Key Points
Rac1 and Cdc42 have redundant functions in platelet biogenesis.
Deficiency of Rac1 and Cdc42 results in highly abnormal megakaryocyte morphology associated with severely defective tubulin organization.
Abstract
Blood platelets are anuclear cell fragments that are essential for blood clotting. Platelets are produced by bone marrow megakaryocytes (MKs), which extend protrusions, or so-called proplatelets, into bone marrow sinusoids. Proplatelet formation requires a profound reorganization of the MK actin and tubulin cytoskeleton. Rho GTPases, such as RhoA, Rac1, and Cdc42, are important regulators of cytoskeletal rearrangements in platelets; however, the specific roles of these proteins during platelet production have not been established. Using conditional knockout mice, we show here that Rac1 and Cdc42 possess redundant functions in platelet production and function. In contrast to a single-deficiency of either protein, a double-deficiency of Rac1 and Cdc42 in MKs resulted in macrothrombocytopenia, abnormal platelet morphology, and impaired platelet function. Double-deficient bone marrow MKs matured normally in vivo but displayed highly abnormal morphology and uncontrolled fragmentation. Consistently, a lack of Rac1/Cdc42 virtually abrogated proplatelet formation in vitro. Strikingly, this phenotype was associated with severely defective tubulin organization, whereas actin assembly and structure were barely affected. Together, these results suggest that the combined action of Rac1 and Cdc42 is crucial for platelet production, particularly by regulating microtubule dynamics.
Introduction
Platelets are synthesized by precursor cells called megakaryocytes (MKs). MKs reside primarily in the bone marrow (BM) and develop by differentiation from pluripotent hematopoietic stem cells.1 MK maturation is initiated by multiple rounds of endomitosis, the synthesis of platelet-specific granules, and the formation of a demarcation membrane system, which seems to function as a membrane reservoir for newly formed (pro)platelets.1,2 Finally, mature MKs extend proplatelet-like protrusions into the sinusoids of the BM, from which platelets are shed and are finally sized by the shear forces present in the blood stream.3,4 Microtubule sliding enables proplatelet elongation and mediates organelle trafficking into future platelets.5 In contrast, actin-dependent mechanisms are thought to be involved in the branching of proplatelet shafts, thereby increasing the number of available proplatelet tips.5
Rho GTPases are small proteins (20-25 kDa) belonging to the superfamily of Ras-related proteins, which are found in all eukaryotic cells.6 They are best known for regulating actin cytoskeletal dynamics in virtually all cell types, as well as for their involvement in the regulation of microtubules.7 The best-characterized Rho GTPases are RhoA, Rac1, and Cdc42, the activation of which is associated with the formation of stress fibers, lamellipodia, and filopodia, respectively. Much knowledge about the functions of Rho GTPases has been gained by overexpression or knockdown studies in cell lines, which, however, has often yielded conflicting results. Therefore, the recent generation of knockout mice for several GTPases has provided new tools to more reliably study the effect of GTPase deficiency with a reduced risk for secondary effects.8,9 Several studies on platelet function using hematopoietic and/or MK- and platelet-specific Rac1 and Cdc42 knockout mice have been performed. The results have revealed that Rac1 is indispensable not only for lamellipodia formation but also for activation of the phospholipase C isoform γ2 (PLCγ2) in murine platelets.10,11 In contrast, Cdc42-deficient platelets, surprisingly, retained the ability to form filopodia and displayed increased agonist-induced secretion.12 However, the role of Rac1 and Cdc42 for MK maturation and platelet production has not been addressed to date. The fact that Rac1-deficient mice display normal platelet count suggests normal MK and platelet production in these animals. MK- and platelet-specific Cdc42 knockout mice display moderate thrombocytopenia, which might, however, be explained by the significantly reduced life span of the mutant platelets.12
Studies using different cell types have revealed that Rac1 and Cdc42 may share different downstream signaling molecules.7 Well-investigated examples among these are the actin-polymerization-promoting proteins mammalian diaphanous 2 (mDia2) and p21-activated kinase (PAK), which in turn induces activation of the LIM kinase (LIMK), leading to phosphorylation-dependent inactivation of cofilin.
In this study, we investigated the potential redundant roles of Rac1 and Cdc42 for platelet production and function by analyzing conditional Rac1 and/or Cdc42 knockout mice. We demonstrate that in contrast to a single-deficiency in either GTPase, double-deficiency in Rac1 and Cdc42 virtually abrogates proplatelet formation in vitro. Interestingly, this phenotype was particularly associated with defective microtubule stabilization. These results demonstrate for the first time a functional redundancy of Rac1 and Cdc42 in the hematopoietic system and point to a novel role of the GTPases in the regulation of microtubule dynamics during platelet production.
Materials and methods
Mice, platelet preparation, platelet spreading, flow cytometry, determination of actin polymerization, platelet life span, tail bleeding, in vivo thrombus formation, western blotting, electron microscopy, histology, determination of ploidy of MKs, in vitro MK differentiation, analysis of proplatelet formation, and statistical data analysis are described in detail in the supplemental Methods, available on the Blood website. A list of antibodies and reagents is also provided in the supplemental Methods. Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken).
Results
Constitutive deletion of either the Rac113 or the Cdc4214 gene results in embryonic lethality in mice. To study the function of Rac1/Cdc42 double-deficiency, mice carrying both the Rac1 and Cdc42 genes flanked by loxP sites15,16 were generated. The resulting Rac1/Cdc42fl/fl mice were crossed with transgenic mice expressing Cre recombinase under the control of either the MK- and platelet-specific platelet factor (Pf) 417 or the Mx promoter,18 which is specific for the hematopoietic system.
