Key Points
The inhibitory effect of platelet function by statins results, at least in part, in action on PECAM-1.
Statins modulate Lyn activation and PECAM-1 tyrosine phosphorylation, resulting in the inhibition of downstream PI3K–dependent signaling.
Abstract
Statins are widely prescribed cholesterol-lowering drugs that are a first-line treatment of coronary artery disease and atherosclerosis, reducing the incidence of thrombotic events such as myocardial infarction and stroke. Statins have been shown to reduce platelet activation, although the mechanism(s) through which this occurs is unclear. Because several of the characteristic effects of statins on platelets are shared with those elicited by the inhibitory platelet adhesion receptor PECAM-1 (platelet endothelial cell adhesion molecule-1), we investigated a potential connection between the influence of statins on platelet function and PECAM-1 signaling. Statins were found to inhibit a range of platelet functional responses and thrombus formation in vitro and in vivo. Notably, these effects of statins on platelet function in vitro and in vivo were diminished in PECAM-1−/− platelets. Activation of PECAM-1 signaling results in its tyrosine phosphorylation, the recruitment and activation of tyrosine phosphatase SHP-2, the subsequent binding of phosphoinositol 3-kinase (PI3K), and diminished PI3K signaling. Statins resulted in the stimulation of these events, leading to the inhibition of Akt activation. Together, these data provide evidence for a fundamental role of PECAM-1 in the inhibitory effects of statins on platelet activation, which may explain some of the pleiotropic actions of these drugs.
Introduction
Platelet activation, aggregation, and thrombosis associated with unstable atherosclerotic lesions represent a serious risk for individuals with coronary artery disease. The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are widely prescribed cholesterol-lowering drugs that are a first-line treatment of coronary artery disease and atherosclerosis, reducing the incidence of thrombotic events such as myocardial infarction and stroke.1-3 Previous reports have also demonstrated antithrombotic activity in hypercholesterolemic patients by statins, but these effects do not correlate with the lipid-lowering activities of these drugs,4-9 Together, this suggests that statins exhibit pleiotropic effects above and beyond their regulation of low-density lipoprotein (LDL) cholesterol levels, which may contribute to their role in decreasing cardiovascular mortality and morbidity.1,10
Statins have been reported to promote improvements in endothelial function and to decrease vascular inflammation and smooth muscle cell proliferation.11-14 Studies to explore the molecular basis of the pleiotropic actions of statins on platelets have been limited to exploring the cholesterol content of platelet membranes, inhibition of thromboxane A2 formation, and increase of nitric oxide (NO) bioavailability by upregulation of endothelial NO synthase with downregulation of markers of platelet reactivity.15-21 The precise mechanisms of drug action, however, are not fully understood.
Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a signaling molecule that plays diverse roles in vascular biology, including modulation of platelet function,22-25 angiogenesis,26 vasculogenesis,27 integrin regulation,28 T-cell and B-cell activation,29 and mediation of leukocyte migration across the endothelium.30 This homophilic receptor functions as a negative regulator of platelet reactivity and thrombosis, at least in part by inhibiting glycoprotein VI (GPVI)–Fc receptor γ-chain collagen receptor signaling following recruitment of protein tyrosine phosphatase-2 (SHP-2) to phosphorylated immunoreceptor tyrosine-based inhibitory motifs in the PECAM-1 cytoplasmic tail.24,31,32 The mechanism underlying PECAM-1–mediated inhibition of GPVI-specific responses results in the recruitment of phosphatidylinositol 3-kinase (PI3K) to PECAM-1–bound SHP-2 complexes, which destabilizes the PI3K association with the activatory signaling molecules Grb-2–associated binding protein-1 (Gab1) and linker for the activation of T cells leading to diminish PI3K signaling.33 Statins have been reported to affect platelet activation by a reduction of platelet thromboxane A2 (TXA2) formation and intracellular platelet calcium, resulting in diminished downstream signaling.16,34,35 Because the inhibition of platelet activation by PECAM-1 cross-linking is also associated with decreased calcium mobilization and TXA2 synthesis, thereby diminishing downstream signaling, in this present study, we investigated whether the effects of statins on platelets are mediated through PECAM-1 signaling.
Methods
Reagents
Detailed reagents for human and mouse platelet aggregation, dense granule secretion, immunoprecipitation, immunoblotting, flow cytometry, and in vivo and in vitro thrombus formation are provided in supplemental Methods (available on the Blood Web site). All protocols involving the use of animals were approved by the University of Reading Local Ethical Review Panel and authorized by a Home Office License.
Human washed platelet preparation, aggregation, dense granule secretion, and immunoblotting
Washed platelets were prepared from fresh blood obtained from healthy, aspirin-free human volunteers for which approval was obtained from the University of Reading Research Ethics Committee. Informed consent was provided according to the Declaration of Helsinki. Platelets were prepared and resuspended in modified Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer to a final density of 4 × 108 cells mL−1 for aggregation assays as described previously.33,36,37 Aggregation studies were performed at 37°C in an optical platelet aggregometer (Chronolog). ATP secretion assays were performed as described previously.37 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting were performed using standard protocols as described previously.33,37
Mouse platelet preparation and aggregation
Blood was obtained from PECAM-1 knockout and control mice via cardiac puncture after termination. Blood (1 mL) was drawn into a syringe containing acidic citrate dextrose as anticoagulant. Platelets were prepared from whole blood by differential centrifugation in the presence of prostacyclin (0.1 μg mL−1), and resuspended in modified Tyrode-HEPES buffer to a final density of 2.5 × 108 cells mL−1 for aggregation assays as described previously.33
Flow cytometric analysis: α-granule secretion and fibrinogen binding to integrin αIIbβ3
Flow cytometry analysis was performed using whole blood with increasing concentrations of statins prior to stimulation with collagen-related peptide (CRP-XL) (1 μg mL−1) at room temperature for 20 minutes with fluorescein isothiocyanate–labeled fibrinogen and phycoerythrin/Cy5 anti-human–CD62P (P-selectin) as described previously.37 Flow cytometric acquisition was performed using a FACSCalibur device, and data were collected from 5000 events analyzed using CellQuest Pro software (Version 3.3; BD Biosciences). Negative controls were set using an appropriate immunoglobulin G1 (IgG1) κ-isotype–matched control for the anti-CD62P antibody, and inclusion of EGTA (10 μM) to prevent fibrinogen binding.
