Key Points
The results met predefined goals in poor-risk older patients with non-M3 AML.
The results in good-risk patients are comparable to those with chemotherapy-based regimens, with a better safety profile.
Abstract
This trial tested the safety and efficacy of a regimen consisting of hydroxyurea followed by azacitidine, 75 mg/m2 for 7 days, and gemtuzumab ozogamicin, 3 mg/m2 on day 8, in older patients with newly diagnosed acute myeloid leukemia. Those achieving a complete remission received 1 consolidation treatment followed by 4 cycles of azacitidine. The patients were stratified into good-risk (age 60-69 years or performance status 0-1) and poor-risk (age ≥70 years and performance status 2 or 3) groups. Specific efficacy and safety goals were defined as being supportive of further study of the regimen. Eighty-three patients were registered in the good-risk cohort and 59 in poor-risk cohort, with median age of 71 and 75 years, respectively. In the good-risk group, 35 patients (44%) achieved a complete remission. Median relapse-free and overall survivals were 8 and 11 months, respectively. Six patients (8%) died within 30 days of registration. In the poor-risk group, 19 (35%) achieved a complete remission. Median relapse-free and overall survivals were 7 and 11 months, respectively. Seven patients (14%) died early. The results of this trial met predefined goals for efficacy and safety for the poor-risk cohort but not the good-risk group. This trial was registered at www.clinicaltrials.gov as #NCT00658814.
Introduction
The median age at the time of diagnosis of acute myeloid leukemia (AML) in the United States is 68 years. With increasing age, the response rates to standard chemotherapy decrease and early mortality increases. Data from 5 SWOG trials show that the complete response (CR) rates to chemotherapy-based treatment regimens fall from 65% for patients <56 years of age to 46%, 39%, and 33% with each additional decade of life. Correspondingly, 30-day death rates increase from 2.7% to 11.2%, 20%, and 31.2%.1 The poor outcomes in the elderly patients with AML are associated with progressive decline in performance status, decrease in the percentage of patients with favorable karyotype, and increase in the frequency of poor-risk cytogenetic abnormalities.1-3
Although patients older than age 60 or 65 years are generally defined as older in most AML trials, there is much debate about what should be the standard treatment of such patients. The recommended therapies for AML in the older age group are diverse,4 including supportive care alone,5 low-dose cytarabine, or intensive chemotherapy.4,6,7 A small number of studies show that the elderly patients tolerate high doses of daunorubicin and similar agents quite well but in patients over the age of 65 years, the outcomes do not appear to be better than those with standard doses of anthracyclines.8,9 Addition of colony-stimulating factors10 or agents to reverse drug resistance11 has also not shown any advantage. Data from large studies conducted by SWOG as well as other major oncology groups show very little improvement in the outcomes in elderly patients with AML over the last 4 decades and only 5% to 10% patients achieve durable remission. These data highlight the need for safer and more effective approaches in these patients.
Azacitidine and gemtuzumab ozogamicin (GO) are active in the treatment of AML. Azacitidine, a pyrimidine nucleoside analog, inhibits DNA methyltransferase and causes hypomethylation in the CpG islands of the promoter region of selected genes.12-15 As a single agent, azacitidine induces an overall response rate of 25% and a CR rate of 10% in elderly patients with AML.16 In patients with low marrow blast count, it prolongs overall survival.17 GO, a recombinant humanized antibody to CD33 conjugated with the cytotoxic antitumor antibiotic calicheamicin, induces responses (CR and complete response with incomplete blood count recovery [CRi]) in about 30% of patients with relapsed AML.18 In newly diagnosed patients who are 70 years of age or older, the CR rate with GO as a single-agent therapy was 14%.19
We chose a combination of 3 agents, hydroxyurea, azacitidine, and GO, as the treatment regimen for this study. Hydroxyurea was used primarily to lower the white blood cell (WBC) count.20 There are several reasons to believe that the combination of azacitidine and GO may be effective against AML. Azacitidine induces maturation of AML blasts and increases CD33 expression which enhances uptake of GO by these cells. It also decreases expression of p-glycoprotein, which mediates resistance to GO.21 Azacitidine increases expression of Syk and SHP1,22,23 which are important for GO-induced cytotoxicity. When CD33 is ligated by a monoclonal antibody, it acts as a down-regulator of growth in a Syk-dependent manner. In vitro experiments show that response of AML blasts to GO depends upon Syk and SHP1 expression. In some instances, azacitidine induced Syk-negative blasts to become Syk positive. Experiments using fresh samples from patients with AML showed that azacitidine increased GO-mediated cytotoxicity significantly.