In the PF4-Cre system, gene deletion occurred intrinsically during MK maturation in Rac1/Cdc42fl/fl Pf4-cre+ mice. In adult Rac1/Cdc42fl/fl Mx-cre+ mice, gene deletion was induced as described in the supplemental Methods. Western blot analysis revealed that both systems efficiently deleted both proteins in platelets (Figure 1A). However, Rac1/Cdc42−/− Mx-cre+ animals died between 9 and 14 days after Cre induction, which is in line with the observation that Mx-Cre-induced Cdc42 deficiency alone results in rapid death of the animals (I.P. and B.N., unpublished data, and Yang et al19 ). In contrast, Rac1/Cdc42−/− Pf4-cre+ mice were outwardly healthy and fertile (not shown). To avoid potential adverse effects on megakaryopoiesis evoked by Mx-Cre-induced gene deletion, we therefore exclusively used Rac1/Cdc42−/− Pf4-cre+ (further referred to as Rac1/Cdc42−/−) animals in this study, with the indicated exception of in vitro platelet adhesion assays.
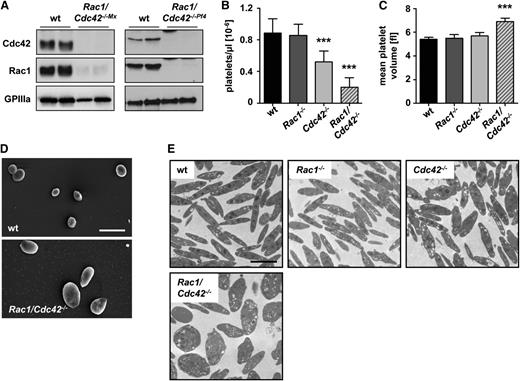
Absence of Rac1 and Cdc42 leads to macrothrombocytopenia. (A) Western blot analysis of Rac1 and Cdc42 expression in wild-type (wt) and Rac1/Cdc42−/− platelets generated by the Mx-Cre (left) or the PF4-Cre (right) system. GPIIIa expression was used as loading control. Analysis of peripheral platelet counts (B) and mean platelet volume (C) of wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice (n = 5 per group; ***P < .001). (D-E) Rac1/Cdc42−/− platelets are increased in size. Representative scanning (D) and transmission (E) electron microscopy pictures from wt and Rac1/Cdc42−/− platelets are depicted. Bars, 5 and 2 µm.
Absence of Rac1 and Cdc42 leads to macrothrombocytopenia. (A) Western blot analysis of Rac1 and Cdc42 expression in wild-type (wt) and Rac1/Cdc42−/− platelets generated by the Mx-Cre (left) or the PF4-Cre (right) system. GPIIIa expression was used as loading control. Analysis of peripheral platelet counts (B) and mean platelet volume (C) of wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice (n = 5 per group; ***P < .001). (D-E) Rac1/Cdc42−/− platelets are increased in size. Representative scanning (D) and transmission (E) electron microscopy pictures from wt and Rac1/Cdc42−/− platelets are depicted. Bars, 5 and 2 µm.
Rac1/Cdc42 double-deficiency leads to macrothrombocytopenia and abnormal platelet morphology
As expected from previous studies,11,12 Rac1−/− mice displayed normal and Cdc42−/− mice only moderately decreased platelet counts. In marked contrast, double-deficiency of Rac1 and Cdc42 resulted in severe thrombocytopenia, with platelet counts lower than 25% those of control mice (wt, 885 × 103/µL [±182 × 103]; Rac1/Cdc42−/−, 202 × 103/µL [±120 × 103]; Figure 1B). The size of Rac1/Cdc42−/− platelets was markedly increased compared with that of the single-deficient platelets, as demonstrated by transmission electron microscopy (TEM) and scanning electron microscopy of resting platelets and determination of the mean platelet volume (wt, 5.5 femtoliter [fL] [±0.2]; Rac1/Cdc42−/−, 6.9 fL [±0.3]; Figure 1C-E).
Detailed analysis by TEM revealed a highly abnormal ultrastructure in Rac1/Cdc42−/− platelets (Figure 2). Whereas Rac1−/− and Cdc42−/− platelets displayed normal granule distribution compared with wt platelets (Figure 2A and data not shown), 51% of the Rac1/Cdc42−/− platelets were overloaded with granules and/or vacuoles, whereas 34% were virtually devoid of granules (Figure 2B), indicating disordered granule formation or trafficking. Interestingly, in the majority of double-deficient platelets, the microtubule coils in the marginal band were not visible; however, if they were present, their numbers were increased compared with the wt (wt, 10.1 [±0.9] microtubule coils/platelet; Rac1/Cdc42−/−, 14 [±0.8] microtubule coils/platelet; Figure 2C). Flow cytometric measurements indicated significantly reduced expression levels of several prominent platelet surface glycoproteins (GPs) in Rac1/Cdc42−/− platelets (Table 1). Together, these results demonstrated a severe defect in platelet production in the absence of both Rac1 and Cdc42.
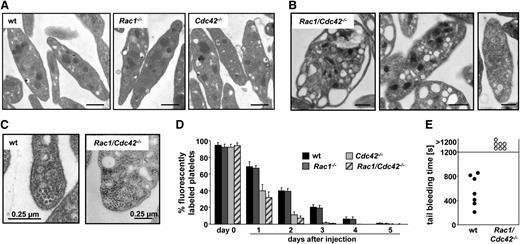
Rac1/Cdc42−/− platelets display abnormal ultrastructure and are rapidly cleared from the circulation. (A-B) Representative TEM pictures are shown. (A) Normal ultrastructure of wt, Rac1−/−, and Cdc42−/− platelets. Bar, 0.5 µm. (B) Abnormal ultrastructure of Rac1/Cdc42−/− platelets. Bar sizes are depicted. (C) Increased number of marginal microtubular rings in Rac1/Cdc42−/− platelets. Bar sizes are depicted. (D) Life span from Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) platelets compared with wt (black) platelets. (E) Infinite tail bleeding times in Rac1/Cdc42−/− (patterned) compared with wt (black) mice (n = 7 per group; ***P < .001).