In vitro thrombus formation
Analysis of thrombus formation under arterial flow conditions was performed as described previously.37,38 Briefly, the DIOC6-labeled human citrated blood was preincubated with vehicle control or increasing concentrations of statins and perfused over a collagen-coated Vena8 BioChip (Cellix Ltd) at a shear rate of 20 dyne/cm2. Z-stack images of thrombi were obtained for every 30 seconds for up to 10 minutes using a Nikon Eclipse microscope (TE2000-U; Nikon Instruments). Thrombus volume and fluorescence intensity were calculated by analyzing data using Slidebook5 (Intelligent Imaging Innovations).
Analysis of thrombosis
Thrombus formation in mice and data analysis were performed as described previously.25,37,38 Briefly, C57BL/6 and PECAM-1 knockout mice were anesthetized by intraperitoneal injection of ketamine (125 mg/kg), xylazine (12.5 mg/kg), and atropine (0.25 mg/kg). Anesthesia was maintained with 5 mg/kg pentobarbital as required and the mouse circulation was accessed via a cannulus placed in the jugular vein, and the platelets were labeled with Alexa 488–conjugated anti-mouse–GPIbβ antibody. The cremaster muscle was exteriorized, connective tissue removed, and an incision was made to allow the muscle to be affixed as a single sheet over a glass slide. Injury to cremaster arterioles was induced with a Micropoint Ablation Laser Unit (Andor Technology PLC). Thrombi were observed using an upright Olympus BX microscope. Images were captured prior to and after the injury by a Hamamatsu charged-coupled device digital camera C9300 in 640 × 480 format (Hamamatsu Photonics UK Ltd.) and analyzed using slidebook5 software (Intelligent Imaging Innovations).
Statistical analysis
Normally distributed data were analyzed using analysis of variance and t test. The nonparametric Mann-Whitney U test was used to analyze nonnormally distributed data.
Results
Statins inhibit platelet function in a concentration-dependent manner
The effect of fluvastatin and simvastatin on platelet aggregation in response to a range of activators of platelet function was explored. Platelet aggregation in response to CRP-XL (0.5 μg mL−1), a GPVI-selective ligand, was found to be inhibited in a concentration-dependent manner by each of the statins (Figure 1A-B). Inhibition of 10%, 33%, and 62% was observed at 90 seconds with fluvastatin (1, 3, and 10 μM), which was slightly more potent than simvastatin in inhibiting platelet aggregation, which may result from differential ability to cross the plasma membrane. Aggregation monitored over an extended period of 5 minutes’ duration confirmed this effect to be inhibition rather than delay in aggregation. Lower levels of inhibition were noted with thrombin-induced platelet aggregation (0.05 U mL−1) (supplemental Figure 1). Thrombin concentration was optimized to ensure a similar level of aggregation to that stimulated by CRP-XL (0.5 μg mL−1). Studies have reported that statins inhibit platelet function by increasing cyclic guanosine monophosphate and cyclic adenosine monophosphate.20,21 The effects of fluvastatin on platelet aggregation in the presence of NO and NO-sensitive soluble guanylyl cyclase inhibitors (L-NAME and ODQ, respectively) upon stimulation with CRP-XL (0.5 μg mL−1) were therefore analyzed. Concentration-dependent inhibition of platelet aggregation by fluvastatin (1-40 μM) was maintained in the presence of such inhibitors (supplemental Figure 2), suggesting alternative mechanisms by which statins inhibit platelet activation.
Statins inhibit platelet activation. Washed human platelets were treated for 5 minutes with increasing concentration of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1) and aggregation measured at 37°C under constant stirring conditions. Numerical data represent the percentage aggregation compared with control, mean ± SEM (n = 4) (A-B). The effect of simvastatin or fluvastatin on fibrinogen binding and P-selectin exposure prior to stimulation with CRP-XL (1 μg mL−1) was measured in human whole blood by flow cytometry. Numerical data represent the percentage of fibrinogen binding and P-selectin exposure compared with control, mean ± SEM (n = 4) (C-D). Washed human platelets were treated for 5 minutes with increasing concentrations of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1). Changes in ATP concentration were used as a measure of dense-granule secretion and monitored simultaneously with aggregation in an optical lumi-aggregometer using a luciferase detection system. Numerical data represent the percentage of ATP release compared with control, mean ± SEM (n = 4) (E-F). Platelets stimulated with CRP-XL (1 μg mL−1) in the presence or absence of simvastatin were analyzed by immunoblotting with anti-phosphotyrosine antibody (4G10) and anti-phospho-site antibodies for Syk (Y323), PLC (Y1197), Fyn (Y59), and Lyn (Y396) (G-I). Blots are representative of 4 different experiments (n = 4), t test, P > .05 (nonsignificant [NS]), *P < .05, **P < .01.
