Key Points
MiR-146a expression is induced by TLR ligation expressed in pDCs.
MiR-146a regulates pDC effector functions, including cytokine production and costimulatory capacity.
Abstract
During microbial infections, plasmacytoid dendritic cells (pDCs) are a main source of type I interferons α/β (IFN-α/-β). Nucleic acids from microbes are sensed by Toll-like receptors 7/9 (TLR7/9), which are selectively expressed in pDCs. Activated pDCs also produce proinflammatory cytokines and upregulate costimulatory molecules. Together, this equips pDCs with the ability to prime T, B, and NK cells and conventional DCs, thereby initiating adaptive immune responses. To avoid deleterious effects to the host, tight regulation of pDC activation is required. Despite data linking aberrant activation of pDCs with autoimmune diseases, little is known about mechanisms controlling pDC activation. Here, we investigated the role of microRNA-146a (miR-146a) in TLR pathway regulation in human pDCs. MiR-146a expression was induced upon TLR7/9 signaling. Furthermore, ectopic miR-146a expression effectively impaired TLR-mediated signaling in pDCs as TLR-induced nuclear factor–κB activation was reduced. This consequently diminished the production of proinflammatory cytokines and reduced pDC survival. Moreover, miR-146a–expressing pDCs had decreased ability to induce CD4+ T-cell proliferation likely due to reduced expression levels of major histocompatibility complex class II and costimulatory molecules. Our data unravel the crucial immunomodulatory role of miR-146a in pDCs and may add to our understanding of aberrant responses in autoimmune diseases.
Introduction
Plasmacytoid dendritic cells (pDCs) form a unique subset within the DC lineage. In contrast to conventional DCs, pDCs selectively express Toll-like receptor 7 (TLR7) and TLR9, which recognize microbial single-stranded RNA or double-stranded DNA, respectively (reviewed in Liu1 ). TLR activation in pDCs leads to rapid secretion of high amounts of type I interferons (IFNs), which prevent viral replication and are involved in regulating antigen-specific immune responses. In addition, TLR-activated pDCs secrete interleukin-6 (IL-6) and mature in response to autocrine production of the proinflammatory cytokine tumor necrosis factor–α thereby upregulating the expression of costimulatory molecules, such as CD40, CD80, and CD86, and major histocompatibility complex class II.2 Collectively, this contributes to activation of T, B, and natural killer (NK) cells.3
Signal transduction via TLR7 and TLR9 depends on recruitment of the myeloid differentiation primary response gene 88 (MyD88) adaptor molecule in complex with IL-1 receptor–associated kinase 1 (IRAK-1) and IRAK-4, tumor necrosis factor receptor-associated 6 (TRAF6) and TRAF3, and the transcription factors IFN response factor 7 (IRF-7) and IRF-5.4 Furthermore, all TLR signaling pathways culminate in activation of the transcription factor nuclear factor–κB (NF-κB), which requires the phosphorylation and degradation of inhibitory κB proteins triggered by 2 kinases, IκB kinase α and IκB kinase β. Several NF-κB members have been identified, including RelA (also known as p65), RelB, c-Rel, p52, and p50. Rel proteins can form homodimers or heterodimers, of which the most frequently activated form after TLR signaling is the RelA/p50 heterodimer.5 RelA/p50 dimers are directly responsible for the expression of costimulatory molecules, while IRF-5 nuclear translocation, together with NF-κB and mitogen-activated protein kinase activation, is crucial for the production of inflammatory cytokines.6 Conversely, the phosphorylation of IRF-7 leads to its translocation into the nucleus where it initiates type I IFN gene transcription.7,8
Unwanted production of IFN-α has been shown to be involved in the pathogenesis of several human autoimmune diseases, such as systemic lupus erythematosus (SLE),9,10 Sjögren syndrome (SS),11,12 and psoriasis.13 In SLE, one of the driving mechanisms of the disease involves uncontrolled and chronic IFN-α and IL-6 production by activated pDCs, which promote survival and differentiation of autoreactive B cells into autoantibody-secreting plasma cells.14 Given the potency of type I IFNs and proinflammatory cytokines to activate a wide range of cells of the innate and adaptive immune system, pDC activation needs to be tightly controlled. Therefore, pDCs express an array of surface receptors, such as the C-type lectin blood DC antigen 2 (BDCA2), DC immunoreceptor, immunoglobulin-like transcript 7, high-affinity immunoglobulin E receptor (FcεRI), and NK partner 44 (NKp44), which counterregulate the prominent TLR signaling pathway.15-18
A more recently discovered and novel layer of gene regulation is mediated by microRNAs (miRNAs), which are an evolutionary conserved class of endogenous ∼19- to 23-nucleotide long noncoding RNAs.19 They act by repressing gene expression through targeting of the 3′-untranslated region (UTR) of messenger RNAs (mRNAs) resulting in either mRNA degradation or translation inhibition, or a combination of both.20 This mode of posttranscriptional regulation of gene expression has recently been shown to play a role in modulating the TLR response in a broad range of human immune cells, including monocytes, macrophages, and T cells.21 Of these miRNAs, miR-146a emerged as a negative master regulator of TLR activation. MiR-146a is a member of the miR-146 miRNA family consisting of 2 evolutionary conserved miRNA genes; miR-146a and miR-146b. Increased expression of miR-146a has been observed in human monocytes in response to TLR4 stimulation by lipopolysaccharides through direct NF-κB–mediated induction.22 Moreover, miR-146a was shown to act as a key regulator of the TLR-MyD88 pathway by directly targeting IRAK1 and TRAF6 mRNAs, suggesting its role as a “brake on immunity.” In line with this, miR-146a−/− mice suffered from myeloproliferative disorders and lipopolysaccharide hypersensitivity.23,24 Furthermore, it has been demonstrated that stimulation of peripheral blood mononuclear cells (PBMCs) from healthy donors with TLR7 and TLR9 agonists induced miR-146a expression and negatively affected type I IFN production, via downregulation of IRF-5 and STAT1.25 Interestingly, in SLE patients, miR-146a was found to be expressed at lower levels as compared with healthy donors, which might well explain the exacerbated type I IFN production by PBMCs of these patients.25 Based on the notion that in human blood only pDCs express TLR7 and TLR9 and secrete type I IFNs after stimulation, it may suggest that the function of pDCs is altered by miR-146a, but this has not been formally addressed. It is also not known whether miR-146a controls other functions of pDCs, such as expression of costimulatory molecules and production of proinflammatory cytokines as well as their survival.