Rac1/Cdc42−/− platelets display abnormal ultrastructure and are rapidly cleared from the circulation. (A-B) Representative TEM pictures are shown. (A) Normal ultrastructure of wt, Rac1−/−, and Cdc42−/− platelets. Bar, 0.5 µm. (B) Abnormal ultrastructure of Rac1/Cdc42−/− platelets. Bar sizes are depicted. (C) Increased number of marginal microtubular rings in Rac1/Cdc42−/− platelets. Bar sizes are depicted. (D) Life span from Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) platelets compared with wt (black) platelets. (E) Infinite tail bleeding times in Rac1/Cdc42−/− (patterned) compared with wt (black) mice (n = 7 per group; ***P < .001).
Platelet GP expression in wt and Rac1/Cdc42−/− mice
| GP . | wt . | Rac1/Cdc42−/− . | Significance . |
|---|---|---|---|
| GPIb | 368 ± 8 | 298 ± 37 | * |
| GPV | 368 ± 7 | 312 ± 35 | * |
| GPIX | 555 ± 20 | 447 ± 58 | * |
| CD9 | 1451 ± 47 | 1380 ± 129 | n.s. |
| GPVI | 52 ± 2 | 43 ± 2 | † |
| α2 | 118 ± 4 | 123 ± 10 | n.s |
| β1 | 193 ± 4 | 205 ± 18 | n.s. |
| αIIbβ3 | 856 ± 45 | 736 ± 47 | * |
| GP . | wt . | Rac1/Cdc42−/− . | Significance . |
|---|---|---|---|
| GPIb | 368 ± 8 | 298 ± 37 | * |
| GPV | 368 ± 7 | 312 ± 35 | * |
| GPIX | 555 ± 20 | 447 ± 58 | * |
| CD9 | 1451 ± 47 | 1380 ± 129 | n.s. |
| GPVI | 52 ± 2 | 43 ± 2 | † |
| α2 | 118 ± 4 | 123 ± 10 | n.s |
| β1 | 193 ± 4 | 205 ± 18 | n.s. |
| αIIbβ3 | 856 ± 45 | 736 ± 47 | * |
Expression of GPs on the platelet surface was determined by flow cytometry. Diluted whole blood from the indicated mice was incubated with fluorescein isothiocyanate–labeled antibodies at saturating concentrations for 15 minutes at room temperature and platelets were analyzed directly using a FACSCalibur. Results are expressed as mean fluorescence intensity ± SD for 4 mice per group.
n.s., no significance.
P < .05.
P < .01.
Rac1/Cdc42−/− platelets display a decreased life span
Nonfunctional or preactivated platelets are constantly cleared from the circulation by the reticulo-endothelial system in spleen and liver.20 To investigate whether the macrothrombocytopenia in the double-deficient mice was caused by an increased platelet turnover, the life span of the cells was determined in Rac1/Cdc42−/− mice. In agreement with normal platelet counts, the life span of Rac1−/− platelets was approximately 5 days and was thus comparable with that of the wt platelets (Figure 2D). In contrast, as shown before,12 the life span of Cdc42−/− platelets was reduced to approximately 3 days. Surprisingly, despite their highly abnormal morphology, the life span of Rac1/Cdc42−/− platelets was not significantly shorter than that of Cdc42−/− platelets. A per se preactivation of the double-deficient and Cdc42−/− platelets was probably not the major cause of their decreased life span, as flow cytometric measurements showed only minor basal integrin αIIbβ3 activation or P-selectin exposure in Rac1/Cdc42−/− and Cdc42−/− platelets under resting conditions or on stimulation with the weak agonist epinephrine in vitro (supplemental Figure 1C).
Rac1/Cdc42 double-deficiency impairs platelet function in vitro and results in defective hemostasis and arterial thrombus formation in vivo
To investigate the effect of Rac1/Cdc42 double-deficiency on platelet function, the response of Rac1/Cdc42−/− platelets toward agonist stimulation was analyzed (supplemental Figure 1). We have previously shown that Rac1−/− platelets display a selective defect when activated with immunoreceptor tyrosine-based activation motif (ITAM)-coupled agonists.11 In Cdc42−/− platelets, degranulation-dependent P-selectin expression was enhanced after stimulation with ITAM as well as G-protein-coupled-receptor (GPCR) agonists, despite a moderate decrease in integrin activation.12
In Rac1/Cdc42−/− platelets, integrin αIIbβ3 activation (supplemental Figure 1A) was markedly decreased, whereas α-granule secretion toward GPCR agonists was preserved (supplemental Figure 1B). Tail bleeding assays revealed that Rac1/Cdc42−/− mice displayed a severe hemostatic defect in vivo characterized by an inability to arrest bleeding within 20 minutes (mean bleeding time in wt, 468 ± 248 seconds) and by a large volume of lost blood (Figure 2E and not shown). Similarly, no occlusive thrombi formed in Rac1/Cdc42−/− mice upon FeCl3-induced injury of mesenteric arterioles in vivo (supplemental Figure 1D).
Taken together, the combination of reduced platelet counts and impaired platelet function translated into defective hemostasis and a profound protection from arterial thrombosis in Rac1/Cdc42 double-deficient animals in vivo.
