Key Points
Recessive mutations in the thrombopoietin gene are a novel cause of aplastic anemia.
Such patients may benefit from treatment with eltrombopag or romiplostim.
Abstract
We recently identified 2 siblings afflicted with idiopathic, autosomal recessive aplastic anemia. Whole-exome sequencing identified a novel homozygous missense mutation in thrombopoietin (THPO, c.112C>T) in both affected siblings. This mutation encodes an arginine to cysteine substitution at residue 38 or residue 17 excluding the 21-amino acid signal peptide of THPO receptor binding domain (RBD). THPO has 4 conserved cysteines in its RBD that form 2 disulfide bonds. Our in silico modeling predicts that introduction of a fifth cysteine may disrupt normal disulfide bonding to cause poor receptor binding. In functional assays, the mutant-THPO–containing media shows two- to threefold reduced ability to sustain UT7-TPO cells, which require THPO for proliferation. Both parents and a sibling with heterozygous R17C change have reduced platelet counts, whereas a sibling with wild-type sequence has normal platelet count. Thus, the R17C partial loss-of-function allele results in aplastic anemia in the homozygous state and mild thrombocytopenia in the heterozygous state in our family. Together with the recent identification of THPO receptor (MPL) mutations and the effects of THPO agonists in aplastic anemia, our results have clinical implications in the diagnosis and treatment of patients with aplastic anemia and highlight a role for the THPO-MPL pathway in hematopoiesis in vivo.
Introduction
Aplastic anemia is a serious and potentially life-threatening hematopoietic disorder affecting the bone marrow tri-lineage cells. The majority of aplastic anemia represents an immunologically mediated acquired disease state.1 Familial aplastic anemia is recognized in patients with disorders such as Fanconi anemia and Dyskeratosis congenita.1 Genes known to be risk factors for aplastic anemia include the telomerase RNA component (TERC), telomerase reverse transcriptase (TERT), interferon-γ (IFNG), Nibrin (NBN), Perforin 1 (PRF1), and Shwachman-Bodian-Diamond syndrome gene (SBDS).2-4 Most recently, homozygous mutations were identified in the receptor for thrombopoietin (THPO), Myeloproliferative-Leukemia virus oncogene (MPL), using homozygosity mapping followed by exome sequencing.5
Human THPO (also previously referred to as TPO) gene maps to chromosome 3q27.1 (184 089,773-184 095,9326 160) and is 6160 bp long with 6 exons that encode 332 amino acids, excluding a 21-amino acid signal peptide (Figure 1A).6-11 It is divided into an N-terminal RBD of 153 amino acids, which contains 4 evolutionarily conserved cysteine residues and a C-terminal domain of 179 amino acids, which contains 6 potential N-linked glycosylation sites. The amino-terminal RBD itself can stimulate human megakaryopoiesis in vitro and is highly conserved with homology to erythropoietin.12 THPO is expressed mainly in hepatocytes, renal convoluted tubular cells, bone marrow stromal cells, spleen,14-16 and in a few other regions such as amygdala and hippocampus in the central nervous system.17-19 The interaction of THPO with its receptor is largely responsible for megakaryopoiesis and platelet activation as well as the maintenance of hematopoietic stem cells. This THPO-MPL interaction initiates several intracellular signaling cascades, including the JAK/STAT, RAS/MAPK, and PI3K/AKT pathways (Figure 1B).13,20 In mice, knockout studies for either Thpo or Mpl show a significant decrease in the numbers of myeloid, erythroid, megakaryocytic, and burst colony-forming units, but only peripheral blood thrombocytopenia.21-24 Heterozygous gain-of-function mutations in THPO cause thrombocythemia, a chronic myeloproliferative syndrome that results in elevated numbers of circulating platelets, thrombotic or hemorrhagic episodes, and occasional leukemic transformation.25-29 Autosomal dominant thrombocytopenia is known to be caused by mutations in the ANKRD26, MASTL, ACBD5, and cytochrome c (CYCS) genes.30-36 Mutations in the THPO receptor (MPL) cause various hematologic disorders, including congenital amegakaryocytic thrombocytopenia37 and thrombocythemia.38,39 More recently, Walne et al5 reported homozygous recessive mutations in MPL causing aplastic anemia in 2 unrelated Tunisian and Pakistani families. However, mono- or bi-allelic loss-of-function mutations in THPO have not been described so far.