In AML, hypermethylation of a number of genes such as p15/Ink4b, p16/Ink4a, Apaf, HIC-1, RAR-β, and E-cadherin has been reported.15 Reversal of this process with demethylating agents allows induction of p21 and re-expression of p73, a tumor-suppressor gene in AML cells. Yang and associates14 have shown that by day 5 of decitabine therapy in patients with AML, methylation of repetitive DNA elements decreases by 9% to 16%, and with continued exposure, about 80% of the samples show a decrease in the methylation by 33%. However, the relationship between methylation status and response to therapy has not been firmly established. Although samples were collected for biomarker studies, the results of these studies will be reported separately.
Our aim was to design a treatment regimen that was effective and had limited toxicity. We had previously tested this therapy in a pilot study24 with promising results, which became the basis of this trial.
Methods
Patients
Patients with a newly diagnosed non-M3 AML under the WHO classification, both de novo and secondary, who had reached their 60th birthday and had a performance status of Zubrod 0-3 were eligible for entry into this study. Patients with a prior history of myelodysplastic syndrome (MDS) were allowed, but previous therapy with azacitidine, decitabine, or GO was not permitted. Patients could have received previous therapies for MDS with hematopoietic growth factors, thalidomide, lenalidomide, arsenic trioxide, and low-dose cytarabine. All previous therapies must have been discontinued for at least 30 days prior to registration. Hydroxyurea, but not systemic therapy for AML, was allowed. Patients with central nervous system involvement and a diagnosis of a malignancy (except for basal cell cancer of the skin) in the previous 2 years were excluded. HIV-positive patients were allowed if they had no AIDS-defining illness, had CD4 cell count 500/μL or higher, and viral load of <50 copies of HIV messenger RNA. Liver and renal functions had to be ≤2 times the upper limit of normal and left ventricular ejection fraction ≥40%.
Study design and treatment
This was a multi-institution phase 2 trial in which the participants were stratified in 2 groups, good risk or poor risk, based on previous results seen in SWOG studies. Good-risk patients were defined as those aged 60 to 69 years or those with performance status of Zubrod 0-1. Poor-risk patients were defined as those who were at least 70 years old and had a performance status of 2 or 3. Both groups were treated in a similar fashion. The objectives of the study were to determine whether the proposed regimen was safe (evaluated with 30-day mortality) and effective (evaluated with CR/CRi rate) enough to warrant a phase 3 study for each cohort.
Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The study was approved by the institutional review boards of participating institutions. In accordance with good medical practice, the following tests and evaluations were recommended in all patients: chest x-ray, electrocardiogram, serum glucose, electrolytes, magnesium and calcium levels, and a dental evaluation. All patients were started on allopurinol 300 mg orally from the time of diagnosis until day 14 of the induction course.
Induction therapy
In stable patients, induction therapy could be given in an outpatient setting. Patients with initial WBC count ≥10 000/mcL were started on hydroxyurea 1500 mg orally twice daily or in higher doses if necessary. In patients with symptoms or signs of hyperleukocytosis or WBC count ≥100 000/mcL, leukapheresis was recommended. Once the WBC count was <10 000/mcL, hydroxyurea was stopped and the patient was started on azacitidine 75 mg/m2 subcutaneously or intravenously daily for 7 days. Hydroxyurea was restarted if the WBC count rose during azacitidine therapy. On day 8, GO 3 mg/m2 was administered with appropriate premedications.