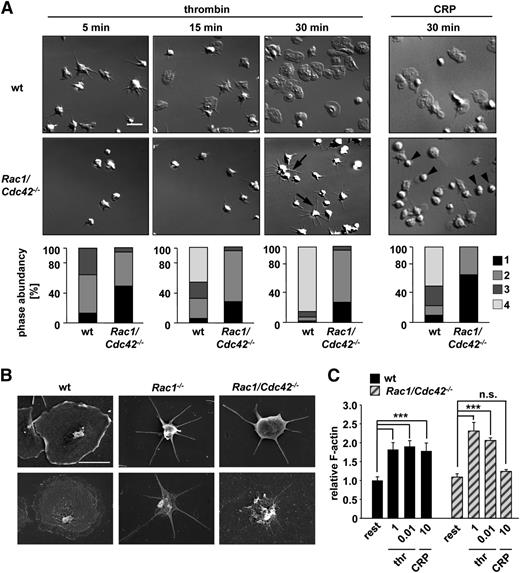
Rac1/Cdc42−/− platelets are unable to spread on fibrinogen but retain the ability to adhere and form filopodia
To analyze the consequences of Rac1/Cdc42 double-deficiency on cytoskeletal rearrangements in platelets, thrombin- or collagen-related peptide (CRP)-activated Rac1/Cdc42−/−Mx platelets were allowed to spread on fibrinogen (Figure 3A). As shown previously,11,12 Rac1−/− platelets were unable to form lamellipodia on fibrinogen upon thrombin activation, whereas Cdc42−/− platelets spread normally. Interestingly, Rac1/Cdc42−/−Mx platelets retained the ability to tightly adhere to the fibrinogen matrix under these conditions (Figure 3A) and, as expected, failed to develop lamellipodia. However, formation of long and thin filopodia was still observed in approximately 70% of Rac1/Cdc42−/−Mx platelets (Figure 3A). Consistently, actin distribution was altered in thrombin-stimulated spread Rac1/Cdc42−/−Mx platelets denudated of the plasma membrane (Figure 3B, bottom). Interestingly, CRP-stimulated Rac1/Cdc42−/−Mx platelets also tightly adhered to fibrinogen; however, the spreading defect was significantly more pronounced than after thrombin activation (Figure 3A). As a result, the majority of adherent double-deficient platelets displayed a resting roundish shape after 30 minutes (Rac1/Cdc42−/−Mx, 64% ± 0.05% vs wild-type, 12.5% ± 0.02%), indicating impaired cellular activation (Figure 3A).
Defective spreading of Rac1/Cdc42−/− platelets on fibrinogen upon activation. (A) Washed platelets from wt and Rac1/Cdc42−/−Mx mice were allowed to adhere on immobilized human fibrinogen (100 µg/mL) upon activation with thrombin (0.01 U/mL, left) or CRP (10 µg/mL, right). (Upper) DIC images were taken at the indicated times (5, 15, and 30 minutes), representative of 4 individual experiments. Bar, 5 µm. (Lower) Statistical analysis of the percentage of spread Rac1/Cdc42−/−Mx and wt platelets. (Bottom) (1) Roundish, no filopodia, no lamellipodia. (2) Only filopodia. (3) Partial spreading. (4) Full spreading. Arrows indicate altered morphology in Rac1/Cdc42−/−Mx platelets. (B) Visualization of defective spreading and actin reorganization of Rac1−/− (middle) and Rac1/Cdc42−/−Mx (right) platelets by scanning electron microscopy. (Upper) Scanning electron microscopy image of intact platelets. (Lower) Visualization of the cytoskeleton after denudation of the plasma membrane. Bar, 2 µm. (C) Determination of relative F-actin contents in resting, thrombin-activated (1 and 0.01 U/mL), and CRP-activated (10 µg/mL) wt (black) and Rac1/Cdc42−/−Mx (patterned) platelets (n = 4 per group; ***P < .001).
Defective spreading of Rac1/Cdc42−/− platelets on fibrinogen upon activation. (A) Washed platelets from wt and Rac1/Cdc42−/−Mx mice were allowed to adhere on immobilized human fibrinogen (100 µg/mL) upon activation with thrombin (0.01 U/mL, left) or CRP (10 µg/mL, right). (Upper) DIC images were taken at the indicated times (5, 15, and 30 minutes), representative of 4 individual experiments. Bar, 5 µm. (Lower) Statistical analysis of the percentage of spread Rac1/Cdc42−/−Mx and wt platelets. (Bottom) (1) Roundish, no filopodia, no lamellipodia. (2) Only filopodia. (3) Partial spreading. (4) Full spreading. Arrows indicate altered morphology in Rac1/Cdc42−/−Mx platelets. (B) Visualization of defective spreading and actin reorganization of Rac1−/− (middle) and Rac1/Cdc42−/−Mx (right) platelets by scanning electron microscopy. (Upper) Scanning electron microscopy image of intact platelets. (Lower) Visualization of the cytoskeleton after denudation of the plasma membrane. Bar, 2 µm. (C) Determination of relative F-actin contents in resting, thrombin-activated (1 and 0.01 U/mL), and CRP-activated (10 µg/mL) wt (black) and Rac1/Cdc42−/−Mx (patterned) platelets (n = 4 per group; ***P < .001).
Despite their effect on platelet-spreading morphology, Rac1/Cdc42−/−Mx platelets were still able to assemble F-actin similar to wt platelets after stimulation with thrombin (0.01 U/mL) (Figure 3C). In contrast, no significant F-actin assembly could be detected in double-deficient platelets after CRP activation (10 µg/mL). Together, these results suggest that defective F-actin assembly and spreading of Rac1/Cdc42−/−Mx platelets on fibrinogen after CRP stimulation represented a direct consequence of defective GP VI/ITAM-induced cellular activation caused by Rac1 deficiency. In contrast, the distinct spreading defect in thrombin-stimulated Rac1/Cdc42−/−Mx platelets was most likely caused predominantly by defective Rac1-mediated cytoskeletal rearrangement, rather than by a general defect in F-actin assembly. This assumption is consistent with the partially preserved activation of double-deficient platelets toward GPCR-coupled agonists observed by flow cytometry (supplemental Figure 1A-B). Interestingly, double-deficient platelets retained the ability to firmly adhere to the fibrinogen substrate, irrespective of the stimulus, indicating that this integrin-mediated process was largely preserved in absence of Rac1 and Cdc42 (supplemental Figure 1D).