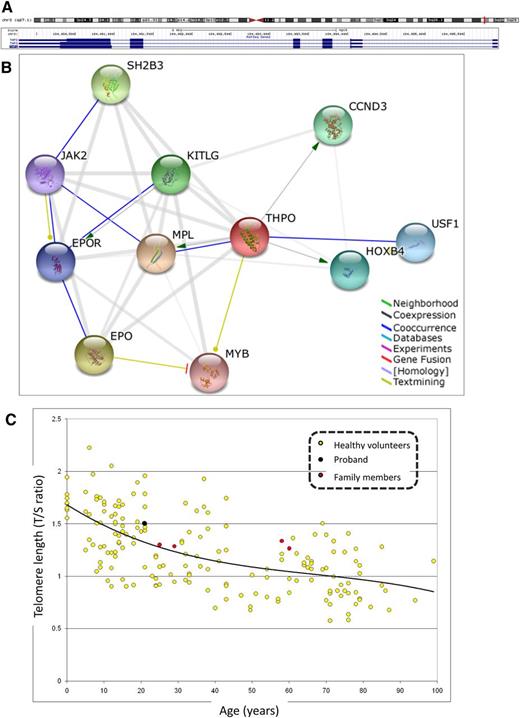
Structure and function of human THPO gene (NM_000460) and telomere length analysis. (A) THPO isoform 1 precursor (chr3q27.1:184 089,773-184 095,9326 160) is extensively alternatively spliced with multiple isoforms (http://genome.ucsc.edu/). Note that THPO is transcribed on the reverse strand. (B) Predicted functional partners of THPO gene (http://string-db.org/). Modes of interaction are shown in different colors with stronger associations represented by thicker lines. Combined score in parentheses. CCND3, cyclin D3 (0.939); EPO, erythropoietin (0.954); EPOR, erythropoietin receptor (0.899); HOXB4, homeobox B4 (0.932); JAK2, Janus kinase 2 (0.895); KITLG, KIT ligand (0.944); MPL, myeloproliferative leukemia virus oncogene (0.999); MYB, v-myb myeloblastosis viral oncogene homolog (avian) (0.892); SH2B3, SH2B adaptor protein 3 (0.944); USF1, upstream transcription factor 1 (0.931). (C) Telomere length in peripheral blood leukocytes is normal in proband and family. Telomere length was determined in the affected proband (black circle) and asymptomatic family members (red circles) by qPCR method and compared with healthy volunteers (yellow circles). No statistical difference was observed in telomere length of the proband or any other family member and control individuals.
Structure and function of human THPO gene (NM_000460) and telomere length analysis. (A) THPO isoform 1 precursor (chr3q27.1:184 089,773-184 095,9326 160) is extensively alternatively spliced with multiple isoforms (http://genome.ucsc.edu/). Note that THPO is transcribed on the reverse strand. (B) Predicted functional partners of THPO gene (http://string-db.org/). Modes of interaction are shown in different colors with stronger associations represented by thicker lines. Combined score in parentheses. CCND3, cyclin D3 (0.939); EPO, erythropoietin (0.954); EPOR, erythropoietin receptor (0.899); HOXB4, homeobox B4 (0.932); JAK2, Janus kinase 2 (0.895); KITLG, KIT ligand (0.944); MPL, myeloproliferative leukemia virus oncogene (0.999); MYB, v-myb myeloblastosis viral oncogene homolog (avian) (0.892); SH2B3, SH2B adaptor protein 3 (0.944); USF1, upstream transcription factor 1 (0.931). (C) Telomere length in peripheral blood leukocytes is normal in proband and family. Telomere length was determined in the affected proband (black circle) and asymptomatic family members (red circles) by qPCR method and compared with healthy volunteers (yellow circles). No statistical difference was observed in telomere length of the proband or any other family member and control individuals.
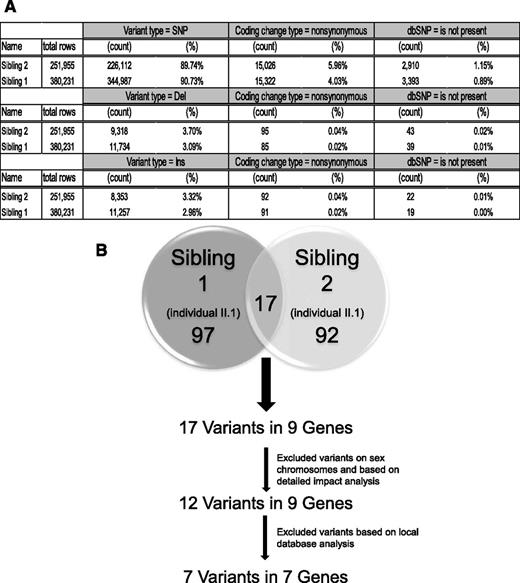
Bioinformatic analysis of exome sequence data to identify candidate homozygous variants. Exome sequence analysis was done in both affected siblings, and the resulting novel, nonsynonymous, homozygous variants were filtered using DNAnexus platform. Only 17 novel nonsynonymous homozygous SNPs were shared between the 2 affected siblings. Using additional filters, novel SNPs were limited to 7.
Bioinformatic analysis of exome sequence data to identify candidate homozygous variants. Exome sequence analysis was done in both affected siblings, and the resulting novel, nonsynonymous, homozygous variants were filtered using DNAnexus platform. Only 17 novel nonsynonymous homozygous SNPs were shared between the 2 affected siblings. Using additional filters, novel SNPs were limited to 7.