Despite the growing evidence involving miRNA in the regulation of TLR functioning, no extensive studies have been performed on purified human pDCs. Therefore, we aimed at clarifying the role of miR-146a in regulating TLR-induced pDC activation and maturation. We made use of both primary pDCs as well as the pDC cell line CAL-1,22 which we previously validated as a model to study human pDC activation and maturation.26,27 We observed strong induction of miR-146a expression upon activation of primary pDCs using either TLR7 or TLR9 agonists. In addition, we investigated the role of miR-146a using overexpression studies in the CAL-1 pDC cell line. Our results suggest that miR-146a is crucially involved in the survival and TLR-induced maturation of pDCs, which has direct consequences for their ability to induce proliferation and IFN-γ production of CD4+ T cells.
Methods
Cells and reagents for functional assay
The pDC cell line CAL-126 was cultured in RPMI 1640 medium (Invitrogen) supplemented with 8% fetal calf serum, and maintained at 37°C, 5% CO2. For activation and maturation of pDCs, cells were cultured in Yssel medium,28 supplemented with 2% human serum (Invitrogen). Oligodeoxynucleotides CpG-A (ODN2216), CpG-B (ODN2006), and R848 were purchased from Invivogen and used at 10 μg/mL.
Lentiviral constructs and transductions
Overexpression of miR-146a in human cells was done using a vector-based miRNA expression system.29 Briefly, the ∼500-bp fragment corresponding to the miR-146a genomic region or the control human telomerase (hTR) genomic region (a nontranslated RNA coding for hTR RNA30 ) were cut out from the pMSCV-Blasticidin vector (kind gifts from R. Agami, The Netherlands Cancer Institute, Amsterdam, The Netherlands) using BamHI-EcoRI restriction sites, and subcloned into the lentiviral vector pCDH1 (System Biosciences) using the copepod Pontellina plumata green fluorescent protein (copGFP) as a reporter gene.
PCR
Total RNA was extracted using TRIzol reagent (Invitrogen). RNA concentration and quality were determined using the Nanodrop spectrophotometer (Thermo Fisher Scientific). MiRNA quantitative real-time reverse transcription polymerase chain reaction (QPCR) was performed using the TaqMan MicroRNA Reverse Transcription kit with TaqMan MicroRNA assay primers for human miR-146a (Applied Biosystems). Total mRNA was reverse transcribed using the RNA-to-cDNA kit (Roche). Complementary DNA (cDNA) was amplified using an iCycler and SYBRgreen supermix (Bio-Rad) for QPCR, using specific primer sets (supplemental Table 1, available on the Blood Web site). The levels of miRNA were normalized to RNA U6 controls, whereas mRNA levels were normalized to the 3 housekeeping genes β-Actin, GAPDH, and HPRT.
ELISA
Enzyme-linked immunosorbent assay (ELISA) was done to detect human IL-6 (U-cytech Biosciences) and IFN-β (PBL Interferon Source) proteins in the supernatants of CAL-1 cells according to the manufacturer’s instructions.
Flow cytometry
For analysis, single-cell suspensions were stained with fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE–cyanine 7 (Cy7), allophycocyanin (APC), APC-Cy7–coupled anti-human monoclonal antibodies targeting the following cell-surface markers: CD40, HLA-DR (Biolegend), CD80, CD86, CCR7, or isotype controls (BD Biosciences). For detection of phosphorylated p65 (phospho-S529; phospho-p65) protein, cells were fixed using cytofix/cytoperm buffer, permeabilized in ice-cold methanol, and washed with Perm/Wash buffer (BD Pharmingen) before incubation with APC-conjugated phospho-p65 antibody (BD Biosciences). For apoptosis staining, we used Annexin V–PE, or Annexin V–APC (BD Biosciences) and 7-aminoactinomycin D (7-AAD) viability staining solution (eBioscience). For intracellular cytokine detection, PE-conjugated anti-human–IFN-γ and IFN-α antibodies (BD Biosciences) were used. Samples were analyzed on an LSRII fluorescence-activated cell sorter (FACS) analyzer (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Isolation of primary human pDCs from tissues
Peripheral blood of healthy volunteers was used for isolation of pDCs (Sanquin Bloodbank, Amsterdam, The Netherlands). PBMCs were isolated via Ficoll-Hypaque density gradient and negatively selected for CD3/CD14/CD16/CD19/CD20 by immunomagnetic bead selection using anti-FITC beads (Miltenyi Biotec). Postnatal thymic tissue was obtained from surgical specimens removed from children up to 3 years of age undergoing open-heart surgery (Leiden University Medical Center, Leiden, The Netherlands), approved by the medical ethical committee of the Academic Medical Center. Informed consent was obtained in accordance with the Declaration of Helsinki. Thymocytes were isolated from a Ficoll-Hypaque density gradient. Subsequently, BDCA4+ cells were enriched by immunomagnetic bead selection using a BDCA4-cell separation kit (Miltenyi Biotec). CD123+CD45RA+ or BDCA4+ pDCs were sorted by flow cytometry on a FACSAria (BD Biosciences). Purity was ≥99% and confirmed by reanalysis of sorted cells.
LNA transfection
Sorted pDCs were transfected using the Neon Transfection system (Invitrogen) with 1 μg of either an FITC-conjugated human-miR-146a locked nucleic acid (LNA) (5′-AACCCATGGAAUTCAGUUCUCA-3′) or control LNA (5′-GUGTAACACGUCTAUACGCCCA-3′) (Ribotask) by electroporation (Microporator, Digital Bio; 1200 V, 20 ms, 1 pulse).