Increased MK number and fragmentation in Rac1/Cdc42 double-deficient mice
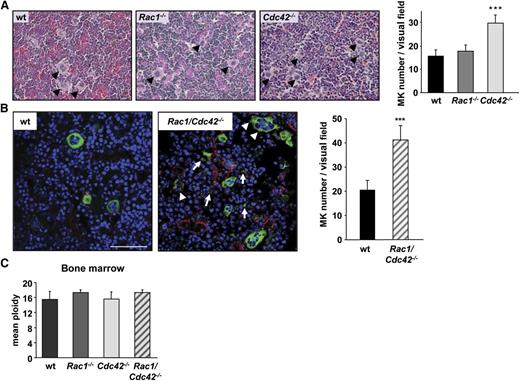
The observation that platelet counts were dramatically reduced in Rac1/Cdc42−/− compared with Cdc42−/− mice despite a similarly decreased platelet life span implied additional functional defects in the double-deficient MKs. We therefore investigated whether impaired MK maturation and/or platelet production contributed to the thrombocytopenia in Rac1/Cdc42−/− (and Cdc42−/−) mice. In accordance with normal platelet count and morphology, MK numbers in hematoxylin and eosin–stained spleen and BM sections of Rac1−/− mice were comparable with the control. In contrast, both Cdc42−/− and Rac1/Cdc42−/− mice displayed increased numbers of MKs in the spleen (not shown). Although BM MK numbers also were markedly increased in Cdc42−/− mice (Figure 4A), Rac1/Cdc42−/− MKs were hardly identifiable in these sections because of their altered morphology and decreased demarcation from the surrounding cells. We therefore visualized Rac1/Cdc42−/− MKs in BM cryosections, using an anti-CD41 antibody clearly revealing increased numbers of MKs per visual field in Rac1/Cdc42−/− mice compared with wt mice (wt, 20 ± 4; Rac1/Cdc42−/−, 41 ± 6; Figure 4B). Furthermore, mature MKs prematurely extending pseudopods, as well as MK fragments, were visible in the BM from double-deficient mice (Figure 4B). Of note, the MK ploidy was similar in all analyzed mouse lines, indicating that lack of Rac1 and/or Cdc42 did not affect endomitosis (Figure 4C and data not shown).
Increased MK numbers in BM of Cdc42−/− and Rac1/Cdc42−/− mice. (A) Determination of MK numbers in hematoxylin and eosin–stained BM sections from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. (Left) Representative images. (Right) Statistical analysis. (B) Determination of MK number and morphology within the BM of anti–CD41-stained (green) cryosections. Endothelial cells are stained with anti-CD105 (red) and nuclei with 4,6 diamidino-2-phenylindole (DAPI) (blue). Results are expressed as mean MK number per visual field ± SD of 3 mice per group. (C) Functional endomitosis in Rac1−/−, Cdc42−/−, and Rac1/Cdc42−/− MKs. Determination of the mean ploidy of BM-derived MKs from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. Results are expressed as mean ploidy ± SD (n = 4 per group; ***P < .001).
Increased MK numbers in BM of Cdc42−/− and Rac1/Cdc42−/− mice. (A) Determination of MK numbers in hematoxylin and eosin–stained BM sections from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. (Left) Representative images. (Right) Statistical analysis. (B) Determination of MK number and morphology within the BM of anti–CD41-stained (green) cryosections. Endothelial cells are stained with anti-CD105 (red) and nuclei with 4,6 diamidino-2-phenylindole (DAPI) (blue). Results are expressed as mean MK number per visual field ± SD of 3 mice per group. (C) Functional endomitosis in Rac1−/−, Cdc42−/−, and Rac1/Cdc42−/− MKs. Determination of the mean ploidy of BM-derived MKs from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. Results are expressed as mean ploidy ± SD (n = 4 per group; ***P < .001).
Together, these results exclude a global defect in MK formation from their progenitors as the reason for the thrombocytopenia in Rac1/Cdc42−/− mice but indicate that the double-mutant MKs are unable to efficiently release proplatelets into the vascular sinusoids.
Rac1/Cdc42 double-deficiency virtually abrogates proplatelet formation
To investigate MK morphology in more detail, BM sections of wt, Rac1−/−, Cdc42−/−, and Rac1/Cdc42−/− mice were analyzed by TEM (Figure 5A). Mature wt and Rac1−/− MKs displayed characteristic membrane invaginations formed by the demarcation membrane system, whereas few invaginations were found in the periphery and around the nucleus (Figure 5Ai-ii). In Cdc42−/− MKs, membrane invaginations and the peripheral zone were partially reduced compared with the wt (Figure 5Aiii). In marked contrast, Rac1/Cdc42−/− MKs displayed a highly abnormal morphology characterized by few demarcation membranes and an overall reduction in granule numbers (Figure 5Aiv). Furthermore, peripheral zones were virtually absent, resulting in their partial fragmentation. This phenotype provides an explanation for the decreased visibility of Rac1/Cdc42−/− MKs in hematoxylin and eosin–stained BM sections and confirms our data from the CD41-stained BM cryosections (Figure 4B).
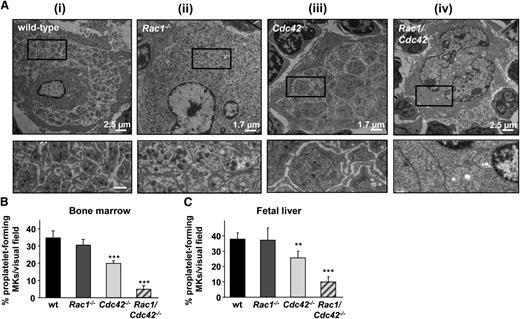
Decreased proplatelet formation and abnormal morphology in Cdc42−/− and Rac1/Cdc42−/− MKs. (A) TEM of MKs in BM of adult (6-week-old) mice. (i-ii) Normal ultrastructure in MKs derived from wt and Rac1−/− mice. (iii-iv) Altered ultrastructure in MKs derived from Cdc42−/− (iii) and Rac1/Cdc42−/− (iv) mice. (Upper) Overview. Bar sizes are depicted. (Lower) Detailed view. Bar, 0.6 µm. (B-C) Determination of proplatelet formation from cultured MKs derived from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. Results are expressed as percentage of proplatelet-forming MKs per visual field ± SD from at least 5 samples per group. (B) Results from BM-derived MKs (day 6 of culture). (C) Results from fetal liver-derived MKs (embryonic day 14.5, day 4 of culture). ***P < .001; **P < .01.