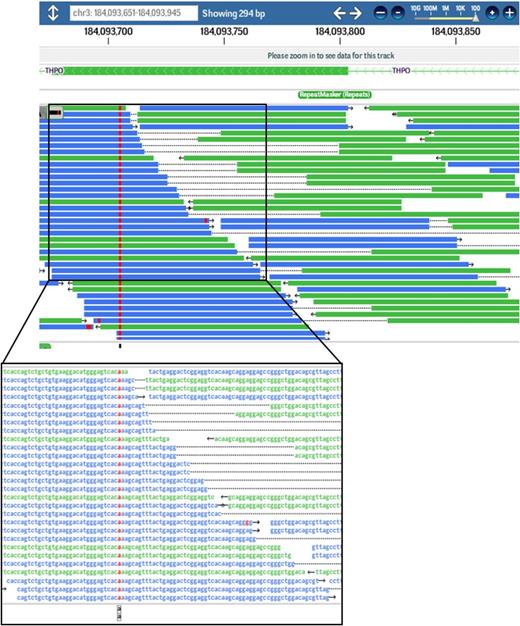
A novel homozygous THPO variant (c.CTG>TGT;p.Arg17Cys) was identified in both siblings affected with aplastic anemia. Screen shot of exome sequence reads (left) and DNA sequence abnormality shown on the reverse strand (red) as visualized by DNAnexus software.
A novel homozygous THPO variant (c.CTG>TGT;p.Arg17Cys) was identified in both siblings affected with aplastic anemia. Screen shot of exome sequence reads (left) and DNA sequence abnormality shown on the reverse strand (red) as visualized by DNAnexus software.
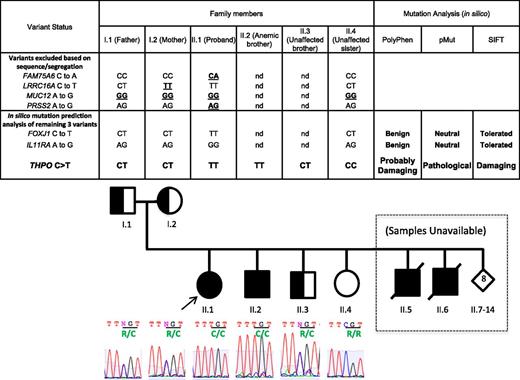
Genetic segregation analysis and in silico mutation prediction analyses of SNP variants identified by exome sequencing. Only the homozygous THPO variant (c.CGT>TGT;p.Arg17C) segregated with the aplastic anemia phenotype in the proband (II.1) and her asymptomatic affected brother (II.2) as shown in A and B. Amino acid substitution is highlighted in green under the affected codon.
Genetic segregation analysis and in silico mutation prediction analyses of SNP variants identified by exome sequencing. Only the homozygous THPO variant (c.CGT>TGT;p.Arg17C) segregated with the aplastic anemia phenotype in the proband (II.1) and her asymptomatic affected brother (II.2) as shown in A and B. Amino acid substitution is highlighted in green under the affected codon.
In this paper, we describe a large Micronesian family with several members with idiopathic aplastic anemia and some members with mild thrombocytopenia. We identify a novel pathogenic loss-of-function mutation in the THPO gene, which segregates with the severe and mild phenotypes in the homozygous and heterozygous state, respectively. This finding has therapeutic implications for this family and other individuals with aplastic anemia and thrombocytopenia due to mutations in THPO. It also highlights a role for the THPO/MPL pathway in hematopoietic stem cell regulation in vivo.
Materials and methods
Patient samples
Blood samples were collected from all patients included in this study after they signed an informed consent in accordance with the Declaration of Helsinki, approved by the Human Subjects Committee at the University of Kansas Medical Center.
Clinical presentation
The proband was a 24-year-old Micronesian female who presented at 16 years of age with menorrhagia and pancytopenia. Her 2 older brothers died at 9 and 18 years of age with idiopathic aplastic anemia. The remaining 11 siblings were reported to be healthy. Parental consanguinity was denied. The proband had a normal physical examination. Her bone marrow was hypocellular and no blasts were seen. She also had normal peripheral blood lymphocyte chromosome breakage and normal bone marrow karyotype (46,XX), serum α fetoprotein, and THPO levels (Quest Diagnostics, San Juan Capistrano, CA).
Telomere length measurement
Telomere length of pretreatment peripheral blood leukocytes was assessed by quantitative polymerase chain reaction (qPCR) as previously described.3,4 Briefly, total leukocyte DNA was extracted using the DNeasy Blood kit (Qiagen, Valencia, CA) and qPCR assays were performed in a Rotor Gene Q (Qiagen, Gaithersburg, MD). Each sample's telomere length (x) was based on the telomere/single copy gene ratio (T/S ratio) and based on the calculation of the ΔCt (Ct[telomeres]/Ct[single gene]). Telomere length was expressed as the relative T/S ratio, which was normalized to the average T/S ratio of reference sample (2−[ΔCtx – ΔCtr] = 2 − ΔΔCt), used for the standard curve, as reference sample, and as validation sample (Figure 1C).
SNP array CGH analysis
The Affymetrix GeneChip system for processing the Genome-Wide SNP 6.0 arrays was used for runs of homozygosity (ROH) and copy number analysis (Affymetrix, Santa Clara, CA). Briefly, the target DNA preparation was initiated with the restriction digestion of separate 250-ng aliquots of each genomic DNA using NspI and StyI restriction endonuclease. The individual restricted fragments were ligated with the appropriate Nsp or Sty adaptor containing a PCR Primer 002 priming site. The ligated sample was diluted and PCR amplified using the PCR Primer 002 and Titanium Taq (Clontech, Mountain View, CA). The Nsp and Sty amplified fragments were then pooled and purified using the SNPClean Magnetic beads (Beckman Coulter, Brea, CA) and the MultiScreen Deep Well Filtration System and plate vacuum manifold (Millipore, Billerica, MA). Following quantification, the combined Nsp and Sty purified target samples were fragmented with DNase I and end labeled with biotin using Terminal deoxynucleotidyl Transferase and DNA labeling reagent (Affymetrix). Biotin labeled-fragmented, single-stranded DNA was hybridized to the Genome-Wide SNP 6.0 arrays according to the manufacturer’s instructions. Hybridized, washed, and R-phycoerythrin-streptavidin–stained arrays were scanned using the GeneChip Scanner 3000. Data collection was performed using the Command Console ver. 1.1 software (Affymetrix). Single nucleotide polymorphism (SNP)-array CGH data were analyzed using Nexus copy number software (Biodiscovery, El Segundo, CA).