A bone marrow biopsy was performed on day 14. If the marrow showed <5% blasts, the patient was monitored closely. Colony-stimulating factors could be given at the discretion of the treating physician. If the day 14 marrow showed blast count of ≥5%, a second induction cycle, identical to the first, was administered. Bone marrow was repeated on day 29. Patients showing a marrow blast count ≥5% on day 29 were removed from the study. If day 29 bone marrow was difficult to interpret, repeat bone marrow on day 35 was permitted.
Consolidation therapy
Patients who achieved CR or CRi were eligible to register for consolidation therapy and given 1 cycle of consolidation therapy within 60 days after completion of induction therapy. Consolidation was identical to induction except that hydroxyurea was not included. Growth factor use was allowed at the discretion of the treating physician. A bone marrow biopsy was repeated between days 28 and 42 after completion of the consolidation therapy.
Maintenance therapy
Patients whose postconsolidation bone marrow biopsy showed continued remission were eligible to register for maintenance therapy, which was started within 42 days after completion of the consolidation treatment. Maintenance therapy consisted of 4 cycles of azacitidine 75 mg/m2 subcutaneously given every 28 days. A bone marrow biopsy was repeated approximately 28 days after completion of the fourth cycle of maintenance therapy. Subsequent management was left to the discretion of the patient’s treating physician.
Supportive care was carried out according to the local institution practice or guidelines.
No dose modifications were allowed during induction therapy. During consolidation, the dose of GO could not be changed, but the treatment could be delayed for abnormality of liver functions over 2 times the institutional upper limit of normal (IULN). The azacitidine dose could be reduced by 50% during consolidation and maintenance therapies for an absolute neutrophil count (ANC) <500 × 10−9/L and platelet count <25 000 × 10−9/L. The azacitidine dose was also reduced by 50% if renal functions became abnormal >2 times IULN. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3 was used to report toxicities.
Response criteria were as follows: a complete response was defined as <5% marrow blasts by morphology, no Auer rods, ANC ≥1000 × 10−9/L, platelet count ≥100 000 × 10−9/L, and no evidence of extramedullary disease. CRi was the same as CR but the ANC may be <1000 × 10−9/L and/or platelet count <100 000 × 10−9/L.
Cytogenetic and molecular studies
Besides standard karyotyping, the participating centers were requested to obtain FLT3 amplification/mutation and NPM1 and CEBPA mutations at the time of diagnosis.
Statistical methods
The primary objective of the trial was to test whether the induction regimen was safe (evaluated via 30-day mortality) and effective (evaluated via CR/CRi rates) enough to warrant a phase 3 study among the older patients with AML. The patients were stratified into 2 risk cohorts based on prior experience in SWOG with older patients treated using standard daunorubicin/cytarabine induction therapy1 : good risk (age between 60-69 years or performance status of 0-1) and poor risk (age 70 years or older and performance status of 2 or more) with independent statistical designs and accrual in each cohort.
The accrual goal for the good-risk cohort was 73 eligible patients, in 2 steps. In the first step, 30 eligible patients were to be accrued. If <12 achieved CR or CRi or <23 survived at least 30 days, the study would be terminated. Otherwise, an additional 43 patients were to be accrued. Based on previous SWOG trials, the null hypothesis for this cohort assumed a CR + CRi rate of 35% and a 30-day survival of 75%. For this cohort, the protocol specified criteria for “success” were (alternative hypothesis) a CR + CRi rate of 55% and 30-day survival of 90%.