Decreased proplatelet formation and abnormal morphology in Cdc42−/− and Rac1/Cdc42−/− MKs. (A) TEM of MKs in BM of adult (6-week-old) mice. (i-ii) Normal ultrastructure in MKs derived from wt and Rac1−/− mice. (iii-iv) Altered ultrastructure in MKs derived from Cdc42−/− (iii) and Rac1/Cdc42−/− (iv) mice. (Upper) Overview. Bar sizes are depicted. (Lower) Detailed view. Bar, 0.6 µm. (B-C) Determination of proplatelet formation from cultured MKs derived from wt (black), Rac1−/− (dark gray), Cdc42−/− (light gray), and Rac1/Cdc42−/− (patterned) mice. Results are expressed as percentage of proplatelet-forming MKs per visual field ± SD from at least 5 samples per group. (B) Results from BM-derived MKs (day 6 of culture). (C) Results from fetal liver-derived MKs (embryonic day 14.5, day 4 of culture). ***P < .001; **P < .01.
Together, these data strongly indicate redundant functions of Rac1 and Cdc42 in the late stages of platelet production in vivo. We therefore investigated the outcome of Rac1 and/or Cdc42 deficiency on proplatelet formation in vitro, using BM- and fetal liver cell (FLC)-derived MKs (Figure 5B-C). Rac1−/− MKs formed proplatelets to a similar extent as wt platelets, whereas in Cdc42−/− MKs, proplatelet formation was moderately, but significantly, reduced, demonstrating that Cdc42 is of greater relevance for platelet production than Rac1. Strikingly, proplatelet formation was markedly reduced in FLC-derived MKs (Figure 5C) and nearly abrogated in BM-derived MKs (Figure 5B) from Rac1/Cdc42−/− mice.
These results were in agreement with our observations from TEM and immunohistochemistry and demonstrated a critical functional redundancy of Rac1 and Cdc42 in the terminal stages of platelet production in vitro and in vivo.
Altered microtubule structure in Rac1/Cdc42−/− MKs
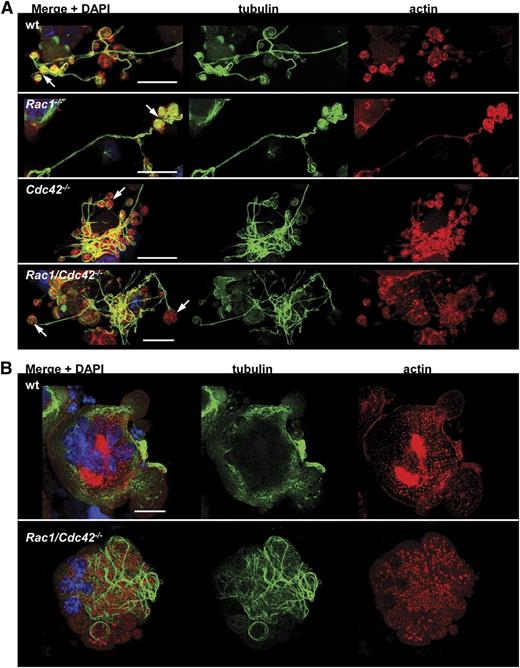
To further analyze the effect of Rac1 and/or Cdc42 deficiency on cytoskeletal rearrangements during proplatelet formation, actin and tubulin distribution was investigated in cultured FLC-derived MKs. In wt MKs, actin and tubulin mostly colocalized in the proplatelet buds, and a similar organization was found in Rac1−/− and most Cdc42−/− MKs (Figure 6A). In contrast, the tips of the few proplatelets present in double-deficient MKs were mostly devoid of the marginal tubulin bundles (Figure 6A, bottom). Very interestingly, further analyses demonstrated that Rac1/Cdc42 double-deficiency severely affected tubulin organization (Figure 6B). In wt MKs at the stage of early pseudopod formation, tubulin was mostly organized in an accurate network (Figure 6B, upper). Early proplatelets from wt MKs displayed a characteristic structure of homogenously distributed actin and of organized tubulin bundles resembling bunches of grapes (Figure 7A, upper). In contrast, in double-deficient MKs, tubulin was organized in thick bundles, which only partially colocalized with actin. The observation that altered tubulin organization was most evident in very mature Rac1/Cdc42−/− MKs supports our hypothesis that specifically, the terminal stages of platelet production are affected by Rac1/Cdc42 double-deficiency, whereas MK maturation is still functional in the absence of both GTPases.
Altered tubulin structure in Rac1/Cdc42−/− MKs. (A-B) Analysis of actin and tubulin structure by confocal microscopy. Representative stainings for actin (red), tubulin (green), and DAPI (blue) from at least 3 different samples per group are shown. (A) Visualization of proplatelets from wt (upper), Rac1−/− (second), Cdc42−/− (third), and Rac1/Cdc42−/− (lower) MKs. Bar, 25 µm. (B) Start of proplatelet formation in mature wt (upper) and Rac1/Cdc42−/− (lower) MKs. Bar, 25 µm.
Altered tubulin structure in Rac1/Cdc42−/− MKs. (A-B) Analysis of actin and tubulin structure by confocal microscopy. Representative stainings for actin (red), tubulin (green), and DAPI (blue) from at least 3 different samples per group are shown. (A) Visualization of proplatelets from wt (upper), Rac1−/− (second), Cdc42−/− (third), and Rac1/Cdc42−/− (lower) MKs. Bar, 25 µm. (B) Start of proplatelet formation in mature wt (upper) and Rac1/Cdc42−/− (lower) MKs. Bar, 25 µm.