DNA preparation and next-generation sequencing
Genomic DNA (gDNA) was extracted from peripheral blood samples using standard methods, and submitted to Otogenetics Corporation (Norcross, GA) for exome capture and sequencing. Briefly, gDNA was subjected to agarose gel and OD ratio tests to confirm the purity and concentration prior to Covaris (Covaris, Inc., Woburn, MA) fragmentation. Fragmented gDNAs were tested for size distribution and concentration using an Agilent Bioanalyzer 2100 and Nanodrop. Illumina libraries were made from qualified fragmented gDNA using NEBNext reagents (New England Biolabs, Ipswich, MA, catalog no. E6040), and the resulting libraries were subjected to exome enrichment using NimbleGen SeqCap EZ Human Exome Library v2.0 (Roche NimbleGen, Inc., Madison, WI, catalog no. 05860482001) following the manufacturer’s instructions. Enriched libraries were tested for enrichment by qPCR and for size distribution and concentration by an Agilent Bioanalyzer 2100. The samples were then sequenced on an Illumina HiSeq2000, which generated paired-end reads of 100 nucleotides. Data were aligned to the hg19 reference genome and analyzed using DNAnexus (DNAnexus, Inc, Mountain View, CA). Briefly, after employing the DNAnexus mapper, the data were subjected to quality-control analysis and exome analysis followed by a variation and small indel analysis.
THPO protein modeling
The structure of the receptor-binding domain of THPO was downloaded from Protein Database (PDB-ID:1V7N). The chain V was applied for modeling using the Molecular Operating Environment (MOE, V. 2011.10, Chemical Computing Group) software suite.40,41 A virtual mutant R17C was created and inspected in MOE for possible disulfide bond formation. To build a reasonable model for the mutant, we used a 2-stage energy minimization process. First, the loop between C144 and C151 was rearranged by an energy minimization, whereas the remaining structure was fixed. Second, a minimization was conducted in which all atoms were unfixed. CHARMM27 force field was used and gradient was set to 0.01 for both energy minimizations.
Cloning and mutagenesis
A pCMV-Sport6 expression vector containing full-length cDNA of murine mThpo (BC003803, Thermo Scientific, Waltham, MA) was used for mutagenesis. The R17C (c.615 CGT>TGT) mutation (designated R17C-Thpo) was generated using the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol.
THPO-conditioned media collection
Human 293T kidney epithelial cells were grown in Dulbecco’s modified Eagle medium (Cellgro, Manassas, VA) and supplemented with 10% heat-inactivated fetal bovine serum (Thermo Scientific HyClone). The transient transfections of 293T cells with the pCMV-Sport6 expression plasmid harboring either wild-type (WT) mThpo or R17C-Thpo were performed in 10-cm cell culture plates with FuGENE reagent (Promega, Madison, WI) according to the manufacturer’s protocol, at a reagent/DNA ratio of 3:1 in 12 mL of the same medium. In parallel, transient transfection of 293T cells with a control pCMV-GFP plasmid (no Thpo) was performed under the same conditions as both a negative control and a measure of transfection efficiency. Transfection efficiency under these conditions was ∼80%. Conditioned media for all 3 transfection conditions were collected at 24 and 48 hours posttransfection, combined, filtered, and stored at −80°C until use.42
Cell culture of UT-7/TPO cells
Megakaryocyte progenitor UT7-TPO cells were cultured according to the protocol developed by Komatsu et al43,44 Briefly, UT7-TPO cells were grown in suspension in Iscove’s modified eagle medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 4 mM l-glutamine (Cellgro), and 10 ng/mL recombinant human THPO (R&D systems). Cells were passaged regularly at a density of ∼5 × 104/mL.
MTT cell proliferation assay
The MTT cell proliferation assay for UT7-TPO cells was adapted from Komatsu et al.43,44 UT7-TPO cells were starved of recombinant THPO for 16 hours before seeding 1 × 104 cells/well in a 96-well plate. The indicated amount (0.5-20 μL) of conditioned medium was added to each well in a total media volume of 250 μL. After 3 days of incubation, 100 μL of cell volume was transferred to a microplate and treated with 10 μL of 5 mg/mL MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Sigma), a yellow tetrazole. After 6 hours of incubation at 37°C, 100 μL of dimethylsulfoxide was added to solubilize the resulting MTT formazan crystals. Plate was mixed for 10 minutes on titer shaker at room temperature and absorbance was measured at 540 nm on an enzyme-linked immunosorbent assay plate-reader (Molecular Devices, Sunnyvale, CA). Background absorbance of medium alone was subtracted from all wells. A standard curve using defined numbers of UT7-TPO cells was used to determine the number of cells following treatment. All treatments were performed in triplicate. Results indicate mean ± standard error of the mean from 4 to 6 independent experiments.
