Abstract
Initial results of the Spanish GEM2005mas65 phase III trial comparing VMP versus VTP as induction therapy in elderly patients with newly diagnosed MM did not show any difference in terms of OS or PFS. The purpose of this study was to update clinical results with a longer follow-up. Secondary objectives were to know the patterns of symptomatic relapse and response to therapy in patients previously exposed to novel agents.
GEM2005mas65 trial lasted from March, 2006, and October, 2008. Overall, 260 patients with untreated MM, 65 years and older, from 63 Spanish centres, were randomly assigned to receive six cycles of VMP (n=130) or VTP (n=130) as induction therapy followed by maintenance therapy with bortezomib plus prednisone (n=87) or bortezomib plus thalidomide (n=91).
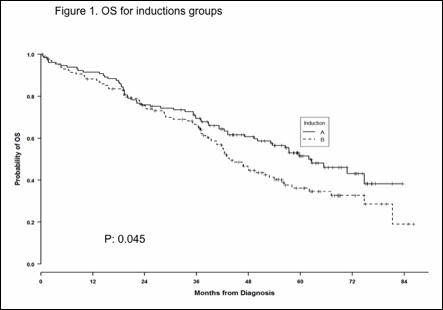
With a median follow-up of 53.2 months, median OS of the entire cohort of patients was 42.45 months. Median OS was 62 months in patients receiving VMP versus 42.6 months in patients receiving induction with VTP (P = 0.045) (Figure 1). No differences were observed in PFS, being 31 and 24.4 months in the VMP and VTP groups, respectively.
At the time of the study, 167 patients had relapsed or progressed and 92 of them received some kind of post relapse treatment. Front-line therapy and clinical and biological characteristics at the time of relapse in this subgroup of patients are summarized in Table 1.
Baseline characteristics of patients with symptomatic relapse
| Characteristic . | N/n (%) . | Median(range) . |
|---|---|---|
| Front-line induction therapy | ||
| VMP* | 40/92 (43.4) | |
| VTP** | 52/92 (56.5) | |
| Exposed to bortezomib and thalidomide | 66/92 (71.7%) | |
| Exposed to bortezomib | 26/92 (28.2%) | |
| Male | 43/92 (46.7) | |
| Age (years) | 74 (65-87) | |
| M protein | ||
| IgG | 51/92 (55.4) | |
| IgA | 32/92 (34.7) | |
| Ligtchain | 9/92 (9.7) | |
| ISS stage | ||
| 1 | 20/74 (27) | |
| 2 | 32/74 (43.2) | |
| 3 | 22/74 (29.7) | |
| Creatinine> 2 mg/dL | 11/92 (11.9) | |
| Hemoglobin | 11.5 (6.7-15.3) | |
| Calcium> 11 mg/dL | 4/92 (4.3) | |
| Plasma cell leukemia | 4/91 (4.4) | |
| Extramedullary plasmacytoma | 15/88 (17) | |
| Multiple bone lesions | 39/80 (48.7) |
| Characteristic . | N/n (%) . | Median(range) . |
|---|---|---|
| Front-line induction therapy | ||
| VMP* | 40/92 (43.4) | |
| VTP** | 52/92 (56.5) | |
| Exposed to bortezomib and thalidomide | 66/92 (71.7%) | |
| Exposed to bortezomib | 26/92 (28.2%) | |
| Male | 43/92 (46.7) | |
| Age (years) | 74 (65-87) | |
| M protein | ||
| IgG | 51/92 (55.4) | |
| IgA | 32/92 (34.7) | |
| Ligtchain | 9/92 (9.7) | |
| ISS stage | ||
| 1 | 20/74 (27) | |
| 2 | 32/74 (43.2) | |
| 3 | 22/74 (29.7) | |
| Creatinine> 2 mg/dL | 11/92 (11.9) | |
| Hemoglobin | 11.5 (6.7-15.3) | |
| Calcium> 11 mg/dL | 4/92 (4.3) | |
| Plasma cell leukemia | 4/91 (4.4) | |
| Extramedullary plasmacytoma | 15/88 (17) | |
| Multiple bone lesions | 39/80 (48.7) |
VMP: Bortezomiob, melphalan, and prednisone;
VTP:Bortezomib, Thalidomide, prednisone
Initial induction treatment consisted of VMP in 40 (43.4%) patients and VTP in 52 (56.5%) patients. Overall, after induction and maintenance therapy 66 (71.7%) patients had been exposed to bortezomib and thalidomide and 26 (28.2%) patients received bortezomib-based therapy without immunomodulatory agents before relapse.
At symptomatic relapse, median age was 74 years (range 65-87) and 43 (46.7%) were male. Serum creatine level > 2 mg/dL was present in 11.9% of the patients and 4.3 % had hypercalcemia. Extramedullary disease and plasma cell leukemia was present in 17% and 4.4% of patients, respectively. Finally, severe bone disease was observed in 39 the patients.
Management of relapse was very heterogeneous. Subsequent drugs used in first clinical relapse included lenalidamide combinations in 40 (43.4%) patients, bortezomib-based therapy in 19 (20.5%) patients, different chemotherapy combinations in 25 (27.1%) patients and bendamustine-prednisone in 2 (2.1%) patients. Six (6.5%) patients received only supportive at time of first relapse. Overall, 45 (52%) patients achieved partial response or better after rescue therapy. Response rates were higher after lenalidomide-based therapy (62.5%) when compared with bortezomib (42.1%) and conventional chemotherapy-based regimens (40.7%),although this difference did not translate into significant PFS advantage.
After a median follow-up of 16.4 months (range 0-50.2) from clinical relapse,58 (63%) patients had died. Median PFS and OS were 10 (CI95%; 10.7-50.2) and 15.6 months (CI95%; 8.5-16.5%), respectively. In patients initially treated with VMP (n = 40) and VTP (n =52) median survival from start of subsequent therapy was 15.6 and 15.4 months, respectively. Among characteristics at relapse, only ISS stage had an impact on survival (P= 0.03).There was no difference in OS or PFS from front-line induction and/or maintenance therapies or salvage therapy.
This updated analysis of the GEM2005mas65trial shows a survival advantage of the VMP arm when compared with patients receiving VTP. Patterns of relapse were similar regardless previous induction and maintenance therapy. Finally, and when analyzing the subgroup of patients receiving active therapy at relapse, only ISS stage seemed to have a prognostic impact on OS.
Group A: VMP, group B : VTD
Mateos:Janssen, Celgene, Onyx, Millennium, Mundipharma: Honoraria. Oriol:Celgene: Consultancy. Off Label Use: Bendamustine, bortezomib and prednisone is a combination not approved for newly diagnosed myeloma patients.
Author notes
Asterisk with author names denotes non-ASH members.