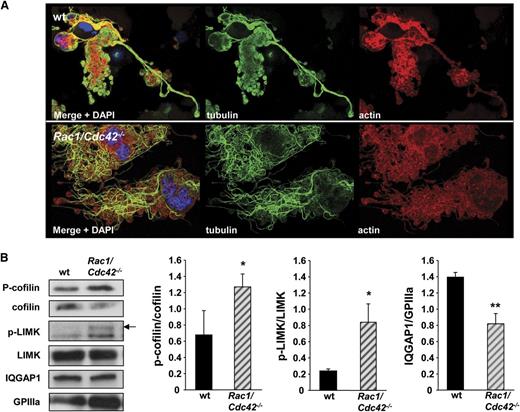
Altered tubulin structure in Rac1/Cdc42-/- MKs is associated with changes in cofilin activation. (A) Analysis of actin and tubulin structure in early proplatelet-forming MKs by confocal microscopy. Representative stainings for actin, tubulin, and DAPI from at least 3 different samples per group are shown. Bar, 25 µm. (B) Increased phosphorylation of cofilin and LIMK, and reduced expression level of IQGAP1 in Rac1/Cdc42−/− MKs. Western blot analysis of FLC-derived MKs. MKs on day 3 of culture were purified with a bovine serum albumin gradient, and protein lysates were obtained. Equal amounts of proteins were loaded, and the expression was detected with specific antibodies and quantified by densitometry with Image J software (National Institutes of Health). Shown blots are representative of at least 3 independent samples. **P < .01; *P < .05.
Altered tubulin structure in Rac1/Cdc42-/- MKs is associated with changes in cofilin activation. (A) Analysis of actin and tubulin structure in early proplatelet-forming MKs by confocal microscopy. Representative stainings for actin, tubulin, and DAPI from at least 3 different samples per group are shown. Bar, 25 µm. (B) Increased phosphorylation of cofilin and LIMK, and reduced expression level of IQGAP1 in Rac1/Cdc42−/− MKs. Western blot analysis of FLC-derived MKs. MKs on day 3 of culture were purified with a bovine serum albumin gradient, and protein lysates were obtained. Equal amounts of proteins were loaded, and the expression was detected with specific antibodies and quantified by densitometry with Image J software (National Institutes of Health). Shown blots are representative of at least 3 independent samples. **P < .01; *P < .05.
To examine potential mechanisms underlying the observed tubulin organization, we investigated the expression of different potential activators and effectors of Rac1/Cdc42 in FLC-derived MKs by western blot. Phosphorylation of the Rac1 and Cdc42 activating protein p70 S6 kinase21 was similar in wt and Rac1/Cdc42−/− MKs (supplemental Figure 3). An intense regulatory crosstalk between the 3 major Rho GTPases Rac1, Cdc42, and RhoA has been described8 ; however, we found that the expression levels of RhoA and its downstream effector mDia1 were not significantly altered in Rac1/Cdc42−/− MKs (supplemental Figure 1). Interestingly, double-deficient MKs displayed a pronounced increase in the amounts of the phosphorylated (inactive) form of the actin turnover-regulating protein cofilin, which is also seen in Cdc42−/− platelets (Figure 7B and Pleines et al12 ). Cofilin phosphorylation is induced downstream of Rac1 and Cdc42 via sequential phosphorylation of PAK and LIMK. Levels of phosphorylated LIMK were indeed significantly increased in double-deficient MKs (P = .04); however, we were not able to detect phosphorylated PAK isoforms in either wt or Rac1/Cdc42−/− MKs (Figure 7B and data not shown).
Another recently emerging effector protein of Rac1 and Cdc42 is the scaffolding protein IQGAP1, which is implicated in the coordination of multiple cellular signaling processes leading to actin polymerization and tubulin multimerization in cell types other than platelets.22 Intriguingly, IQGAP1 expression was significantly reduced in Rac1/Cdc42−/− MKs compared with in the wt MKs (P = .007). Together, these results indicate potential roles for cofilin and IQGAP1 in regulating actin and microtubule dynamics downstream of Rac1/Cdc42 during platelet production.
Discussion
To date, little is known about the detailed roles of Rho GTPases in the regulation of cytoskeletal rearrangements during MK maturation and platelet formation. However, the observations that deficiency of Rac1,11 Cdc42,12 or RhoA23 has differential effects on platelet counts indicate important roles for Rho GTPases in platelet production. Our data now show that whereas deletion of either Rac1 or Cdc42 had no or only a mild effect on platelet counts, respectively, combined deletion of both Rho GTPases resulted in severe macrothrombocytopenia. The few circulating Rac1/Cdc42−/− platelets displayed a severely abnormal morphology and multiple functional defects, resulting in a bleeding phenotype and abrogated thrombus formation in vivo (Figure 2E; supplemental Figure 2). The thrombocytopenia in the double-deficient mice was associated with normal MK ploidy (Figure 4C), suggesting that Rac1 and Cdc42 are not essentially required to establish a polyploid nucleus during MK maturation.
Importantly, our findings indicate that overlapping Rac1- and Cdc42-mediated signaling is crucial, specifically for the final stages of platelet production, as loss of both Rac1 and Cdc42 virtually abrogated proplatelet formation in vitro (Figure 5B-C). Likewise, mature BM MKs from Rac1/Cdc42−/− mice exhibited a highly abnormal morphology in vivo (Figure 5A). Thus, Rac1- and Cdc42-induced signaling seems to be required not only for efficient formation of demarcation membranes and synthesis and trafficking of granules but also for the maintenance of the structural integrity of mature MKs. The resulting uncontrolled fragmentation of Rac1/Cdc42−/− MKs within the BM provides a further explanation for the severe thrombocytopenia seen in the double-deficient mice (Figure 4B).