Immunodepletion of THPO
Antibodies specific to mouse THPO (R and D Systems, AF-488-NA) and human THPO (R and D Systems, MAB288) were conjugated to magnetic Dynabeads (Invitrogen) at 10 μg/mL concentration. A total of 5 μg of conjugated antibody was incubated with 500 μL of WT or R17C conditioned media or cell lysate overnight at 4°C. A magnetic stand was used to remove the Dynabeads, and the remaining immunodepleted conditioned media or lysate was used in experiments outlined in supplemental Figure 2.
Results
Clinical evaluation of the Micronesian family with aplastic anemia
We identified a proband of Micronesian descent with idiopathic severe aplastic anemia. Known genetic causes of aplastic anemia and bone marrow failure syndromes were excluded based on the normal clinical physical examination, absence of increased chromosome breakage, and normal telomere length. Subsequent routine hematological studies identified aplastic anemia in the proband’s older brother (individual II.2) (Table1, Figure 4). The plasma THPO level in the proband was normal as well. In this family of 14 siblings, 2 additional children reportedly died soon after birth of unknown cause and 2 older brothers succumbed to fatal aplastic anemia in their adolescence. The parents and 1 nonanemic brother had mild and asymptomatic thrombocytopenia (Table 1). We were able to obtain DNA samples from the second anemic brother (II.2) and 2 nonanemic siblings, one with low (II.3) and one with normal (II.4) platelet counts (Table 1).
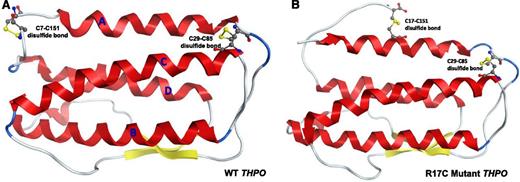
In silico model showing the effect of R17C mutation on disulfide bonding in THPO receptor-binding domain. (A) The THPO receptor-binding domain consists of a 4-helix bundle (red, shown as letters A-D) and a short antiparallel β sheet formed by 2 strands. The domain contains 4 highly conserved cysteine residues (C7, C29, C85, and C151), which form 2 disulfide bonds. Due to position constraints, the mutant C17 cannot form a disulfide bond with any of C7, C29, and C85 residues without disrupting the overall structure but can form a disulfide bond with C151 as shown in B.
In silico model showing the effect of R17C mutation on disulfide bonding in THPO receptor-binding domain. (A) The THPO receptor-binding domain consists of a 4-helix bundle (red, shown as letters A-D) and a short antiparallel β sheet formed by 2 strands. The domain contains 4 highly conserved cysteine residues (C7, C29, C85, and C151), which form 2 disulfide bonds. Due to position constraints, the mutant C17 cannot form a disulfide bond with any of C7, C29, and C85 residues without disrupting the overall structure but can form a disulfide bond with C151 as shown in B.
Although consanguinity was denied, we began our analysis with an SNP array CGH to identify possible ROH or copy number abnormalities to determine the candidate gene locus for aplastic anemia in this family. No regions of clinically significant ROH or copy number changes were found in this analysis.
Next we tested telomere length, because short telomeres are a recognized feature of patients with aplastic anemia. Mutations in genes encoding the telomerase complex result in short telomeres and some of these have been described in patients with apparently acquired severe aplastic anemia as well.1-3 In both affected siblings and their parents, we did not find significantly different telomere lengths compared with those for normal control individuals (Figure 1C). Therefore, we excluded the possibility that short telomeres were involved in the pathology of aplastic anemia in this family.
Exome analysis reveals a homozygous THPO variant
Exome sequencing was carried out with 100-bp paired-end reads at 40× coverage. The sequence data were analyzed using the DNAnexus cloud- and Web-based online DNA data analysis tools (Figure 2). We filtered for variants that were homozygous in the coding sequence and found in both affected siblings but not in dbSNP or in other databases (including 1000 Genomes and all publicly available Complete Genomics human genome datasets) (Figure 2). Following filtering, a total of 7 homozygous variants in 7 genes were identified that were common between the 2 affected siblings (Figure 2).
Next we sequenced these 7 variants to verify proper segregation in the proband, parents, and an unaffected sibling. Two of the variants in FAM75A6 and PRSS2 turned out to be false-positive and were actually heterozygous (Figure 4). Two additional variants were excluded, because they were also homozygous in one (LRRC16A) or both (MUC12) parents. Segregation for the remaining 3 variants in FOXJ1, IL11RA, and THPO genes remained consistent with the phenotypes. However, further in silico analysis using PolyPhen, SIFT, and pMUT mutation prediction algorithms strongly indicated that the FOXJ1 and IL11RA variants are benign in nature. The THPO variant (CGT>TGT; p.R17C) (Figures 3 and 4B) not only segregates properly but is also predicted by all three algorithms to be damaging and pathological. Therefore, we carried out functional analysis of the THPO variant. In addition, manual inspection of MPL (THPO receptor) exome sequence did not show any pathogenic variants.