The accrual goal for the poor-risk cohort was 66 eligible patients, in 2 steps. In the first step, 15 eligible patients were to be registered; if 2 or more patients had a CR/CRi and 8 or more patients survived at least 30 days, an additional 51 patients were to be enrolled. Based on previous SWOG trials, the null hypothesis for this cohort assumed a CR + CRi rate of 10% and a 30-day survival of 50%. For this cohort, the protocol specified criteria for supporting a phase 3 study (alternative hypothesis) were CR + CRi rate of 30% and 30-day survival of 70%.
Patient characteristics were examined using descriptive statistics. Overall survival and relapse-free survival were estimated using the Kaplan-Meier method. Differences in survival between cohorts were evaluated using the log-rank test and differences in categorical variables between cohorts were evaluated using the Fisher exact test. This study was not powered for any formal comparisons between cohorts.
Data collection and analyses for the trial were performed by the SWOG Statistical Center. All authors had access to primary clinical trial data.
Results
Among the 99 patients with de novo AML registered to the study, the median time between pathologic diagnosis and registration was 6 days. Among the 30 patients with MDS-related AML, the median time between pathologic diagnosis and registration to the study was 13 days. For the 2 patients with treatment-related AML, the interval between pathologic diagnosis and registration was 1 and 14 days.
Good-risk group
A total of 83 patients were accrued in the good-risk category. One was ineligible due to concurrent diagnosis of myeloma and 3 other patients were ineligible because they did not receive treatment. The remaining 79 evaluable patients are included in this analysis. Three patients were taken off the study during induction therapy, 1 for “declining health,” 1 for persistent high WBC count, and 1 for toxicity. Treatment was completed as planned in 75 patients. The median age was 71 years (range, 60-88 years) and 49 were men. Seventy-seven were white, 3 Hispanic, 1 black, and 1 Asian. Fifty-two (66%) had de novo and 27 (34%) had secondary AML. Cytogenetic data and molecular data are shown in Table 1. These data were not available on all patients.
Efficacy
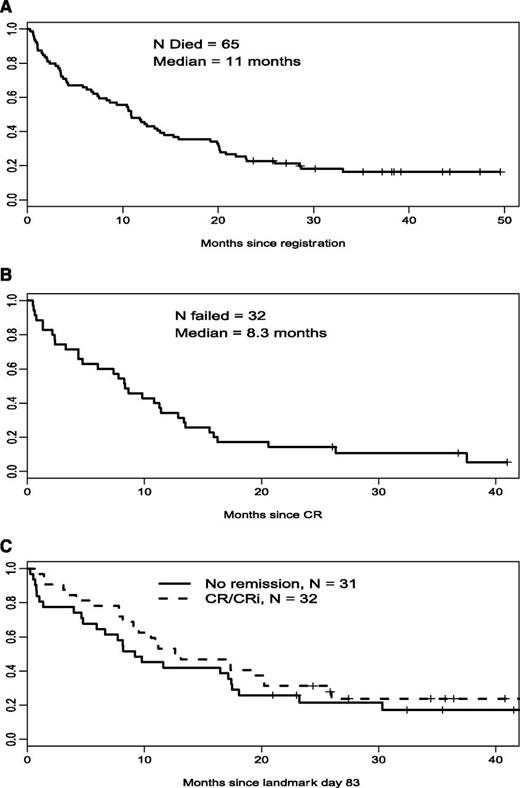
A total of 35 good-risk patients (44%) achieved CR (23 patients) or CRi (12 patients). Forty-one patients (52%) had resistant disease, 1 died <7 days after the first course, and in 1 patient assessment was inadequate. Median relapse-free survival was 8.3 months and median overall survival was 11 months (Figure 1).
Overall survival, relapse-free survival, and landmark overall survival in good-risk patients. (A) Good-risk cohort, overall survival (n = 79). (B) Good-risk cohort, relapse-free survival (n = 35). (C) Good-risk cohort, landmark survival.
Overall survival, relapse-free survival, and landmark overall survival in good-risk patients. (A) Good-risk cohort, overall survival (n = 79). (B) Good-risk cohort, relapse-free survival (n = 35). (C) Good-risk cohort, landmark survival.
Toxicity
In the good-risk group, 54 patients reported a grade 3 or higher nonhematologic event. The most frequent toxicity was neutropenic fever (28 grade 3 and 3 grade 4). Other common events included infections (21 grade 3); thromboembolism, 2; central nervous system hemorrhage, 1; and anorexia and hyperbilirubinemia. There were 4 fatal toxicities: disease progression, 2; infection, 1; and sudden death, 1. There were no reports of veno-occlusive disease. Six patients died within 30 days of registration
Poor-risk group
A total of 59 patients were registered of whom 2 were ineligible and 3 did not receive protocol therapy and were not analyzable, leaving 54 evaluable patients available for analysis. Median age was 75 years (70-87 years) and 33 were men. Five patients had preexisting MDS or had received myelotoxic treatment. Fifty patients were white and 4 were black. Thirty-eight had a performance status (PS) of 2 and the other 16 had a PS of 3. The cohort met the protocol-defined criteria for efficacy before reaching full accrual and the study was closed before accruing 66 evaluable patients.
Efficacy
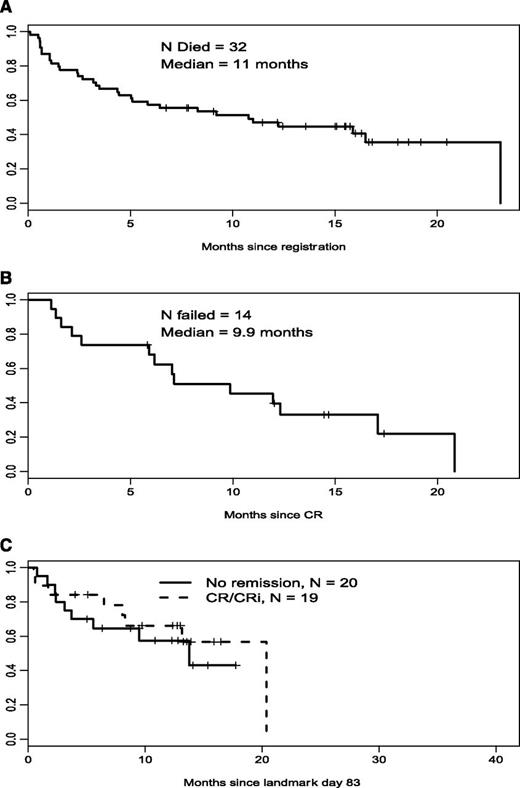
Nineteen patients (35%) achieved a CR or a CRi. One patient achieved a partial remission (recovery of neutrophil count to >1000 × 10−9/L, platelet count to >100 000 × 10−9/L, and at least a 50% decrease in percentage of marrow blasts, or marrow blasts <5% with persistence of Auer rods). Median relapse-free survival for this group was 7 months and median overall survival was 11 months (Figure 2).
Overall survival, relapse-free survival, and landmark overall survival in poor-risk patients. (A) Poor-risk cohort, overall survival (n = 54). (B) Poor-risk cohort, relapse-free survival (n = 19). (C) Poor-risk cohort, landmark survival.
Overall survival, relapse-free survival, and landmark overall survival in poor-risk patients. (A) Poor-risk cohort, overall survival (n = 54). (B) Poor-risk cohort, relapse-free survival (n = 19). (C) Poor-risk cohort, landmark survival.