Of note, our study also provides novel insights into the individual roles of Rac1 and Cdc42 in platelet production. Whereas our results confirm that Rac1 is dispensable for platelet production, we interestingly found that proplatelet formation of Cdc42 single-deficient MKs was significantly reduced in vitro, and BM MKs showed a partially altered morphology. In line with these observations, Cdc42 also may positively regulate proplatelet formation in human MKs.24 Together with the similarly reduced platelet life span upon Cdc42 single- and Rac1/Cdc42 double-deficiency, this indicates that Cdc42 represents the major regulator of platelet production and function, whereas Rac1 fulfills supporting functions. This is reflected by the complex phenotype of Cdc42-deficient platelets.12
Our results reveal that defective proplatelet formation in double-deficient MKs (and to some extent also in Cdc42 single-deficient MKs) was associated with a severely altered microtubule structure, whereas actin distribution was not dramatically affected (Figures 6 and 7A).
To our knowledge, our results for the first time demonstrate redundant roles of Rac1 and Cdc42 in the regulation of microtubule dynamics during platelet production. Microtubules are crucial for the movements of granules and organelles within proplatelets,25 as well as for final platelet sizing in the blood,26 and mice lacking the hematopoietic-specific β tubulin isoform display defective platelet production, reduced platelet counts, and roundish platelet morphology.27 Thus, the defect in microtubule stabilization provides a plausible explanation for the macrothrombocytopenia in Rac1/Cdc42−/− platelets. Supporting our hypothesis, we observed that tubulin was virtually absent in most proplatelet tips from double-deficient MKs and that the marginal tubulin band was abnormally organized in double-deficient platelets (Figures 2C, 6A, and 7A).
The exact mechanism by which Rac1 and Cdc42 regulate microtubule dynamics in MKs remains to be investigated. Interestingly, our results reveal altered regulation/expression of 2 Rac1 and Cdc42 downstream signaling molecules: cofilin and IQGAP1. We found significantly increased amounts of phosphorylated (inactive) cofilin and its upstream regulator LIMK in Rac1/Cdc42−/− MKs and platelets (Figure 7B), a phenotype to some extent also present in Cdc42−/− platelets.12 As Cdc42 single-deficiency alone already significantly reduced proplatelet formation (Figure 5B-C), this supports our assumption of the involvement of cofilin in proplatelet formation and platelet production, potentially by regulating microtubule stabilization in MKs. It is noteworthy in this context that cofilin-deficiency in MKs significantly impairs platelet production while not leading to obvious defects in MK microtubule morphology and proplatelet formation.28 However, it is difficult to directly compare the outcome of the complete absence of cofilin with that of altered protein activity present here.
In line with our findings in MKs, knockout studies demonstrated that both Rac1 and Cdc42 play important roles in neurite polarity and outgrowth, processes that are in many respects similar to proplatelet formation and are also highly dependent on microtubule stabilization.29,30 Intriguingly, increased cofilin phosphorylation was also observed in Cdc42−/−-deficient neurons, and cofilin knockdown in wt neurons resulted in a phenotype similar to Cdc42 deficiency.29
One important Rac1/Cdc42 effector mediating cofilin phosphorylation is PAK, via activation of the downstream effector LIMK. However, whereas LIMK phosphorylation was significantly increased, we were not able to detect significant amounts of (phosphorylated) PAK in double-deficient or wt MKs (Figure 7B and not shown). This might indicate that as observed in neurons, increased cofilin and LIMK phosphorylation is mediated by decreased phosphatase activity, rather than increased PAK activity.29 Of note, cofilin phosphorylation can also occur by RhoA-mediated activation of LIMK. However, levels of RhoA and its downstream effector mDia1 were not significantly altered in double-deficient compared with wt MKs, which is in line with observations made in Rac1- or Cdc42-deficient neurons (supplemental Figure 3).29,30 It therefore seems unlikely that a compensatory upregulation of RhoA is responsible for the observed phenotype in our system.
A direct role of cofilin in modulating tubulin rearrangements has not been described to date. Recently, however, a pharmacologic approach demonstrated that inhibition of the cofilin phosphorylating enzyme LIMK stabilizes microtubules.31 Moreover, the formation of aggregates consisting of active cofilin and actin (so-called cofilin rods) were observed in the brains of patients with Alzheimer disease.32 Studies using mice later demonstrated that cofilin rods might influence microtubule organization and intracellular trafficking by affecting the redistribution and phosphorylation of microtubule associated protein.33,34
Together, these studies strongly support the hypothesis that cofilin may be involved in the regulation of tubulin dynamics and stability, and thereby platelet production in MKs.
In addition to cofilin deregulation, our study interestingly reveals reduced expression of the scaffolding protein IQGAP122 in Rac1/Cdc42−/− MKs, for the first time indicating IQGAP1 as a regulator of actin/microtubule dynamics during platelet production. The exact function of IQGAP1 in these processes remains to be investigated. However, its established function as a linker of actin and microtubule dynamics downstream of Rac1 and Cdc42, but not RhoA,35 makes it a promising novel candidate for the regulation of these processes in MKs.
In summary, the results presented here demonstrate for the first time that Rac1/Cdc42-controlled regulation of microtubule dynamics, potentially via effectors such as cofilin and IQGAP1, is critical for the terminal stages of platelet production in vivo.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylvia Hengst and Jonas Müller for excellent technical assistance. The authors also thank the microscopy platform of the Bioimaging Center (Rudolf Virchow Center).
This work was supported by the Deutsche Forschungsgemeinschft (NI 556/9-1 and Sonderforschungsbereich 688 [B.N.]) and the Rudolf Virchow Center.
Authorship
Contribution: I.P. and S.D. performed experiments, analyzed data, and wrote the paper; D.C., A.E., I.M., and M.M. performed experiments and analyzed data; G.K., H.S., C.G., N.D., and C.B. provided vital new reagents and contributed to the writing of the paper; and B.N. designed research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Department of Experimental Biomedicine-Vascular Medicine, University Hospital and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.
References
Author notes
I.P. and S.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal