase C. Mechanistically, treatment with KPT-330 resulted in the nuclear accumulation of SET, CIP2A, IkBα, FoxO3a, p53, and p21, with the redistribution of SET and CIP2A subsequently leading to reactivation of PP2A. Taken together, the data suggest that reactivation of PP2A was responsible for significantly reduced Bcr-abl levels and accounted for at least 50% of the catastrophic apoptosis. KPT-330 showed promise in an allograft model of CML-BC and in a single CML-AP patient in whom KPT-330 therapy led to a reduction in bone pain, splenomegaly, and immature myeloid blasts in the peripheral blood. Unfortunately, the patient refused more than a single week of therapy, and duration of response was unclear.
Previous studies have attempted to achieve similar nuclear protein entrapment, using leptomycin B. One such study exploited the observation that Bcr-abl translocates to the nucleus once bound by imatinib.6 The authors performed experiments demonstrating that once Bcr-abl was located in the nucleus under imatinib exposure, treatment with leptomycin B trapped nuclear Bcr-abl. By excluding Bcr-abl from the cytoplasm, it was unable to aberrantly phosphorylate multiple proteins with resultant apoptosis. Although theoretically elegant, these studies were performed in Bcr-abl overexpressing fibroblasts and mouse bone marrow cells. Subsequent studies using normal and CML human CD34+ cells demonstrated that this strategy conferred mild cytostatic effects, but little cytotoxicity, to leukemic progenitors.7 Other clinical studies with leptomycin B (elactocin) determined that this drug was unsuitable for further development, as it induced severe gastrointestinal toxicities, leading to anorexia and malaise.5
The new studies completed by Walker et al provide an encouraging basis for more fundamental preclinical examinations of KPT-330 in conjunction with tyrosine kinase inhibitors. The synergistic potential of these drugs should be assessed in primary samples and in a more disease-relevant mouse model. It would also be important to determine whether this drug combination can affect tumor-initiating populations in advanced-phase CML and ALL. This study fits with the ongoing paradigm shift in thinking away from single-targeted agents to those agents able to abrogate multiple aberrant pathways simultaneously, as represented here by alterations in tumor suppressors PP2A and p53 and oncogenes Bcr-abl, Akt, NF-kB, and c-Myc.8
Conflict-of-interest disclosure: The authors declare no competing financial interests.