Statins inhibit platelet activation. Washed human platelets were treated for 5 minutes with increasing concentration of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1) and aggregation measured at 37°C under constant stirring conditions. Numerical data represent the percentage aggregation compared with control, mean ± SEM (n = 4) (A-B). The effect of simvastatin or fluvastatin on fibrinogen binding and P-selectin exposure prior to stimulation with CRP-XL (1 μg mL−1) was measured in human whole blood by flow cytometry. Numerical data represent the percentage of fibrinogen binding and P-selectin exposure compared with control, mean ± SEM (n = 4) (C-D). Washed human platelets were treated for 5 minutes with increasing concentrations of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1). Changes in ATP concentration were used as a measure of dense-granule secretion and monitored simultaneously with aggregation in an optical lumi-aggregometer using a luciferase detection system. Numerical data represent the percentage of ATP release compared with control, mean ± SEM (n = 4) (E-F). Platelets stimulated with CRP-XL (1 μg mL−1) in the presence or absence of simvastatin were analyzed by immunoblotting with anti-phosphotyrosine antibody (4G10) and anti-phospho-site antibodies for Syk (Y323), PLC (Y1197), Fyn (Y59), and Lyn (Y396) (G-I). Blots are representative of 4 different experiments (n = 4), t test, P > .05 (nonsignificant [NS]), *P < .05, **P < .01.
Platelet aggregation is dependent on conformational changes on integrin αIIbβ3 through inside-out signaling to increase its affinity for fibrinogen binding.37 Flow cytometry was used to measure fibrinogen binding to platelets, as marker for activation of the integrin αIIbβ3. Human whole citrated blood was stimulated with CRP-XL (1 μg mL−1) in the absence or presence of different concentrations of statins. CRP-XL–stimulated fibrinogen binding was reduced in the presence of either fluvastatin or simvastatin in a concentration-dependent manner (Figure 1C), consistent with reduced aggregation and indicating the ability of these statins to interfere with inside-outside signaling to integrin αIIbβ3 in platelets.
Thrombus generation is supported and enhanced by the release of a number of substances from the α and dense granules stored within platelets, which are critical for the recruitment of additional platelets and for the stabilization of the aggregate.25,37,38 To analyze the effects of statin treatment on platelet granule secretion, both α and dense-granule secretion were assayed in the absence and presence of statins. α-granule secretion was assessed by measuring the levels of P-selectin exposed on the surface of platelets after stimulation with CRP-XL (1 μg mL−1) by flow cytometry in human whole blood. P-selectin exposure was inhibited by either statin in a concentration-dependent manner (Figure 1D). Similarly, ATP secretion from the dense granules was measured using a luciferin-luciferase luminescence. Simvastatin or fluvastatin were found to reduce ATP secretion after CRP-XL stimulation (Figure 1E-F). Lower levels of inhibition of ATP release were found with statins treatment followed by stimulation with thrombin (0.05 U mL−1) (supplemental Figure 1).
GPVI signaling results in the sequential assembly and activation of a tyrosine kinase–dependent signaling pathway.22,24,33 Due to the inhibitory effect by statins on CRP-XL–induced aggregation, the phosphorylation levels of proteins involved early in the GPVI signaling pathway were assessed. Platelet lysates were prepared after stimulation with CRP-XL (1 μg mL−1) in the absence or presence of simvastatin (1-10 μM). The levels of platelet protein tyrosine phosphorylation, and the site-specific phosphorylation of the tyrosine kinase Syk and phospholipase C (PLC) γ2 protein (Figure 1G-H) were unaffected after simvastatin treatment, indicating that statins are not involved in the control of GPVI-proximal signaling. Lyn and Fyn are implicated in phosphorylation of PECAM-1 and regulation of its function; indeed, Lyn and PECAM-1 have been reported to act as interdependent inhibitors of platelet function.39 Treatment with simvastatin did not affect the phosphorylation of Fyn, however, it was able to increase Lyn phosphorylation on an activation-dependent site (Y396) (Figure 1I), demonstrating that statins activate Lyn. The precise mechanism(s) through which simvastatin exerts its effects on Lyn, whether through direct effects on Lyn kinase, or upstream signaling molecules, or through enhancing PECAM-1 clustering, remains to be established. The fact that Fyn is not activated by simvastatin may suggest effects on Lyn signaling are more probable. Furthermore, statin may, however, modulate signaling further downstream including signaling steps that are common to platelet stimulation by other agonists, consistent with the ability of these drugs to inhibit thrombin-mediated platelet activation. It is well established that statins inhibit platelet functions through peroxisome proliferator-activated receptors (PPARs).34 To establish whether the effects of statins on PECAM-1 are mediated through PPARs, we performed aggregation assays using the PPARγ antagonist, GW9662 (supplemental Figure 4). Our results suggest that concentrations at which the effects of simvastatin are PECAM-1 dependent (≤10 μM), aggregation was not affected by GW9662. At higher concentrations of statin (>10 μM) where PECAM-1–independent effects are observed, GW9662 caused the inhibition of platelet function to be diminished.
Statins have antithrombotic actions
Thrombosis associated with unstable atherosclerotic plaques represents a serious risk of myocardial infarction for individuals with coronary artery disease.1-3 Given the ability and importance of statins in regulating platelet function, we sought to determine the potential implications of the pleiotropic effects of statins on thrombus formation. Analysis of thrombus formation in vitro was performed by fluorescence microscopy using whole blood under arterial flow conditions (20 dyne/cm2) through Vena8Biochip coated with collagen with and without prior incubation with simvastatin (1-20 μM) for 5 minutes. Captured images were analyzed by calculating the size and number of thrombi formed and the sum intensity of fluorescence. The size, number, and volume of thrombi were reduced by simvastatin over a 10-minute period. The sum intensity of thrombi was decreased by 40% with 1 μM simvastatin following perfusion for 10 minutes (Figure 2A), whereas 10 μM simvastatin was able to inhibit the thrombi by 75% (Figure 2B). Similar data were obtained with fluvastatin (data not shown).
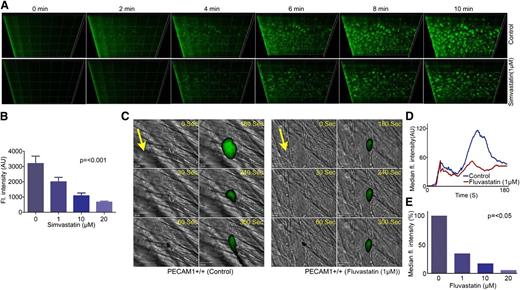
Statins have antithrombotic actions. Human whole blood labeled with the lipophilic dye 3,3′-dihexyloxacarbocyanine iodide was treated with increasing concentrations of simvastatin (1-20 μM) or vehicle for 5 minutes and perfused through collagen-coated (400 μg mL−1) Vena8 Biochips at a shear rate of 20 dyne/cm2. Thrombi were recorded over a 10-minute period through a series of images in the Z-plane through their full depth using a Nikon Eclipse (TE2000-U) microscope, and thrombus fluorescence intensity was calculated using Slidebook, Version 5 (A). Numerical data represent sum fluorescence intensities, mean ± SD of 4 separate experiments (n = 4), P < .001 (nonparametric Kruskal-Wallis global) (B). In vivo thrombosis was assayed using a laser injury model by intravital microscopy. Fluvastatin (1-20 μM) or vehicle was administered intravenously to mice, and platelets were fluorescently labeled by injection of Alexa 488–conjugated anti-GPIb antibody. After laser-induced injury of the cremaster muscle arterioles, accumulation of platelets was assessed. Representative images of thrombi obtained from mice treated with or without fluvastatin (1 μM) at different time intervals are shown. Arrows indicate the direction of the blood flow (C). Median fluorescence intensity was measured from 21 thrombi from 4 mice from each of control and fluvastatin (1-20 μM)–treated groups (D-E). P values calculated by nonparametric Mann-Whitney test (P < .05).
Statins have antithrombotic actions. Human whole blood labeled with the lipophilic dye 3,3′-dihexyloxacarbocyanine iodide was treated with increasing concentrations of simvastatin (1-20 μM) or vehicle for 5 minutes and perfused through collagen-coated (400 μg mL−1) Vena8 Biochips at a shear rate of 20 dyne/cm2. Thrombi were recorded over a 10-minute period through a series of images in the Z-plane through their full depth using a Nikon Eclipse (TE2000-U) microscope, and thrombus fluorescence intensity was calculated using Slidebook, Version 5 (A). Numerical data represent sum fluorescence intensities, mean ± SD of 4 separate experiments (n = 4), P < .001 (nonparametric Kruskal-Wallis global) (B). In vivo thrombosis was assayed using a laser injury model by intravital microscopy. Fluvastatin (1-20 μM) or vehicle was administered intravenously to mice, and platelets were fluorescently labeled by injection of Alexa 488–conjugated anti-GPIb antibody. After laser-induced injury of the cremaster muscle arterioles, accumulation of platelets was assessed. Representative images of thrombi obtained from mice treated with or without fluvastatin (1 μM) at different time intervals are shown. Arrows indicate the direction of the blood flow (C). Median fluorescence intensity was measured from 21 thrombi from 4 mice from each of control and fluvastatin (1-20 μM)–treated groups (D-E). P values calculated by nonparametric Mann-Whitney test (P < .05).
To determine the potential impact of statins on the acute regulation of platelet function in a pathological setting in vivo, the effect of fluvastatin on laser-induced thrombosis in mouse cremaster muscle arterioles was assessed. Parallel experiments were performed to establish that the injury resulted in exposure of subendothelial collagen (data not shown). We chose to use fluvastatin in the in vivo experimental thrombosis model due to the ability of this drug to achieve peak plasma concentrations, in micromolar concentrations, in humans following oral administration, which could implicate potential clinical relevance as an antithrombotic drug.40 The ability of platelets fluorescently labeled with an anti-mouse GPIb Alexa Fluor 488–conjugated antibody to form thrombi was measured. Thrombus formation was monitored over a period of 180 seconds at the site of the injury and the size of thrombi formed was analyzed by calculating the fluorescence intensity. The effect of fluvastatin infused intravenously prior to thrombus formation was explored and compared with thrombosis in vehicle control-treated mice. Data analysis was performed for multiple thrombi formed in 4 control and 4 mice treated with fluvastatin (1-20 µM). The initial adherence of platelets at the site of injury was unaffected in both control and fluvastatin-treated mice (Figure 2C-D). After 100 seconds, thrombus size was reduced by 65% in fluvastatin-treated mice (1 µM), whereas 10 µM fluvastatin was able to inhibit the thrombi by 80% (Figure 2E). Similar levels of inhibition of thrombus formation in the absence of endothelial cells (in vitro model) suggests that the inhibitory effects of statins on thrombi formed in vivo are principally the result of diminished platelet function. The possibility of potential additional effects of statins on the endothelium cannot be ruled out. Indeed, the effect of statin on thrombus formation in vivo (Figure 2C-E) was greater than in vitro (Figure 2A-B) or aggregation (Figure 1B), which is suggestive of the importance of other vascular mechanisms of action. Taken together, these data establish a role of statins in the regulation of thrombosis, which may contribute to the beneficial effects of statin therapies.
Statins modulates PECAM-1 signaling
PECAM-1 is an adhesion receptor responsible for moderate inhibition of platelet function.33 It is likely to be more physiologically important in localities where the endothelium is damaged or diseased, and therefore where local concentrations of NO and prostaglandin I2 are reduced. To assess whether statins play a role in regulating the inhibitory effect of platelet function by PECAM-1 signaling, tyrosine phosphorylation levels of PECAM-1 were measured in human platelets under conditions that disfavor aggregation (presence of EGTA 1 mM, indomethacin 10 mM [to prevent thromboxane A2 synthesis], apyrase 2 U mL−1 [to prevent the actions of secreted ADP]). Simvastatin was found to cause a marked and concentration-dependent increase in the levels of tyrosine phosphorylation of PECAM-1 (Figure 3A). PECAM-1 tyrosine phosphorylation and subsequent activation of signaling molecules is stimulated following PECAM-1 clustering following homophilic ligation, leading to the inhibition of platelet function.22-24 To further explore the role of statins on PECAM-1 signaling, aggregation assays were performed in human washed platelets after incubation with PECAM-1 antibody-mediated clustering (PECAM-1 XL) or isotype control (IgG XL) (0.8 μg mL−1) prior to CRP-XL (0.5 μg mL−1) stimulation in the absence and presence of simvastatin (10 μM). PECAM-1 cross-linking inhibited platelet aggregation upon CRP-XL stimulation by ∼20%, as previously described.33 Treatment with simvastatin (10 μM) in addition to PECAM-1 cross-linking enhanced inhibition of aggregation by ∼20% (Figure 3B). These observations were associated with an increase in the level of tyrosine phosphorylation of PECAM-1 in the presence of simvastatin (Figure 3C), suggesting that the inhibitory effect of statins may be mediated through the initiation or enhancement of PECAM-1 signaling.
Statins modulate PECAM-1 tyrosine phosphorylation. Washed human platelets were incubated with simvastatin or control for 5 minutes under conditions that disfavor aggregation (presence of EGTA [1 mM], indomethacin [10 mM], apyrase [2 U mL-1]). PECAM-1 was immunoprecipitated from cell lysates using anti–PECAM-1 antibody (WM59) and immunoblotted with an anti-phosphotyrosine antibody (4G10). Blots are representative of 3 separate experiments and normalized to loading control. Numerical data represent the percentage increase above phosphorylation levels in nontreated platelets (A). Washed human platelets were treated with PECAM-1 antibody to mediate clustering (PECAM-1 XL, 0.8 μg mL-1) or isotype control (IgG-XL, 0.8 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (10 μM) and aggregation was measured (B). Washed platelets were incubated with PECAM-1 antibody to mediate clustering (PECAM-1 XL) or isotype control (IgG-XL) (1 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (5-10 μM). PECAM-1 was immunoprecipitated from cell lysates. Immunoprecipitates were divided in 2, with half probed for tyrosine phosphorylation and half probed for PECAM-1, to ensure equivalent levels of protein isolation between samples. Blots are representative of 3 separate experiments and normalized to loading control (C), t test, *P ≤ .05, ***P ≤ .001. A vertical line has been inserted to indicate the position where a gel lane was removed.
Statins modulate PECAM-1 tyrosine phosphorylation. Washed human platelets were incubated with simvastatin or control for 5 minutes under conditions that disfavor aggregation (presence of EGTA [1 mM], indomethacin [10 mM], apyrase [2 U mL-1]). PECAM-1 was immunoprecipitated from cell lysates using anti–PECAM-1 antibody (WM59) and immunoblotted with an anti-phosphotyrosine antibody (4G10). Blots are representative of 3 separate experiments and normalized to loading control. Numerical data represent the percentage increase above phosphorylation levels in nontreated platelets (A). Washed human platelets were treated with PECAM-1 antibody to mediate clustering (PECAM-1 XL, 0.8 μg mL-1) or isotype control (IgG-XL, 0.8 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (10 μM) and aggregation was measured (B). Washed platelets were incubated with PECAM-1 antibody to mediate clustering (PECAM-1 XL) or isotype control (IgG-XL) (1 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (5-10 μM). PECAM-1 was immunoprecipitated from cell lysates. Immunoprecipitates were divided in 2, with half probed for tyrosine phosphorylation and half probed for PECAM-1, to ensure equivalent levels of protein isolation between samples. Blots are representative of 3 separate experiments and normalized to loading control (C), t test, *P ≤ .05, ***P ≤ .001. A vertical line has been inserted to indicate the position where a gel lane was removed.
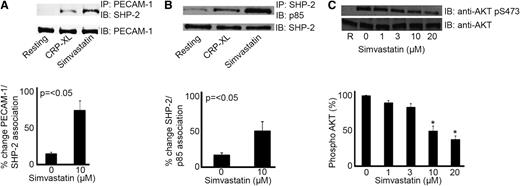
PECAM-1 activation by clustering, ligand binding, or following platelet activation results in the recruitment and activation of tyrosine phosphatase-2 (SHP-2), which plays a role in the ensuing negative regulation of platelet function.22-24,31 Recently, we characterized a mechanism that explains the ability of PECAM-1 to inhibit PI3K-dependent signaling and platelet function. The ability of SHP-2 to interact with the p85 subunit of PI3K following PECAM-1 activation results in the relative redistribution of PI3K and exclusion from lipid raft compartments leading to diminished PI3K signaling.33 Therefore, we sought to establish whether this mechanism is also stimulated by statins. Simvastatin treatment enhanced the level of SHP-2 associated with immunoprecipitated PECAM-1 upon CRP-XL stimulation (Figure 4A) and thrombin (supplemental Figure 5). This was also associated with a concomitant increase in p85 association with SHP-2 following statin treatment (Figure 4B).