Allogeneic T-cell stimulation
Peripheral blood CD4+ T cells were enriched by immunomagnetic bead selection using a CD4-cell separation kit (Miltenyi Biotec). CAL-1 cells transduced with miR-146a or hTR control were incubated with or without the TLR7 ligand R848 for 48 hours, irradiated (60 Gy), and subsequently cocultured with T cells at a 1:1 ratio for 6 days in Yssel medium supplemented with 2% human serum. T-cell proliferation was assessed by flow cytometry using the CellTrace-Violet Proliferation kit. T cells activated with human T-expander CD3/CD28 beads (Dynabeads; Dynal/Invitrogen) were used as a positive control. For detection of intracellular IFN-γ, CD4+ T cells were restimulated with phorbol myristate acetate (PMA)/ionomycin (1 μg/mL each) at day 6 for 6 hours in the presence of Brefeldin A and stained with anti–IFN-γ PE (BD Pharmingen).
Statistical analyses
Data were subjected to 2-tailed paired Student t test analysis using GraphPad Prism 5 for Windows (GraphPad Software) and considered significant when at least P < .05.
Results
MiR-146a expression is induced in human pDCs in response to TLR7 and TLR9 activation
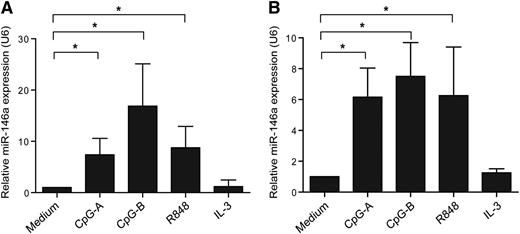
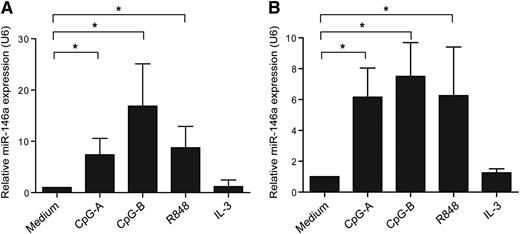
To study whether miR-146a has a role in pDCs, we first set out to analyze whether ex vivo pDCs expressed miR-146a after stimulation with TLR9 agonists (CpG-A, CpG-B) or TLR7 agonist (R848). After 16 hours, miR-146a levels were measured by QPCR. We observed that both TLR7 and TLR9 engagement induced miR-146a expression as compared with medium-cultured pDCs (Figure 1A). The leukemic pDC cell line CAL-1, which represent a valid model to study certain aspects of pDC biology,27 expressed miR-146a as well upon TLR7 or TLR9 ligation (Figure 1B). Stimulation with IL-3 did not upregulate expression of miR-146a in primary pDCs or CAL-1 cells (Figure 1). Collectively, this suggests that miR-146a may have a role in TLR-induced responses in pDCs.
MiR-146a is upregulated upon activation of pDCs. (A) Freshly isolated pDCs or (B) CAL-1 cells were activated with the TLR9 agonists CpG-A or CpG-B, the TLR7 agonist R848 (each 10 μg/mL), IL-3 (10 ng/mL), or cultured in medium alone for 16 hours. The relative expression of the mature form of miR-146a was assessed by QPCR using specific TaqMan primers and the QPCR TaqMan kit. MiR-146a levels were normalized to the level of the small nuclear RNA U6, and the medium control condition was set to 1. Data are shown as means ± SD of independent pDC donors (CpG-A, n = 5; CpG-B, n = 4; R848, n = 5; IL-3, n = 2) or independent experiments using CAL-1 cells (CpG-A and CpG-B, n = 3; R848 and IL-3, n = 4). *P < .05.
MiR-146a is upregulated upon activation of pDCs. (A) Freshly isolated pDCs or (B) CAL-1 cells were activated with the TLR9 agonists CpG-A or CpG-B, the TLR7 agonist R848 (each 10 μg/mL), IL-3 (10 ng/mL), or cultured in medium alone for 16 hours. The relative expression of the mature form of miR-146a was assessed by QPCR using specific TaqMan primers and the QPCR TaqMan kit. MiR-146a levels were normalized to the level of the small nuclear RNA U6, and the medium control condition was set to 1. Data are shown as means ± SD of independent pDC donors (CpG-A, n = 5; CpG-B, n = 4; R848, n = 5; IL-3, n = 2) or independent experiments using CAL-1 cells (CpG-A and CpG-B, n = 3; R848 and IL-3, n = 4). *P < .05.
MiR-146a impairs expression of components in the NF-κB signaling pathway
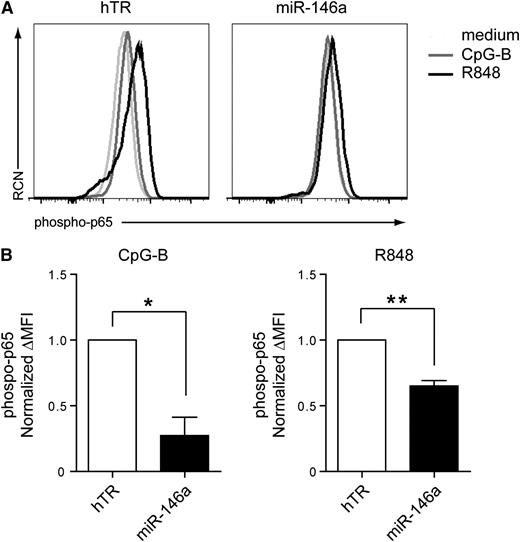
To further characterize the role of miR-146a in pDCs, we transduced CAL-1 cells with a lentiviral vector expressing either miR-146a or hTR as a control. The vector also allows expression of GFP to trace transduced cells by flow cytometry. GFP+ cells were sorted and western blot analysis was performed to assess the protein levels of IRAK1, which was shown to be a direct miR-146a target.22 MiR-146a overexpression resulted in 25% decreased IRAK1 levels as compared with hTR control-transduced cells (supplemental Figure 1). In line with these results, we observed reduced nuclear phospho-p65 (RelA) levels in miR-146a–transduced CAL-1 cells as compared with hTR-transduced cells after stimulation with CpG-B (n = 4, *P = .014) or R848 (n = 4, **P = .004) (Figure 2). This shows that miR-146a inhibits NF-κB activation in response to TLR engagement in pDCs, possibly through targeting of components in the NF-κB pathway, such as IRAK1.