R17C mutation likely disturbs the receptor binding domain structure
To examine the possibility that this novel missense variant in the THPO ligand affects receptor binding, we carried out in silico protein modeling. THPO receptor-binding domain consists of a 4-helix bundle and a short antiparallel β sheet formed by 2 strands (Figure 5). The domain contains 4 highly conserved cysteine (C) residues at positions 7, 29, 85, and 151 (C28, C50, C106, and C172, including the cleaved 21 aa signal peptide). These 4 cysteines form 2 disulfide bonds between C7-C151 and C29-C85 (Figure 5A). The mutant C17 is in the middle of helix A, while C7 and C29 are close to the 2 termini of the helix, making it sterically unlikely that these cysteines form disulfide bonds. Similarly, C85 is close to the end of helix C in proximity to C29 (Figure 5A). Although there is a possibility that helix A containing the mutant C17 could be shifted to allow an interaction with C85, we consider it highly disruptive and unlikely. However, it is reasonable for C151, which is in the middle of an unstructured loop, to form a disulfide bond with mutant C17 instead of normal C7 (Figure 5A vs 5B). Therefore, we created a structural model to test the possibility of a disulfide bond between mutant C17 and C151. We broke the existing disulfide bond between C7 and C151 and then formed one between mutant C17 and C151 (Figure 5B). For comparison, the WT loop was also subjected to an energy minimization using the same parameters as for the mutant. MOE potentials for the WT protein and R17C mutant are −580.32 and −594.42 kcal/mol, respectively. It is noteworthy that energy minimization used in the study did not consider solvent effects. In addition, the unstructured terminal regions of both the WT and mutant were not modeled. Nevertheless, our modeling analysis indicates that disulfide bond formation between mutant C17 and C151 (Figure 5B) is at least as likely as that between WT C7 and C151 (Figure 5A). Thus, our analysis suggests that the mutant C17 residue can disrupt normal disulfide bonding in the THPO RBD, thereby affecting normal THPO-MPL signaling.
R17C mutant THPO protein is functionally defective
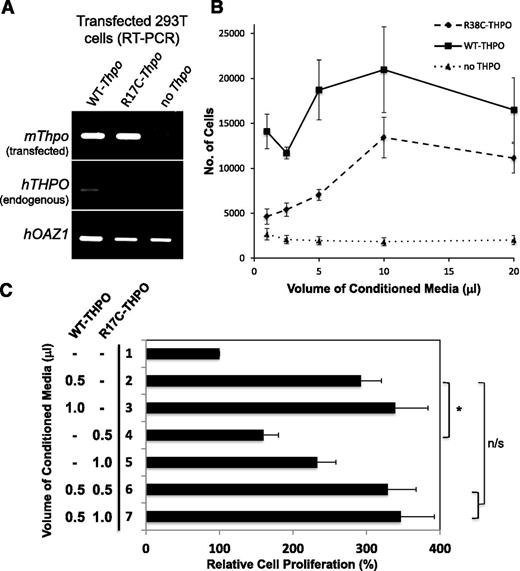
To determine the effect of the R17C mutation on THPO function, we used a cell line (UT7-TPO) that is dependent on exogenous THPO ligand for its proliferation.43,44 UT-7 is a human megakaryoblastic leukemia cell line with absolute dependence on interleukin-3, granulocyte-macrophage colony-stimulating factor, or erythropoietin for growth and survival. The cell line UT-7/TPO was established from UT-7/GM and has morphologically mature megakaryocytic characteristics with absolute dependence on THPO for growth and survival. It has been used as a model to analyze the gene regulation of megakaryocytic maturation-associated proteins and study the specific actions of THPO.20 We first mutagenized a murine WT-Thpo expression construct to generate the R17C (CGT>TGT) mutation. The control (no Thpo), WT Thpo, and R17C mutant Thpo expression constructs were transfected into human embryonic kidney 293T cells. The transfected cells were cultured for 48 hours and the conditioned media were collected. The transfected cells were analyzed by RT-PCR for transfected murine mThpo and endogenous human hTHPO expression (Figure 6A upper panel) relative to hOAZ1 housekeeping gene expression (Figure 6A lower panel). Both WT and R17C mutant Thpo show equivalent expression, whereas there is no expression with the control construct, as expected (Figure 6A upper panel). Also, no significant expression of endogenous hTHPO was detected in human embryonic kidney 293T cells at the RNA (Figure 6A middle panel) or protein (supplemental Figure 1) levels; thus, the conditional media contained only murine THPO ligand derived from the WT and mutant transfected constructs.
R17C mutant THPO protein is deficient in maintaining UT7-TPO cells. (A) Conditioned media were collected from human embryonic kidney (293T) cells transfected with murine WT-Thpo, mutagenized R17C-Thpo, and control (no Thpo) constructs. The transfected cells were analyzed by RT-PCR. In the top, both WT and R17C mutant mThpo transfections show equivalent expression and none with the control construct (no Thpo). There is no significant endogenous expression of hTHPO in mutant transfected human embryonic kidney cells (middle). The faint hTHPO background band visible in the WT-Thpo transfected sample is due to increased overall transcript levels for WT-Thpo compared with R17C-Thpo and no-Thpo, as seen with control hOAZ1 transcript levels (bottom). (B) 1 × 104 UT7-TPO cells, starved for commercial THPO for 16 hours, were treated with indicated volumes of WT, R17C mutant, or control conditioned media in a 96-well plate. Following 3 days of growth, proliferation was measured using MTT assays. WT THPO-containing conditioned media is able to maintain significantly increased UT7-TPO cell proliferation than the R17C mutant conditioned media at volumes of 1 μL (P < .03), 2.5 μL (P < .0002), and 5 μL (P < .02). Conditioned media with no exogenous THPO were not able to maintain cell proliferation. All treatments were done in triplicate. Results indicate mean ± standard error of the mean from 4 independent experiments. (C) 1 × 104 UT7-TPO cells were treated with indicated amounts of WT and R17C mutant conditioned media as above. MTT assay results following 3 days of growth are shown compared with baseline proliferation in the absence of THPO (lane 1). WT THPO shows significantly greater proliferation of UT7-TPO cells than mutant R17C THPO (lane 2 vs 4; *P < .01). Mutant R17C THPO is not able to reduce proliferation by WT THPO (lane 2 vs 6) even when twice as much mutant THPO is added (lane 2 vs 7). n/s, not significant. All treatments were done in triplicate. Results indicate mean ± standard error of the mean from 6 independent experiments.
R17C mutant THPO protein is deficient in maintaining UT7-TPO cells. (A) Conditioned media were collected from human embryonic kidney (293T) cells transfected with murine WT-Thpo, mutagenized R17C-Thpo, and control (no Thpo) constructs. The transfected cells were analyzed by RT-PCR. In the top, both WT and R17C mutant mThpo transfections show equivalent expression and none with the control construct (no Thpo). There is no significant endogenous expression of hTHPO in mutant transfected human embryonic kidney cells (middle). The faint hTHPO background band visible in the WT-Thpo transfected sample is due to increased overall transcript levels for WT-Thpo compared with R17C-Thpo and no-Thpo, as seen with control hOAZ1 transcript levels (bottom). (B) 1 × 104 UT7-TPO cells, starved for commercial THPO for 16 hours, were treated with indicated volumes of WT, R17C mutant, or control conditioned media in a 96-well plate. Following 3 days of growth, proliferation was measured using MTT assays. WT THPO-containing conditioned media is able to maintain significantly increased UT7-TPO cell proliferation than the R17C mutant conditioned media at volumes of 1 μL (P < .03), 2.5 μL (P < .0002), and 5 μL (P < .02). Conditioned media with no exogenous THPO were not able to maintain cell proliferation. All treatments were done in triplicate. Results indicate mean ± standard error of the mean from 4 independent experiments. (C) 1 × 104 UT7-TPO cells were treated with indicated amounts of WT and R17C mutant conditioned media as above. MTT assay results following 3 days of growth are shown compared with baseline proliferation in the absence of THPO (lane 1). WT THPO shows significantly greater proliferation of UT7-TPO cells than mutant R17C THPO (lane 2 vs 4; *P < .01). Mutant R17C THPO is not able to reduce proliferation by WT THPO (lane 2 vs 6) even when twice as much mutant THPO is added (lane 2 vs 7). n/s, not significant. All treatments were done in triplicate. Results indicate mean ± standard error of the mean from 6 independent experiments.
Increasing amounts of control (no THPO), WT THPO, and R17C mutant THPO containing conditioned media were added to 1 × 104 UT7-TPO cells, which were starved for commercial THPO for 16 hours prior to the experiment. Cell proliferation was measured after 3 days of culture by using the MTT assay. Indeed, WT THPO-containing conditioned media is able to maintain UT7-TPO cell proliferation ∼2.5-fold better than the R17C mutant-containing conditioned media (Figure 6B) at lower volumes. The difference is less pronounced at high volumes, likely due to oversaturation of available THPO receptor sites. Consistently, 10 ng/mL of commercial THPO ligand, used to propagate UT7-TPO cells, maintained an average of ∼7000 cells (data not shown) compared with ∼14 000 cells with 1 μL of WT THPO-containing conditioned media and ∼4600 cells with 1 μL of R17C mutant THPO-containing conditioned media. As control, the identically prepared conditioned media with no exogenous THPO (no THPO) was not able to maintain UT7-TPO cells, showing that the observed cell proliferation is due to exogenously produced THPO ligand. We also confirmed by immunodepletion that the WT and R17C mutant THPO activity is specifically due to overexpression of murine THPO (supplemental Figure 2A-B) and that any residual human THPO does not have an effect (supplemental Figure 2A-C). Our results show that the R17C mutant THPO ligand is defective in its ability to activate the THPO receptor (MPL) signaling cascade. To determine if R17C mutation has a dominant-negative effect on WT THPO function, we added increasing amounts of R17C mutant THPO (0.5 or1.0 μL) to a fixed amount (0.5 μL) of WT THPO (Figure 6C). Again, 0.5 μL of WT THPO showed approximately twofold better proliferation of UT7-TPO cells than did the R17C mutant (Figure 6C, lane 2 vs 4; P < .01). Despite addition of equal (0.5 μL; Figure 6C, lane 6) or even twice as much mutant THPO (1.0 μL; Figure 6C, lane 7), WT THPO (0.5 μL) proliferation of UT7-TPO cells was not affected (Figure 6C, lane 2 vs 6 and 7). Thus, R17C mutant THPO has reduced function but is not dominant-negative in our assay.
Discussion
The cytokine THPO is known to play an important role in stimulating the proliferation of megakaryocytes and platelets. Activating gain-of-function mutations in THPO have been shown to cause increased platelet production,25-29 wheras loss-of-function mutations in MPL receptor have been reported to result in thrombocytopenia, pancytopenia, and recently aplastic anemia.5,37-39 No loss-of-function mutations have been reported in THPO thus far. However, Mandrile et al recently reported 3 unrelated patients with similar syndromic phenotypes carrying heterozygous 3q26.33q27.2 microdeletions that include THPO gene.45 Two of these 3 patients also have mild thrombocytopenia. We have further identified a fourth patient with an overlapping 3q27.1 microdeletion, who also shows mild thrombocytopenia among other phenotypes (Majed Dasouki, Jennifer Roberts, Angela Santiago, Irfan Saadi, Karine Hovanes; submitted manuscript, August 2013). These findings lend additional support to our identification of mild thrombocytopenia in the parents and siblings with heterozygous R17C THPO mutation in our Micronesian family. Together with the aplastic anemia phenotype of our patients with homozygous R17C THPO mutation, these results suggest that megakaryocyte proliferation and platelet production are most sensitive to THPO gene dosage; however, THPO signaling is also required for erythroid and myeloid proliferation. Furthermore, as expected, it is important to note that the THPO point mutation in our proband did not alter the plasma THPO level. Thus, in addition to plasma THPO levels, mutation analysis of THPO gene may be a useful diagnostic tool in a subset of patients with aplastic anemia.
Our mutation converts an arginine residue to a cysteine at position 17 in the MPL binding domain (RBD) of THPO. This position 17 in THPO RBD was previously identified in site-directed mutagenesis studies to be important for receptor binding.42,46,47 Pearce et al46 showed a 2.54-fold reduction in receptor binding by an R17A mutant THPO. Park et al47 showed that an R17N, but not an R17A, substitution resulted in a significantly reduced activity, indicating that R17 lies close to residues that directly interact with the receptor. Thus, our R17C mutation affects an important residue and introduces a potentially highly disruptive fifth cysteine in the RBD. In silico modeling analysis indicates slightly better than equal plausibility of a mutant C17-C151 disulfide bond to the WT C7-C151 bond. Together, these analyses are consistent with the observed two- to threefold reduced activity of mutant R17C THPO in UT7-TPO proliferation assays.
Exome sequence analysis shows that the MPL receptor is normal in our patients with the THPO mutation (see Methods). Thus, THPO-mimetics might serve as potential therapy in our patients. Romiplostim and eltrombopag are FDA-approved THPO mimetics (MPL agonists) used in the treatment of patients with idiopathic thrombocytopenic purpura48,49 and MYH9 related inherited thrombocytopenia.50,51 More recently, eltrombopag was reported to improve hematopoiesis in patients with refractory aplastic anemia.52 However, these patients with aplastic anemia were not genotyped for possible mutations in THPO or MPL. We speculate that some of these patients who responded to treatment with eltrombopag might carry recessive mutations in the THPO gene, whereas those with mutations in MPL or other unrelated pathways would not benefit from this therapy. If true, this “personalized medicine” approach will greatly enhance therapeutic responsiveness and prevent unnecessary treatment and associated side effects.
The data reported in this article have been deposited in the NCBI ClinVar (accession number not yet available).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Drs. Ian Hitchcock and Kenneth Kaushansky (Stony Brook University, Stony Brook, NY) for the valuable gift of the UT7-TPO cell line, Dr Kenneth Peterson (University of Kansas Medical Center, Kansas City, KS) for K562 cell lysate, and Diana Acevedo (University of Kansas Medical Center, Kansas City, KS) for assistance with experiments. We sincerely thank the family reported here for their willingness to participate in this research and the University of Kansas Medical Center-Research Institute for its support.
This project was supported in part by the National Institutes of Health Kansas IDeA Network for Biomedical Research Excellence grant (National Center for Research Resources P20 RR016475 and National Institute of General Medical Sciences P20 GM103418, M.J.D. and I.S.), the Clinical and Translational Science Awards grant “Frontiers: The Heartland Institute for Clinical and Translational Research” (National Center for Research Resources, UL1 RR033179 and National Center for Advancing Translational Sciences, UL1TR000001, M.J.D.), Kansas Intellectual and Developmental Disabilities Research Center grant (P30 Eunice Kennedy Shriver National Institute of Child Health and Human Development, HD 002528, I.S.), and Center of Biomedical Research Excellence grant (National Institute of General Medical Sciences P20 GM104936, I.S.).
Authorship
Contribution: S.A. and M.J.D. conducted clinical evaluation; M.J.D. performed the SNP aCGH study; M.J.D., B.G, and L.M.F. performed exome analysis; J.F. performed the in silico modeling; R.T.C. conducted telomere length analysis; S.R., A.O.S., N.R.W., and I.S. conducted functional studies and DNA Sanger sequencing; and M.J.D. and I.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Majed Dasouki, Departments of Pediatrics and Internal Medicine, University of Kansas Medical Center, Kansas City, KS; e-mail: mdasouki@kumc.edu; and Irfan Saadi, Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS; e-mail: isaadi@kumc.edu.