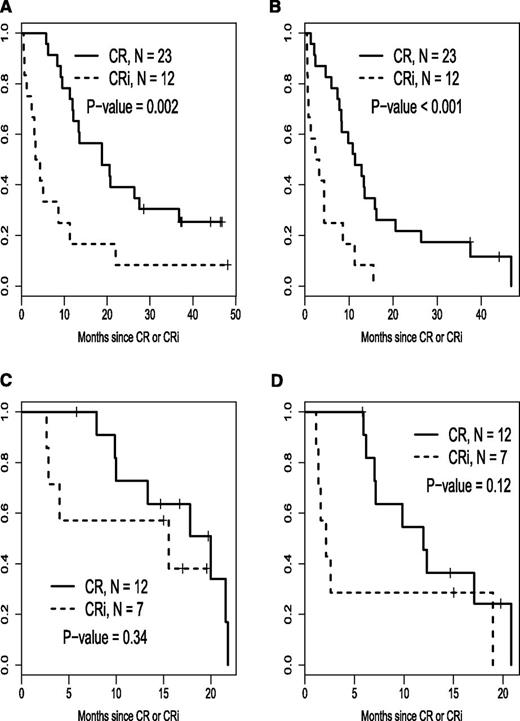
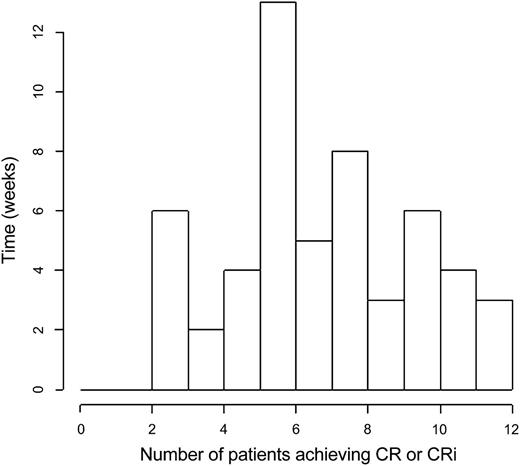
Relapse-free survival stratified by CR vs CRi for each risk cohort is illustrated in Figure 3. Time to best response, calculated from the start of induction treatment to the date of bone marrow examination documenting the best response (CR or CRi) is depicted in a histogram in Figure 4.
Relapse-free and overall survival stratified by CR vs CRi for each risk cohort. (A) Good-risk cohort, overall survival after response. (B) Good-risk cohort, relapse-free survival. (C) Poor-risk cohort, overall survival after response. (D) Poor-risk cohort, relapse-free survival.
Relapse-free and overall survival stratified by CR vs CRi for each risk cohort. (A) Good-risk cohort, overall survival after response. (B) Good-risk cohort, relapse-free survival. (C) Poor-risk cohort, overall survival after response. (D) Poor-risk cohort, relapse-free survival.
Histogram of time to best response, calculated from the start of induction treatment to the date of bone marrow examination documenting the best response (CR or CRi).
Histogram of time to best response, calculated from the start of induction treatment to the date of bone marrow examination documenting the best response (CR or CRi).
Toxicity
In the poor-risk group, 37 patients reported grade 3 or higher nonhematologic events. Five had fatal toxicities: 2 due to infection, 1 due to multiorgan failure, 1 due to neutropenic fever and multiorgan failure, and 1 due to respiratory failure and hypoxia. Nine additional patients had grade 4 nonhematologic toxicities. There were no reports of veno-occlusive disease. Seven patients (13%) died within 30 days of registration. Nonhematologic toxicity during induction therapy for both risk groups is summarized in Table 2.
Effect of age and performance status
The study was not powered to make formal comparisons between the 2 risk cohorts. The CR/CRi rate in the good-risk group was 44% and in the poor-risk group was 35%. The difference between the 2 groups was not statistically different (Fisher exact test, P = .37). The early death rates were 8% in the good-risk group and 13% in the poor-risk group. This difference was also not significant (Fisher exact test, P = .38)
Median overall survival was 11 months in both cohorts (log rank, P = 0.51). The median relapse-free survival in the good-risk group and poor-risk groups was 8 months and 10 months, respectively, and the difference was not significant (log rank, P = .78). Survival after landmark date 83 was not significantly different between patients who had a CR/CRi and those who did not (good risk, P = .24; poor risk, P = .65). We note that this study was not powered for this comparison.