Statins modulate PECAM-1 signaling. Platelet cell lysates were immunoprecipitated for PECAM-1 or SHP-2 and immunoblotted using anti–SHP-2 and anti-p85 antibodies, respectively. Blots are representative of 3 separate experiments and quantification normalized to loading control. Numerical data represent the percentage of change of PECAM-1/SHP-2 and SHP-2/p85 association (A-B). Phosphatidylinositol 3-kinase signaling was measured through assessment of Akt phosphorylation. Platelets stimulate with CRP-XL (1 μg mL−1) in the presence of increasing concentrations of simvastatin (1-20 μM) were analyzed by immunoblotting with an Akt/PKB phospho-specific antibody (Ser473). Equivalent protein loading was verified by reprobing for total Akt (C), t test, *P ≤ .05.
Statins modulate PECAM-1 signaling. Platelet cell lysates were immunoprecipitated for PECAM-1 or SHP-2 and immunoblotted using anti–SHP-2 and anti-p85 antibodies, respectively. Blots are representative of 3 separate experiments and quantification normalized to loading control. Numerical data represent the percentage of change of PECAM-1/SHP-2 and SHP-2/p85 association (A-B). Phosphatidylinositol 3-kinase signaling was measured through assessment of Akt phosphorylation. Platelets stimulate with CRP-XL (1 μg mL−1) in the presence of increasing concentrations of simvastatin (1-20 μM) were analyzed by immunoblotting with an Akt/PKB phospho-specific antibody (Ser473). Equivalent protein loading was verified by reprobing for total Akt (C), t test, *P ≤ .05.
To explore whether statins affect PECAM-1 downstream signaling, Akt/protein kinase B (PKB) activation (a marker of PI3K signaling) was assessed by measuring the levels of phosphorylation (Ser473) upon CRP-XL stimulation in the presence of simvastatin (1-20 μM). Treatment with simvastatin was found to result in concentration-dependent inhibition of Akt phosphorylation (Ser473), where 10 μM simvastatin was able to achieve ∼50% inhibition (Figure 4C). Taken together, these data indicate that the actions of these statins may be mediated through PECAM-1.
Antiplatelet function of statins involve PECAM-1
To confirm whether PECAM-1 is required for the inhibitory effect of statins on platelet function, the ability of statins to inhibit platelet function in mice deficient in PECAM-1 was explored. CRP-XL concentration was optimized to ensure similar levels of fibrinogen binding on platelets derived from PECAM-1+/+ and PECAM-1−/− mice (supplemental Figure 3A). Statin treatment inhibited aggregation, fibrinogen binding, and P-selectin exposed on the surface of both PECAM-1+/+ and PECAM-1−/− platelets upon stimulation with CRP-XL; however, a lower concentration of statins was required to inhibit responses of PECAM-1+/+ relative to PECAM-1−/− platelets (Figure 5A-C). PECAM-1−/− platelets were similarly less sensitive to the effects of statin treatment than were PECAM-1+/+ platelets following stimulation with thrombin and ADP (supplemental Figure 3B-E). These data indicate that the inhibitory effects of simvastatin may be attributed in part to the ability to activate PECAM-1 signaling, and are consistent with the ability of PECAM-1 to inhibit collagen, thrombin, and ADP-mediated platelet activation.24,32,33 Similar data were obtained with fluvastatin (data not shown).
Antiplatelet function of statins involves PECAM-1. Platelets derived from PECAM-1+/+ and PECAM-1−/− mice were treated with simvastatin (1-20 μM) for 5 minutes prior to stimulation with CRP-XL (1 μg mL−1) and aggregation measured at 37°C under constant stirring conditions (A). Platelets derived from PECAM-1+/+ and PECAM-1−/− mice were treated with simvastatin (1-20 μM) for 5 minutes prior to stimulation with CRP-XL (1 μg mL−1) and fibrinogen binding and P-selectin exposure were assessed by flow cytometry (B-C). Data are representative of 4 different experiments (n = 4); P values were calculated by nonparametric Mann-Whitney test (*P ≤ .05, **P ≤ .01) (A-C). Laser-induced thrombosis in PECAM-1−/− mice in the presence and absence of fluvastatin (1-20 μM) or vehicle was investigated. Representative images of thrombi obtained from mice treated with or without fluvastatin (1 μM) at different time intervals after laser injury are shown. Arrows indicate the direction of blood flow (D). Median fluorescence intensity was measured from 21 thrombi from 4 mice from each of control and fluvastatin (1-20 μM)–treated groups (E-F). P values calculated by the nonparametric Mann-Whitney test, P > .05 (NS).
Antiplatelet function of statins involves PECAM-1. Platelets derived from PECAM-1+/+ and PECAM-1−/− mice were treated with simvastatin (1-20 μM) for 5 minutes prior to stimulation with CRP-XL (1 μg mL−1) and aggregation measured at 37°C under constant stirring conditions (A). Platelets derived from PECAM-1+/+ and PECAM-1−/− mice were treated with simvastatin (1-20 μM) for 5 minutes prior to stimulation with CRP-XL (1 μg mL−1) and fibrinogen binding and P-selectin exposure were assessed by flow cytometry (B-C). Data are representative of 4 different experiments (n = 4); P values were calculated by nonparametric Mann-Whitney test (*P ≤ .05, **P ≤ .01) (A-C). Laser-induced thrombosis in PECAM-1−/− mice in the presence and absence of fluvastatin (1-20 μM) or vehicle was investigated. Representative images of thrombi obtained from mice treated with or without fluvastatin (1 μM) at different time intervals after laser injury are shown. Arrows indicate the direction of blood flow (D). Median fluorescence intensity was measured from 21 thrombi from 4 mice from each of control and fluvastatin (1-20 μM)–treated groups (E-F). P values calculated by the nonparametric Mann-Whitney test, P > .05 (NS).
To explore the potential involvement of PECAM-1 in the antithrombotic actions of statins, a laser-induced cremaster muscle arteriole injury model in PECAM-1−/− mice was used to measure the effect of statin on arterial thrombus formation in vivo. Consistent with in vitro data (Figure 5A-C), PECAM-1−/− mice treated with fluvastatin (1 µM) showed no significant difference in the initial adherence of platelets at the site of injury and thrombus formation growth kinetics in comparison with untreated PECAM-1−/− mice (Figure 5D-E), whereas at higher concentrations (≥10 µM) where PECAM-1–independent effects of statins are observed, fluvastatin caused diminished thrombus formation (Figure 5F). On the basis of these results, we propose that the inhibitory effect of platelet function by statins results, at least in part, in action on PECAM-1. Given the very high levels of PECAM-1 on the surface of endothelial cells, the possibility of additional effects of statins on PECAM-1–dependent endothelial effects cannot be ruled out.
Discussion
Arterial thrombosis occurs on ruptured atherosclerotic plaques and involves platelet activation and the generation of rapid and coordinated intracellular signals resulting in cell-matrix and cell-cell adhesion and thrombus formation.3 The potential importance of the pleiotropic effects of statins has been demonstrated in clinical trials in patients with atherosclerotic disease,10 suggesting that the antiatherosclerotic effects of statins are likely to be attributable both to a potential lipid-lowering action (inhibition of 3-hydroxy-3-methylglutaryl coenzyme A [HMG-CoA] reductase) and to their wide range of additional pleiotropic effects on proatherogenic processes, such as improvement of endothelial function, reduction of LDL oxidation and oxidative stress, with decreased vascular inflammation.1,2,7-12
The inhibition of thrombus generation in hypercholesterolemic patients by statins does not correlate with the lipid-lowering effect.1,2,16 The possibility was therefore explored that lipid-independent effects of statins may regulate the inhibition of the platelet function and thrombus formation through known inhibitory pathways in platelets.1 The inhibitory effect on platelets of statins has been suggested in several reports.16-18,20,21,34,35 Some of these studies demonstrated that statins may exert a direct antiplatelet effect independent from cholesterol lowering potentially by (1) NADPH oxidase downregulation, followed by inhibition of Rac1 and p47phox phosphorylation, calcium mobilization, and GPIIb/IIIa activation; (2) downregulation of PLA2-mediated TXA2; and (3) NO signaling.1,16,21,34,35 In this study, we suggest an alternative mechanism that could contribute to antithrombotic effects of statins, at least in part, to be due to the stimulation of PECAM-1 signaling resulting in diminished platelet activation and thrombus formation.
Statins were found to inhibit a range of platelet functions, including aggregation, α and dense-granule secretion, and thrombus formation in vitro and in vivo. The PECAM-1−/− platelets were less susceptible to the actions of statins on platelet function and thrombosis. PECAM-1 tyrosine phosphorylation and subsequent activation of signaling molecules is stimulated following PECAM-1 clustering (due to homophilic ligation, although this can be achieved experimentally by antibody-mediated cross-linking) or following platelet activation.22-24,33 Phosphorylation of PECAM-1 mediated by src-family kinases such as Fyn and Lyn, is associated with the inhibition of platelet function, secretion, and adhesion responses.23,24 The recruitment of SHP-2 to PECAM-1 downregulates GPVI-stimulated activatory signals where GPVI-proximal signaling is unaffected; however, downstream signaling is affected as noted by reduced Akt/PKB phosphorylation.31-33 Treatment with statins enhanced Lyn activation (pY396), PECAM-1 tyrosine phosphorylation, and subsequent recruitment of the protein tyrosine phosphatase SHP-2, which in turn recruits PI3K resulting in the inhibition of downstream (Akt/PKB) PI3K-dependent signaling.
Recent studies provide compelling evidence linking hypercholesterolemia with elevated tissue factor–dependent coagulation and thrombosis in mice and monkeys.41 Indeed, these effects may be alleviated through administration of statins and therefore diminish plasma LDL cholesterol levels. Our study introduces a new dimension to the relationship between statin treatment, hemostasis, and thrombosis, through the acute effects of statin treatment on platelet signaling and function. Whether the effects of statins on PECAM-1 signaling are related to their shared abilities to regulate HMG-CoA or other target molecules remains to be established.
Our data are consistent with previous studies that highlight the ability of statins to modulate platelet function,16,21,34,35,42 and reveal a new receptor-dependent target mechanism that is required for platelet inhibition at lower statin concentrations. It is important to note that PECAM-1 signaling is regulated in an acute manner by statins. The chronic antiplatelet effects of statins appear to be closely associated with LDL cholesterol lowering1,35 and multiple potential targets may play a role, such as endothelial cell and NO signaling; however, our results suggest that the acute effects of statins on platelets are independent of NO signaling.
Although recent clinical observations indicate that statins demonstrate beneficial effects on cardiovascular complications, long-term follow-up studies are clearly necessary to elucidate precisely the pleiotropic effects by which statins might influence mortality and morbidity in patients with atherosclerosis. Our study provides evidence for a fundamental role of PECAM-1 in the inhibitory effects of statins on platelet activation, through a novel mechanism for the pleiotropic and specifically antithrombotic actions of these drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr T. Mak (Amgen Institute, Toronto, ON, Canada) for generating that PECAM-1–deficient mouse.
This work was supported by the British Heart Foundation (grants RG/09/011/28094, PG FS/11/86/29137, PG/11/125/29320).
J.M.G. is a visiting professor at the King Saud University, Riyadh, Saudi Arabia.
Authorship
Contribution: L.A.M. designed the research, performed experiments, analyzed results, made figures, and wrote the paper; S.V. and P.S. performed experiments, analyzed results, and made figures; M.S.A., N.K., and T.S. performed experiments; and J.M.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonardo Augusto Moraes, Institute for Cardiovascular and Metabolite Research, School of Biological Sciences, Harborne Building, Whiteknights Campus, University of Reading, Reading, United Kingdom, RG6 6AS; e-mail: l.a.moraes@reading.ac.uk.
References
Author notes
S.V. and P.S. have equal contribution in this work.

![Figure 1. Statins inhibit platelet activation. Washed human platelets were treated for 5 minutes with increasing concentration of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1) and aggregation measured at 37°C under constant stirring conditions. Numerical data represent the percentage aggregation compared with control, mean ± SEM (n = 4) (A-B). The effect of simvastatin or fluvastatin on fibrinogen binding and P-selectin exposure prior to stimulation with CRP-XL (1 μg mL−1) was measured in human whole blood by flow cytometry. Numerical data represent the percentage of fibrinogen binding and P-selectin exposure compared with control, mean ± SEM (n = 4) (C-D). Washed human platelets were treated for 5 minutes with increasing concentrations of simvastatin or fluvastatin, prior to stimulation for 90 seconds with collagen-related peptide CRP-XL (0.5 μg mL−1). Changes in ATP concentration were used as a measure of dense-granule secretion and monitored simultaneously with aggregation in an optical lumi-aggregometer using a luciferase detection system. Numerical data represent the percentage of ATP release compared with control, mean ± SEM (n = 4) (E-F). Platelets stimulated with CRP-XL (1 μg mL−1) in the presence or absence of simvastatin were analyzed by immunoblotting with anti-phosphotyrosine antibody (4G10) and anti-phospho-site antibodies for Syk (Y323), PLC (Y1197), Fyn (Y59), and Lyn (Y396) (G-I). Blots are representative of 4 different experiments (n = 4), t test, P > .05 (nonsignificant [NS]), *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/18/10.1182_blood-2013-04-491845/4/m_3188f1.jpeg?Expires=1768561991&Signature=FukOHHwso27ztfeabWSERnMEa2Jut5khrfrYsZieG5iRYTHuC4C3HcK3bnCA6WJfbl9hnc88JZqFGs~n~7vTDnreFvtf6oxJAynZNrhEwTvCaKAwlXapRHAa6zYuriN0gaCAprd1zrdRdsYWyEiFHxf6zG5x6fFeH3TU5xVIgcklt7ukbv7Z6RyoOmNNg0hSvXetbjGHD5EQ9GRRtPs3LVDa4AV6aWQjWeP5ANzYbHVDX6MymL5wu0bQToixL0WoHgjnHqj1jwgwmckgzZTUJ1Fznvmh-p7oM47POg6sHYf8OFHizjchlwn-nJLft3npyKUlbwtDN4~UjLqUFQK1MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Statins modulate PECAM-1 tyrosine phosphorylation. Washed human platelets were incubated with simvastatin or control for 5 minutes under conditions that disfavor aggregation (presence of EGTA [1 mM], indomethacin [10 mM], apyrase [2 U mL-1]). PECAM-1 was immunoprecipitated from cell lysates using anti–PECAM-1 antibody (WM59) and immunoblotted with an anti-phosphotyrosine antibody (4G10). Blots are representative of 3 separate experiments and normalized to loading control. Numerical data represent the percentage increase above phosphorylation levels in nontreated platelets (A). Washed human platelets were treated with PECAM-1 antibody to mediate clustering (PECAM-1 XL, 0.8 μg mL-1) or isotype control (IgG-XL, 0.8 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (10 μM) and aggregation was measured (B). Washed platelets were incubated with PECAM-1 antibody to mediate clustering (PECAM-1 XL) or isotype control (IgG-XL) (1 μg mL-1) prior to CRP-XL (1 μg mL-1) stimulation in the absence or presence of simvastatin (5-10 μM). PECAM-1 was immunoprecipitated from cell lysates. Immunoprecipitates were divided in 2, with half probed for tyrosine phosphorylation and half probed for PECAM-1, to ensure equivalent levels of protein isolation between samples. Blots are representative of 3 separate experiments and normalized to loading control (C), t test, *P ≤ .05, ***P ≤ .001. A vertical line has been inserted to indicate the position where a gel lane was removed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/18/10.1182_blood-2013-04-491845/4/m_3188f3.jpeg?Expires=1768561991&Signature=LhvQ63VW8XhptirTisxSs9mJMJTdc6irnFOCLwSbGEB-1ouLUbOOeFpnnSkFELwz8lPqsXWvtzh9GJL0Z6JyA4JfYw8kxF7CS19POVbn~XkwIeSrSwog8luuAt~VvdACD67qhBt~wcHj-03sX7MPpUhgNuVvg-PPWDNeIRqEKTkkSwqIYtA1cGIjrT4UOMNy2cUCjFGVIfEgSLjfVtGyeZ2Q7QFxB0-DCo73cEwvLAjsGKpResj4xb1N5yX7wc14FhRgj0pYK-SJKFU2KyVRZ~nsCcnWGbo4I5O8UEtpc-q80xGBrE3xeMuaJfP7vm~fFVxtYGAnKO61e39MJPz2AQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)