Overexpression of miR-146a in CAL-1 cells blocks TLR-induced NF-κB activation. (A) Flow cytometric analysis of phospho-p65 levels after intracellular staining of CAL-1 cells. CAL-1 cells were transduced with miRNA-146a or with control hTR-expressing vectors, which also drive expression of the marker GFP. Levels of phosphorylation of the NF-κB subunit p65 were measured in GFP-sorted transduced cells after activation for 15 minutes with 10 μg/mL CpG-B (dark gray line), 10 μg/mL R848 (black line), or medium as control (light gray line). RCN, relative cell number. (B) Statistical analysis of phospo-p65 levels was assessed by plotting the difference in mean fluorescence intensity (ΔMFI) between activated cells and medium-cultured cells of 4 different experiments. Data are normalized to phospho-p65 levels in activated hTR-transduced cells, which was set to 1. *P = .014; **P = .004.
Overexpression of miR-146a in CAL-1 cells blocks TLR-induced NF-κB activation. (A) Flow cytometric analysis of phospho-p65 levels after intracellular staining of CAL-1 cells. CAL-1 cells were transduced with miRNA-146a or with control hTR-expressing vectors, which also drive expression of the marker GFP. Levels of phosphorylation of the NF-κB subunit p65 were measured in GFP-sorted transduced cells after activation for 15 minutes with 10 μg/mL CpG-B (dark gray line), 10 μg/mL R848 (black line), or medium as control (light gray line). RCN, relative cell number. (B) Statistical analysis of phospo-p65 levels was assessed by plotting the difference in mean fluorescence intensity (ΔMFI) between activated cells and medium-cultured cells of 4 different experiments. Data are normalized to phospho-p65 levels in activated hTR-transduced cells, which was set to 1. *P = .014; **P = .004.
MiR-146a overexpression induces apoptosis in CAL-1 cells
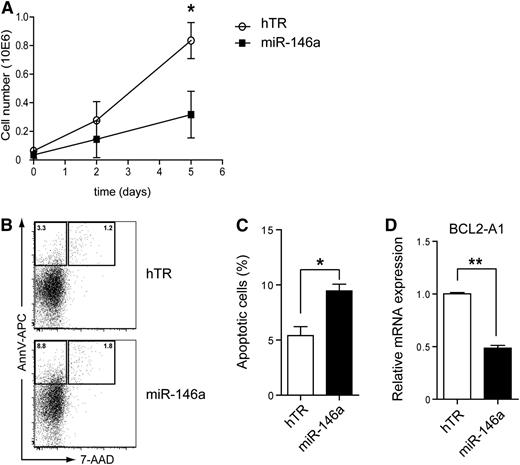
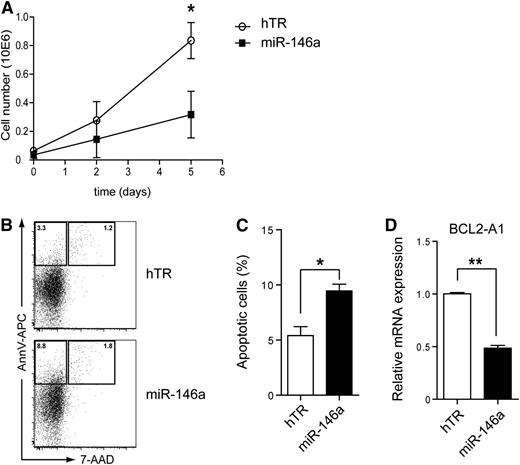
The antiapoptotic gene BCL2-A1 is a direct target of Spi-B involved in human pDC development and survival.27 In addition, NF-κB directly induces BCL2-A1 expression.31 This prompted us to investigate the role of miR-146a in pDC survival. CAL-1 cells, which depend on constitutive NF-κB activity for their survival (supplemental Figure 2), were transduced with lentiviral vectors marked with GFP to overexpress miR-146a or hTR as a control. The absolute number of GFP+ cells as measured by flow cytometry was reduced when miR-146a–transduced CAL-1 cells were cultured over time as compared with control-transduced CAL-1 cells (Figure 3A). Furthermore, the percentages of apoptotic cells as assessed by flow cytometry after Annexin V and 7-AAD staining was on average 1.8-fold higher when CAL-1 cells were cultured after transduction with miR-146a as compared with hTR control (Figure 3B-C; n = 3, *P < .05). A similar increase in percentage of apoptotic miR-146a–transduced CAL-1 cells was notable at several time points analyzed (supplemental Figure 3). Consistent with this, BCL2-A1 mRNA levels were significantly lower in GFP-sorted CAL-1 cells in which miR-146a was overexpressed as compared with hTR control cells (Figure 3C; n = 4, **P < .01). Thus, our data provide evidence for a specific role of miR-146a in the induction of apoptosis in pDCs, which may correlate with downregulation of BCL2-A1 levels.
MiR-146a overexpression induces apoptosis of CAL-1 cells. (A) CAL-1 cells were transduced with hTR control RNA (open symbols) or miR-146a (closed symbols) and cultured in medium. GFP expression was determined at the indicated days by flow cytometry. Shown are the absolute numbers of GFP+ CAL-1 cells. (B) GFP+ cells were sorted and cultured for 2 days. Cells were stained with Annexin V and 7-AAD to assess for apoptosis. Numbers in the dot plots represent percentages of cells that fall within the indicated gates. (C) The percentages of apoptotic cells were determined as in panel B. Shown are mean percentages ± SD of 3 independent experiments (*P < .05). (D) QPCR analysis of BCL2-A1 mRNA levels in CAL-1 cells transduced with hTR control or with miR-146a. Expression levels in hTR-transduced CAL-1 cells were set to 1. Shown are mean values ± SD of an analysis of QPCR done in triplicate. This is a representative experiment of 4. **P < .01.
MiR-146a overexpression induces apoptosis of CAL-1 cells. (A) CAL-1 cells were transduced with hTR control RNA (open symbols) or miR-146a (closed symbols) and cultured in medium. GFP expression was determined at the indicated days by flow cytometry. Shown are the absolute numbers of GFP+ CAL-1 cells. (B) GFP+ cells were sorted and cultured for 2 days. Cells were stained with Annexin V and 7-AAD to assess for apoptosis. Numbers in the dot plots represent percentages of cells that fall within the indicated gates. (C) The percentages of apoptotic cells were determined as in panel B. Shown are mean percentages ± SD of 3 independent experiments (*P < .05). (D) QPCR analysis of BCL2-A1 mRNA levels in CAL-1 cells transduced with hTR control or with miR-146a. Expression levels in hTR-transduced CAL-1 cells were set to 1. Shown are mean values ± SD of an analysis of QPCR done in triplicate. This is a representative experiment of 4. **P < .01.
MiR-146a affects pDC activation and maturation after TLR stimulation
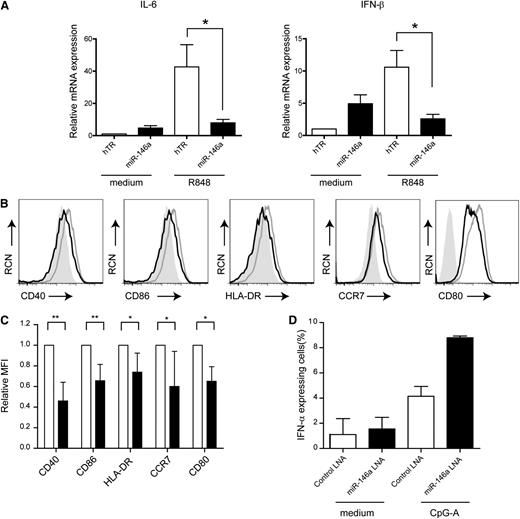
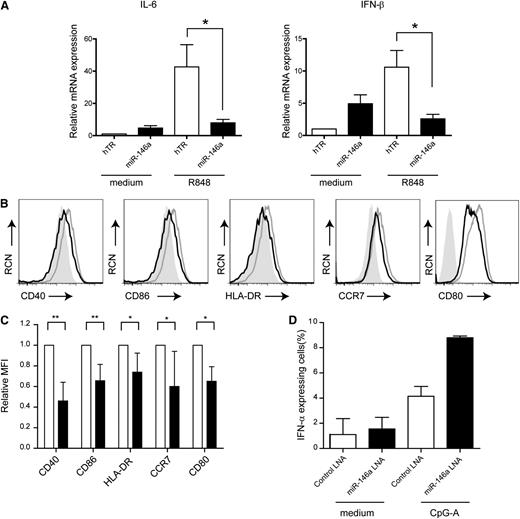
To substantiate the role of miR-146a in CAL-1 cells, we activated the cells with the TLR7 agonist R848 or the TLR9 agonist CpG-B. Sorted GFP+ cells were activated with R848 for 4 hours, and analyzed for IFN-β and IL-6 mRNA levels by QPCR. Notably, ectopic expression of miR-146a resulted in a fourfold decrease in the expression of IFN-β mRNA and a fivefold decrease in IL-6 mRNA levels as compared with TLR7-activated hTR-CAL-1 cells (Figure 4A; n = 2, P < .05). In line with this, IFN-β and IL-6 protein levels as measured by ELISA were reduced in the supernatants of miR-146a CAL-1 cells as compared with control cells (supplemental Figure 4). It was unexpected that IL-6 and IFN-β levels in miR-146a CAL-1 cells cultured in medium are increased as compared with hTR-CAL-1 cells, but as yet this remains unexplained. Furthermore, flow cytometric analysis revealed that miR-146a CAL-1 cells after electronic gating on GFP+ cells were significantly impaired to upregulate CD40, CD80, CD86, HLA-DR, and CCR7 after TLR7 stimulation as compared with control cells (Figure 4B-C; n = 4-6, *P < .05, **P < 0.01). A similar reduction in expression of CD40 and CD80 was observed after TLR9 stimulation, though no difference in CD86 and HLA-DR expression and no consistent effect on CCR7 expression was observed between miR-146a cells and control cells (supplemental Figure 5).
Ectopic miR-146a expression affects pDC activation and maturation. (A) QPCR analysis of IL-6 and IFN-β mRNA levels in CAL-1 cells transduced with hTR control or with miR-146a cultured in medium or activated with TLR7 ligand R848 (10 μg/mL) for 4 hours. Shown are mean values ± SD of 2 independent experiments. The values for hTR-transduced cells cultured in medium are set to 1. *P < .05. (B) Flow cytometric analysis of CAL-1 cells after transduction with hTR control (gray line) or miR-146a (black line). Cells were activated overnight with the TLR7 ligand R848 and stained for expression of CD40, CD80, CD86, HLA-DR, and CCR7. The filled gray histograms represent isotype control antibody-stained cells. RCN, relative cell number. (C) Cells were analyzed as in panel B. Statistical analysis of the relative MFIs ± SD of 4 to 6 different experiments. MFIs of proteins expressed on hTR control cells are set to 1. *P < .05; **P < .01. (D) Primary pDCs isolated from blood were transfected with FITC-conjugated LNA-miR146a or LNA-control followed by activation with CpG-A (10 μg/mL) or medium only for 18 hours. Cells were analyzed by flow cytometry after staining with an anti–IFN-α antibody or isotype control antibody. Shown are the mean percentages ± SD of IFN-α–expressing pDCs of 2 independent experiments.
Ectopic miR-146a expression affects pDC activation and maturation. (A) QPCR analysis of IL-6 and IFN-β mRNA levels in CAL-1 cells transduced with hTR control or with miR-146a cultured in medium or activated with TLR7 ligand R848 (10 μg/mL) for 4 hours. Shown are mean values ± SD of 2 independent experiments. The values for hTR-transduced cells cultured in medium are set to 1. *P < .05. (B) Flow cytometric analysis of CAL-1 cells after transduction with hTR control (gray line) or miR-146a (black line). Cells were activated overnight with the TLR7 ligand R848 and stained for expression of CD40, CD80, CD86, HLA-DR, and CCR7. The filled gray histograms represent isotype control antibody-stained cells. RCN, relative cell number. (C) Cells were analyzed as in panel B. Statistical analysis of the relative MFIs ± SD of 4 to 6 different experiments. MFIs of proteins expressed on hTR control cells are set to 1. *P < .05; **P < .01. (D) Primary pDCs isolated from blood were transfected with FITC-conjugated LNA-miR146a or LNA-control followed by activation with CpG-A (10 μg/mL) or medium only for 18 hours. Cells were analyzed by flow cytometry after staining with an anti–IFN-α antibody or isotype control antibody. Shown are the mean percentages ± SD of IFN-α–expressing pDCs of 2 independent experiments.
To enforce our findings in a more physiologically relevant manner, we silenced miR-146a activity using an LNA that specifically binds miR-146a. We transfected primary human pDCs with an FITC-conjugated LNA-miR-146a or LNA-control miRNA. After stimulation with the TLR9 agonist CpG-A, a known inducer of type I IFNs, we observed a 2.2-fold increase in the percentage of IFN-α–expressing pDCs when LNA-miR-146a was transfected as compared with LNA-control miRNA (Figure 4D). Collectively, these data support the notion that miR-146a controls the TLR-induced upregulation of costimulatory molecules, major histocompatibility complex II, and proinflammatory cytokines in pDCs.
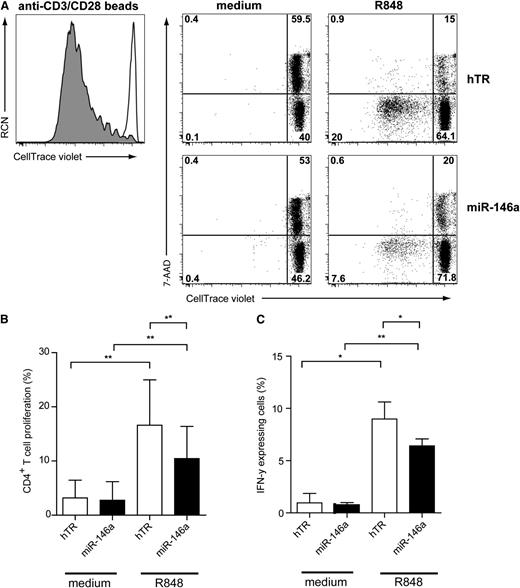
CAL-1 cells ectopically expressing miR-146a are impaired in inducing alloreactive T-cell proliferation
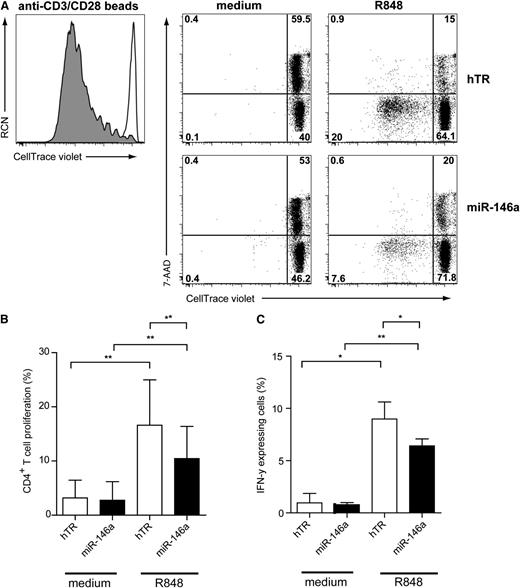
Because maturation of miR-146a–transduced CAL-1 after TLR7 stimulation was impaired as compared with hTR-control–transduced cells, we investigated their ability to activate CD4+ T cells. Therefore, in vitro allogeneic T-cell stimulation assays using transduced CAL-1 cells were performed. Sorted GFP+ CAL-1 cells were preactivated for 48 hours with R848, irradiated to avoid further cell growth, and cocultured with resting allogeneic CD4+ T cells for 6 days. T-cell proliferation was assessed by measuring the loss of the CellTrace Violet membrane dye upon cell division as detected by flow cytometry (Figure 5A). We included 7-AAD in the staining to measure cell death. We observed that TLR7-preactivated CAL-1 cells induced T-cell proliferation and prevented T-cell death as compared with medium-cultured CAL-1 cells (Figure 5A, upper panels). Allogeneic T-cell proliferation induced by TLR7-activated CAL-1 cells was reduced compared with polyclonal stimulation using anti-CD3/CD28 beads (Figure 5A, left panel). Strikingly, miR-146a CAL-1 cells preactivated by the TLR7 agonist were less capable of inducing T-cell proliferation (Figure 5A: 7.6%) as compared with activated hTR CAL-1 cells (Figure 5A: 20%). Like nonactivated hTR-transduced CAL-1 cells, nonactivated miR-146a-transduced CAL-1 cells were also unable to induce T-cell proliferation. When analyzing several independent donors (n = 6), we observed a low, but significant, reduction in T-cell stimulatory capacity of miR-146a CAL-1 cells as compared with hTR CAL-1 cells (Figure 5B; n = 6, *P < .01). To assess whether CD4+ T cells were affected in their capacity to produce cytokines, we analyzed intracellular IFN-γ levels by flow cytometry upon restimulation of the T cells after 6 days (Figure 5C). We observed a reduced percentage of IFN-γ–producing CD4+ T cells after coculture with miR-146a CAL-1 cells as compared with control CAL-1 cells (n = 3, *P < .05). Taken together, these results suggest that ectopic expression of miR-146 impairs efficient TLR-induced maturation of pDCs resulting in compromised ability to activate CD4+ T cells.
MiR-146a inhibits CD4+T-cell proliferation induced by TLR7-preactivated CAL-1 cells. (A) Flow cytometric analysis of T-cell proliferation induced by CAL-1 cells transduced with hTR control RNA or with miR-146a–expressing vectors that were preactivated for 48 hours with or without TLR7 agonist R848 (10 μg/mL). Preactivated CAL-1 cells were cocultured together with freshly isolated allogeneic CD4+ T cells (ratio CAL-1:CD4+ T cells = 1:1) after labeling with the CellTrace Violet membrane dye. After 6 days, T cells were analyzed for expression of CellTrace Violet and 7-AAD in CD3+ T cells. Percentages of CellTrace-Violetlo7-AAD−CD3+ cells represent T cells that proliferated and are alive (lower left quadrant). CD4+ T cells activated with anti-CD3/CD28 beads are shown as a positive control for proliferation (gray histogram) as compared with CD4+ T cells cultured only with medium (white histogram). Shown is 1 representative experiment of 3. Numbers in plots represent percentages of cells that fall within the indicated quadrant. (B) Cells were analyzed as in panel A. Statistical analysis of CD4+ T-cell proliferation of 3 independent experiments. Only the mean percentages ± SD of CellTrace-Violetlo7-AAD−CD3+ T cells are depicted. *P < .05. (C) After 6 days of coculture, CD4+ T cells were restimulated with PMA/ionomycin for 6 hours in the presence of Brefeldin A and analyzed by flow cytometry for IFN-γ expression. Shown are the mean percentages of IFN-γ+ T cells of 1 representative of 3 independent experiments. Error bars indicate SD values of measurements done in triplicate.
MiR-146a inhibits CD4+T-cell proliferation induced by TLR7-preactivated CAL-1 cells. (A) Flow cytometric analysis of T-cell proliferation induced by CAL-1 cells transduced with hTR control RNA or with miR-146a–expressing vectors that were preactivated for 48 hours with or without TLR7 agonist R848 (10 μg/mL). Preactivated CAL-1 cells were cocultured together with freshly isolated allogeneic CD4+ T cells (ratio CAL-1:CD4+ T cells = 1:1) after labeling with the CellTrace Violet membrane dye. After 6 days, T cells were analyzed for expression of CellTrace Violet and 7-AAD in CD3+ T cells. Percentages of CellTrace-Violetlo7-AAD−CD3+ cells represent T cells that proliferated and are alive (lower left quadrant). CD4+ T cells activated with anti-CD3/CD28 beads are shown as a positive control for proliferation (gray histogram) as compared with CD4+ T cells cultured only with medium (white histogram). Shown is 1 representative experiment of 3. Numbers in plots represent percentages of cells that fall within the indicated quadrant. (B) Cells were analyzed as in panel A. Statistical analysis of CD4+ T-cell proliferation of 3 independent experiments. Only the mean percentages ± SD of CellTrace-Violetlo7-AAD−CD3+ T cells are depicted. *P < .05. (C) After 6 days of coculture, CD4+ T cells were restimulated with PMA/ionomycin for 6 hours in the presence of Brefeldin A and analyzed by flow cytometry for IFN-γ expression. Shown are the mean percentages of IFN-γ+ T cells of 1 representative of 3 independent experiments. Error bars indicate SD values of measurements done in triplicate.
Discussion
In the present study, we highlight the immunomodulatory role of miR-146a during human pDC activation and maturation induced by TLR engagement. We show that miR-146a expression was induced in primary pDCs upon TLR7 and TLR9 triggering. Furthermore, ectopic expression of miR-146a in the CAL-1 pDC model cell line downregulated expression of IRAK1, enforcing earlier findings that the transcript encoding this protein is a bona fide target of miR-146a.22 Overexpression of miR-146a increased the level of apoptosis induction in CAL-1 cells, which correlated with lower BCL2-A1 mRNA levels, as well as impaired expression of costimulatory molecules and proinflammatory cytokines in response to TLR ligation. Consistent with this, miR-146a–transduced CAL-1 cells after activation with the TLR7 agonist R848 were hampered to induce optimal T-cell expansion in an allogeneic setting as compared with control-transduced cells.
It has been demonstrated that miR-146a was induced upon TLR2, TLR4, or TLR5 signaling in the human acute monocytic leukemia cell line THP.22 While miR-146a expression was not affected after ligation of TLR3, TLR7, and TLR9 in this cell line,22 others reported that miR-146a expression was induced in PBMCs upon TLR7 and TLR9 triggering.25 It is currently unclear how to interpret the differential TLR7/9 responsiveness, but it may be attributed to the fact that in one study, a monocytic cell line22 was used, and in the other, case primary PBMCs were used.25 Our results here confirm and extend the findings in primary cells as we demonstrated that miR-146a expression is induced in freshly isolated pDCs both in response to the TLR7 ligand R848 as well as the TLR9 ligands CpG-A and B. Our results are consistent with the notion that in human peripheral blood only pDCs selectively express TLR7 and TLR9.32 To more extensively analyze the role of miR-146a in pDCs, we used the leukemic pDC cell line CAL-1.26 Recently, we reported that this cell line has many phenotypical and functional overlapping characteristics compared with primary pDCs, including their responsiveness to TLR7 and TLR9 ligation, which validates the use of CAL-1 cells to study the role of miRNAs in gene regulation in pDCs.27
In line with earlier observations that IRAK1, a key adaptor molecule in the TLR signaling cascade required for activation of NF-κB, is a target of miR-146a,22 we show that IRAK1 levels were reduced in miR-146a CAL-1 cells. As expected. this correlated with decreased levels of phospho-p65 induced by activation of both TLR7 and TLR9 signaling pathways. Moreover, this correlated with reduced levels of BCL2-A1, costimulatory molecules, and cytokines, which all depend on NF-κB activity in pDCs.
An important role of miR-146a in regulating myeloid and lymphoid development, growth/proliferation, and survival of leukocytes in mice was demonstrated in miR-146a−/− mice.23,24 These miR-146a−/− mice suffered from severe splenomegaly, which was mostly due to increased myeloid proliferation in comparison with wild-type animals. Interestingly, several studies in humans reported that the levels of miR-146a were reduced in various acute myeloid leukemias (AMLs) as compared with healthy CD34+ progenitor cells.33,34 When enforcing expression of miR-146a in these AML cell lines, this resulted in a significant block in cell proliferation and in the induction of apoptosis, thereby implicating this miRNA in the pathogenesis of AML.34 The mechanism underlying these observations remained unaddressed. Interestingly, our data demonstrated that overexpression of miR-146a in CAL-1 cells induced apoptosis as well, and correlated with reduced levels of BCL2-A1 as compared with control-transduced cells. While we speculated that miR-146a may control cell survival through direct targeting of this antiapoptotic gene, using the in silico prediction software TargetScan (release 6.2, www.targetscan.org), no miR-146a binding sites in the 3′-UTR of the BCL2-A1 mRNA were found (data not shown). We also studied whether the transcript of Spi-B is targeted by miR-146a because Spi-B is highly expressed in pDCs and directly regulates BCL2-A1 gene expression.27 Despite the fact that the 3′-UTR of Spi-B mRNA is predicted to contain miR-146a binding sites, however, we were unable to observe a consistent reduction in Spi-B protein levels in miR-146a CAL-1 cells (J.J.K., unpublished findings). This, together with the notion that bona fide targets of miR-146a are TRAF6 and IRAK1,22 suggests that in pDCs, and possibly also AML, cell survival by miR-146a is more likely to be regulated at the level of NF-κB activation instead of its target genes.
Excessive or inappropriate immune activation can be deleterious for the organism, requiring the need for various molecular mechanisms ensuring tight control of the immune response. A significant number of studies have linked alteration of miR-146a expression with initiation, development, and severity of several autoimmune diseases.35 Studies in mice showed that the serum of aged miR-146a−/− mice contained on average a 60-fold higher titer of autoantibodies directed against double-stranded DNA as compared with wild-type animals.23 This, together with the observation that these mice develop a pathological condition, strongly suggests the involvement of miR-146a in preventing autoimmune disease. In humans, miR-146a expression levels in PBMCs isolated from SLE patients were found to be decreased as compared with healthy control individuals.25 Furthermore, low miRNA-146a levels were correlated with increased severity of the disease. Given the accepted role of pDCs in the pathogenesis of SLE via production of elevated levels of type I IFNs,9 it has been speculated that downregulation of miR-146a in these cells may be one of the leading mechanisms driving their uncontrolled activation. This notion is enforced by our results showing that miR-146a controls the levels of type I IFNs in pDCs. Conversely, contrasting data were reported on patients suffering from SS and rheumatoid arthritis (RA), 2 autoimmune diseases in which pDCs have been implicated as well.11,12,36 These studies showed that miR-146a was overexpressed in PBMCs37,38 and synovial fluid,39 as compared with healthy control individuals. It is noteworthy that the levels of miR-146a in RA and SS patients have only been analyzed in total PBMCs and not in individual cell types, including pDCs. As pDCs represent only a minor population of total PBMCs, a putative downregulation of miR-146a specifically in pDCs of these patients may have remained unnoticed. Moreover, miR-146a levels are increased in CD4+ T cells from RA patients,40 and since the number of CD4+ T cells in blood is much higher compared with pDCs, this more likely reflects the observed difference when analyzing total PBMCs. Furthermore, elevated miR-146a levels in total PBMCs may be the consequence of chronic inflammation leading to constitutive NF-κB activation. Hence, it is important to perform expression profiling of miRNAs, but also of mRNAs, in purified leukocyte subsets to correlate the level of expression to their putative role in a disease setting.
Previously, it was demonstrated that T cells from lupus patients exhibited a marked increase in proliferation as assessed by Ki67 staining.41 Hence, T cells in these patients may have received substantial activation signals in vivo. The molecular mechanisms underlying excessive T-cell activation in SLE have not been fully understood, but may be attributed to abnormal CTLA-4 expression in lupus responder T cells41 or aberrant signaling downstream of the T-cell receptor.42 Here, we provide evidence that miR-146a regulates the maturation status of pDCs, thereby impairing their capacity to stimulate proliferation of T cells. Hence, it is reasonable to assume that underexpression of miR-146a in pDCs, as observed in lupus,25 leads to increased levels of costimulatory molecules on pDCs that will enhance T-cell proliferation. Therefore, reduced levels of miR-146a in lupus pDCs may not only drive the overproduction of type I IFNs, but in addition may increase the expansion of lupus T cells. These T cells may subsequently contribute in the generation of autoantibody-producing B cells.
Collectively, our results further clarify the crucial role of miR-146a as a brake of the TLR-induced activation and maturation specifically in pDCs. Given the growing evidence indicating that pDCs are key players in the initiation and/or maintenance of autoimmune diseases, our work enforces that therapies aimed at targeting the miR-146a–dependent regulation of immunity and inflammation in pDCs has potential for future interventions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof R. Agami for providing the miRNA-expressing vectors (Division of Tumor Biology, The Netherlands Cancer Institute, Amsterdam, The Netherlands). The authors thank Berend Hooibrink and Toni van Capel for maintaining the FACS facility. The authors acknowledge Prof Dr Hazekamp, and staff from the Leiden University Medical Center (Leiden, The Netherlands), for providing human thymus tissue. The authors thank Dr R. Schotte for critically reading the manuscript and Femke Muller for technical assistance.
J.J.K. is supported through a personal Innovational Research Incentives Scheme (VIDI) grant to B.B. (Dutch Science Foundation, no. 917.66.310). L.C.M.J. and C.H.U. are supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI 080564, University of California Los Angeles, Center for AIDS Research).
Authorship
Contribution: J.J.K. and L.C.M.J. designed research, performed experiments, analyzed data, and wrote the manuscript; M.L., A.I., K.B. M.N., and E.W.T.-K. performed experiments and analyzed data; E.C.d.J. and C.H.U. analyzed data; and B.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bianca Blom, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; e-mail: b.blom@amc.uva.nl.
References
Author notes
J.J.K. and L.C.M.J. contributed equally to this study.