Association of cytogenetic and molecular abnormalities with outcomes
The patients were divided into 4 prognostic categories (favorable, intermediate, unfavorable, and indeterminate) by their cytogenetic abnormalities.25 There was no evidence that the CR rates varied by cytogenetic risk (good risk, P = .54; poor risk, P = 0.23).
Forty-four patients had FLT3 data (29 good risk; 15 poor risk). No poor-risk patients were positive for FLT3 tandem repeats or mutations, 3 good-risk patients (10%) had FLT3 abnormality. Among the 3 FLT+ patients in the good-risk group, 2 achieved a CR and among the 27 patients who were FLT3 negative, 13 (48%) achieved a CR/CRi (P = 1.00)
Forty-two patients had NPM1 data (28 good risk; 14 poor risk). No poor-risk patients were NPM1+. Three (11%) good-risk patients were NPM1+ (2 were FLT3 negative and 1 FLT3 positive). All 3 NPM1 patients in the good-risk group achieved a CR and among the 26 patients with negative NPM1 mutation, 11 (42%) achieved a CR/CRi (P = .10).
Other results
Four patients, 2 from the good-risk and 2 from the poor-risk group, achieved a complete remission after being removed from the study for persistent disease on day 28. These patients had shown significant reduction in the marrow blast counts and were continued on azacitidine therapy.
Eighty-nine patients received a second induction, 56 (71%) in the good-risk group and 33 (61%) in the poor-risk group. The median time to best response was 35 days (range, 13-83 days), the 25th percentile was 30 days and 75th percentile 55 days. About 30% of all patients in both good-risk and poor-risk cohorts received their induction therapy in the outpatient setting. The consolidation and maintenance therapies were given in the outpatient area.
To compare survival of patients who achieved a CR/CRi to those who did not, we considered a landmark survival analysis among patients who were alive on day 83 (the latest date a patient achieved a CR/CRi). There were no significant differences between survival among these subsets of patients (good risk, P = .33; poor risk, P = .55).
Discussion
In this trial, patients were categorized as good risk or poor risk based on age and performance status. Predefined thresholds based on prior experience in SWOG trials were established to determine whether the treatment regimen yielded sufficiently encouraging results to be considered for further study. The results show that the regimen reached its goal in the poor-risk group but not in the good-risk group. Thus, our results support further study of the SWOG S0703 treatment regimen in patients with AML who are 70 years old or older and have a performance status of 2-3. Our previous experience in SWOG with such patients treated with standard “7 + 3” induction was a CR rate of 29% and a 30-day mortality of 48% (Appelbaum et al1 ; F.A., unpublished data). The results of the current study show a CR rate of 35% and a 30-day mortality of 14%, suggesting that the current regimen provides a CR rate at least comparable to standard therapy, but with a much better safety profile. Median overall survival for both risk groups was 11 months which is higher than 4 to 9 months reported in previous SWOG studies.1 The historical experience that formed the basis for statistical goals of this study involved SWOG clinical trials for AML that had identical entry criteria and were carried out at the same institutions as S0703. Of course, short of a truly randomized trial, we cannot be assured that there were not unapparent differences between patient population on which the study was based and the actual patients seen. We also do not know how much advances in supportive care may have accounted for this improvement. Nor do we formally know whether these results are better than what could be achieved with either GO or azacitidine alone, although published data would suggest that neither drug is that active as a single agent.17,20
In contrast to the results in poor-risk patients, S0703 failed to meet its goal in good-risk patients (ie, those age 60-69 years, or over age 60 years with a performance status of 0-1). Prior experience in SWOG with such patients using a standard “7 + 3” regimen has been a CR rate of 45% and a 30-day mortality of around 17% (Appelbaum et al1 ; F.A., unpublished data). The results of S0703 showed a CR rate of 44% and a 30-day mortality rate of 8%. Although these results suggest activity that is comparable to our prior experience with standard “7 + 3” and a safety profile that may be somewhat better, these results were not felt to be enough of an advancement to warrant further study, based on our predefined protocol goals.
Whether it is possible to further identify particular subgroups within our patient population who are more or less likely to specifically benefit from this approach is uncertain. Comparison between the good-risk and poor-risk patients entered into SWOG S0703 did not show a statistically significant difference in efficacy and early death rates. The results were not associated with cytogenetic risk, FLT3, or NPM1 status among patients with these data.
There were 4 patients who, after being removed from the study for persistent disease on day 28, were continued on azacitidine therapy and achieved complete remission. The reason for continuation of therapy was a significant reduction in the disease burden in the marrow, which did not reach criteria for remission. This suggests that by allowing longer duration of azacitidine therapy, an additional number of patients may achieve complete remission. Our treatment schema also included only 4 cycles of maintenance therapy with azacitidine. In future trials, prolongation of azacitidine therapy may extend progression-free and overall survival. This may be an attractive option in patients presenting with low marrow blast count, as has been suggested by others.17
Currently, there is no widely accepted standard of care for patients over 70 years of age with AML with a performance status of 2 or higher. Commonly used agents include hydroxyurea, low-dose cytarabine, hypomethylating agents, or standard “7 + 3”. Unfortunately, none of these approaches are particularly active, with low response rates or high treatment-related mortalities. Newer approaches are necessary and the results of this trial offer a reasonable alternative for this group of patients.
Our results add to the recent appeal26,27 for bringing GO back as a treatment option for patients with AML. There have been 5 major trials that added GO to their treatment regimens.28-32 Of these, 4 showed an increase in progression-free and/or overall survival. A retrospective study33 has shown that GO as the first-line treatment of AML in patients over the age of 70 years who have intermediate-risk cytogenetic abnormalities may be beneficial.
In summary, the results of the SWOG S0703 trial demonstrate that it is possible to use a potentially outpatient regimen of hydroxyurea, azacitidine, and GO for induction and postremission therapy in older patients with AML and obtain complete response rates that are comparable to those obtained with chemotherapy-based regimens. The toxicity of this new combination is low. Prolonged “maintenance therapy” with azacitidine may improve the results further. The results of this trial are sufficiently encouraging to warrant a phase 3 study, particularly in patients who are 70 years old or older with a poor performance status.
Presented in part at the American Society of Clinical Oncology annual meeting (Chicago, IL, June 1-5, 2012) and at the American Society of Hematology annual meeting (Atlanta, GA, December 8-11, 2012).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Patricia Arlauskas for her assistance in the preparation of this manuscript.
This work was supported in part by the following Public Health Service (PHS) Cooperative Agreement grant numbers awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (DHHS): CA32102, CA38926, CA46282, CA46136, CA27057, CA45377, CA14028, CA45807, CA86780, CA35176, CA11083, CA46441, CA12644, CA35431, CA45808, CA35178, CA63848, CA35261, CA67575, CA16385, CA58882, and in part by Celgene Corporation and Pfizer, Inc.
Authorship
Contribution: S.N., F.R.A., M.O., J.E.G., C.L.W., and T.H.N. conceived and designed the study; S.N., J.E.G., D.S.H., S.E.C., and H.P.E. provided study materials or patients; S.N., M.O., T.H.N., F.R.A., and H.P.E. collected and assembled data; S.N., M.O., F.R.A., and H.P.E. analyzed and interpreted data; and all authors wrote the manuscript.
Conflict-of-interest disclosure: S.N. performed a consultant/advisory role and received honoraria and research funding from Celgene. H.P.E. received honoraria from Celgene for their speakers bureau. The remaining authors declare no competing financial interests.
Correspondence: Sucha Nand, Loyola University Medical Center, Cardinal Bernardin Cancer Center, 2160 South First Ave, Room 345, Maywood, IL 60153; e-mail: snand@lumc.edu.